Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista colombiana de Gastroenterología
versão impressa ISSN 0120-9957
Rev Col Gastroenterol vol.31 no.3 Bogotá jul./set. 2016
Most Relevant Pathology Issues in the Late Post Liver Transplant Period
Rocío del Pilar López Panqueva MD (1)
(1) Pathologist at the Hospital Universitario Fundación Santa Fe de Bogota and the University of the Andes. Bogotá, Colombia.
Received: 10-07-16 Accepted: 25-07-16
Abstract
One year survival rates of liver transplant patients exceed 90% while five year survival rates exceed 75%. Understanding the causes of graft losses and patient deaths is essential for further improvement of long-term results. Evaluation of liver biopsies has an important role in explaining liver graft dysfunction that occurs more than one year after transplantation, and thus is key for post-transplant patient management. The interpretation of these biopsies can be very difficult especially because of the high incidence of recurrent diseases that sometimes have clinical and histopathological features that resemble various other conditions. This is especially true for acute and chronic rejection which can overwhelm an existing condition and which can develop simultaneously with other conditions that contribute to late graft dysfunction. Analysis of the biopsy can help determine the main component of a lesion. Clinical findings must be correlated to pathological findings, and the correlation must take into account the original disease, the type of immunosuppression, liver function tests, viral serology, autoantibodies and radiological findings. In this article I will discuss the most common diseases and those that cause the most problems for diagnosis during the late post-transplant period.
Keywords
Liver biopsy, liver transplantation, chronic rejection, ductopenia, recurrent viral hepatitis C, recurrent autoimmune hepatitis, de novo autoimmune hepatitis, recurrent primary biliary cirrhosis, recurring primary sclerosing cholangitis.
INTRODUCTION
The numerous non-surgical complications observed in liver transplantation include alcoholic and non-alcoholic steatohepatitis, drug toxicity, Budd-Chiari syndrome, tumors, pseudotumors, sarcoidosis, granulomatous hepatitis, idiopathic diseases and post infantile giant cell hepatitis. Usually they are not difficult to diagnose given their histopathology and clinical similarity to pathologies unrelated to transplantation Recurrent or de novo disease, especially infectious viral hepatitis B and C, pathologies that involve dysregulation of autoimmunity such as autoimmune hepatitis, primary biliary cirrhosis, primary sclerosing cholangitis, and chronic graft rejection, are those that pose the greatest diagnostic difficulties and challenges both clinically and from the morphological point of view. These topics will be reviewed in this article. (1)
IS A LIVER BIOPSY USEFUL?
Evaluating needle biopsies and appropriate clinical correlation in the late post-transplant period play important roles in diagnosing graft dysfunction. Whether biopsies should be part of the protocol is controversial, some medical groups perform them in totally asymptomatic patients or in patients with minimal changes in liver function tests. The interpretation of these biopsies can be very difficult because morphological analysis to demonstrate atypical, complex or mixed patterns is difficult. This is especially true because more than one factor can contribute to graft dysfunction. They influence the severity of histopathological findings, levels of immunosuppression, autoimmune processes, hepatotoxicity, and also the high incidence of recurrence. These are some of the factors that can hide acute or chronic rejection as well as the true underlying condition that affects the body from the clinical serological and histopathological points of view. (1, 2)
A liver biopsy may be useful for early detection of disease that is not clinically apparent disease, for identifying receptors that might be used to reduce or remove immunosuppression successfully, for recognizing the rapid progression of recurrent hepatitis B or C, for guiding therapy according to response to treatment, for assessing the impact of alcohol consumption, for confirming ductopenia, and for clarifying whether ductopenia is part of chronic rejection or conversely indicates recurrence of chronic cholestatic disease. Among others, these are some of the benefits of post-transplant biopsies. (3, 4)
Histological abnormalities present in most transplant patients cannot be identified with liver function tests which are usually normal. This lack of sensitivity and specificity justifies protocol biopsies. (5) The incidence and the true meaning of morphological abnormalities observed in protocol biopsies from asymptomatic patients is dependent on the underlying disease of the recipient of the transplanted organ. Viral hepatitis C, primary biliary cirrhosis and autoimmune hepatitis recur most frequently. (5, 6) Findings are not yet well understood and require further study in about 75% of patients without recurrent disease, without abnormalities in liver function tests, who have mild symptoms found histopathologically in a biopsy, especially those attributable to effects of immunosuppression such as nodular regenerative hyperplasia or hyalinization of hepatic arteries, and patients with non-specific inflammatory portal or lobular alterations. Most findings for symptomatic patients and patients with abnormal liver function tests patients are attributable to chronic rejection, recurrence of the underlying disease or events secondary to surgical complications such as strictures of the bile duct and vascular anastomoses. (7, 8)
CHRONIC REJECTION
Chronic transplant rejection results from chronic injuries of small bile ducts and/or vascular endothelial injury in arteries of medium caliber. Its incidence is 3% to 5%, and most cases occur simultaneously. In biopsies it is usually possible to corroborate ductopenia but not vascular disease and arterial pathologies of the graft which can practically never be observed in biopsies due to the lack of representation of medium and large vessels which are most often compromised in the hepatic hilum. It should be added that very occasionally alterations have been described in medium-sized arterioles, portal veins and sinusoid veins in biopsies. Diagnosis must consider a number of inflammatory, infectious and recurrent entities which affect bile ducts and which are particularly difficult to identify in early stages. (9) The main risk factors include patient history of episodes of acute cellular rejection, rejection refractory to treatment, inadequate immunosuppression, and use of immune response activators such as interferon which increase the likelihood of chronic rejection and which have also implicated in the pathogenesis of persistent cytomegalovirus infections. (10, 11)
The criteria for the diagnosis of chronic rejection are:
Injury to ducts
- Degenerative changes in the epithelium of more than 50% of the bile ducts
- Loss of over 50% of bile ducts
Vascular injury
- Obliterative arterial disease with accumulation of foamy cells in the arterial wall (1,9)
Chronic Ductopenic Rejection or Vanishing Bile Duct Syndrome
Vanishing bile duct syndrome, defined as the loss of bile ducts in the post-transplant period, is typical of chronic rejection. Bile ducts are one of the main targets of the immune response. There is a dual mechanism of bile duct loss in allograft rejection: first ductal inflammation produces marked cytotoxicity mediated by T cells, then ischemia caused by obliterating arteriopathy develops. (10, 11) Clinically, this manifests as chronic cholestasis in a patient for whom there is no evidence of obstruction, injury to the extrahepatic bile ducts, drug toxicity or recurrence of cholestatic pretransplant disease.
Ductopenic Histopathology Findings Of Chronic Rejection (12)
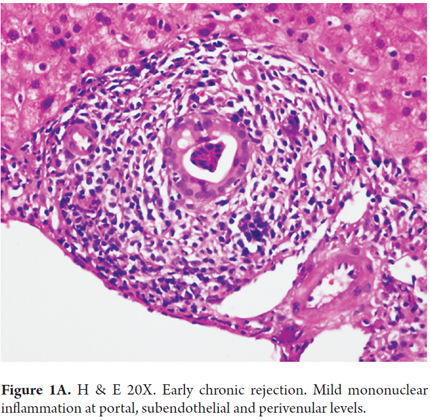
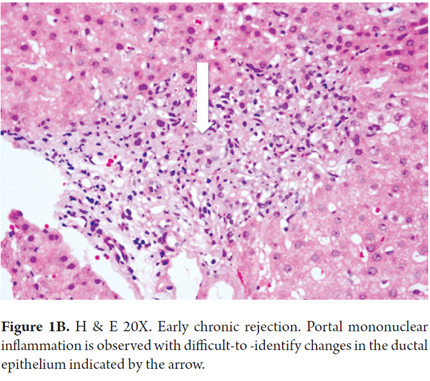
Early changes which occur mostly in small bile ducts include atrophy and flattening of the ductal epithelium with the presence of only 2-3 epithelial cells, enlarged hyperchromatic nuclei and cytoplasmic eosinophilia. This is generally accompanied by mild portal inflammation. The terminal hepatic venules and the surrounding parenchyma are characterized by subendothelial mononuclear inflammation and are accompanied by lipid-laden macrophages, bile pigment, centrilobular cholestasis, hepatocellular ballooning and isolated necrosis with mild perivenular fibrosis (Figures 1A and B). These changes are described as transitional hepatitis during progression to late chronic rejection. A finding of perivenular necrosis and fibrosis in repeated biopsies is considered to be a sign of poor prognosis. All of these findings are potentially reversible.
Late changes include loss or disappearance of bile ducts and destructive cholangitis in at least half (50%) of the portal tracts adequately represented. This is usually accompanied by loss of hepatic arterioles, little or no portal inflammation and ductile reaction (Figure 2). Complementary studies of cytokeratin can corroborate absence of ducts. Late changes can also include perivenular fibrosis, central-portal bridges, isolation of hepatocytes with canalicular cholestasis, regenerative hyperplasia and sinusoidal foam cell clusters (Figure 3).
Table 1 summarizes morphological findings of chronic rejection as established at the 5th Banff Conference of 1999 and which remain in force to date. (12)
Differential diagnosis for the pattern of ductal damage in chronic rejection should include complications not related to rejection such as obstructive cholangiopathy, stenosis of the hepatic artery, arterial thrombosis, adverse drug reactions and cytomegalovirus infections. The final diagnosis should be based on clinical, radiological, laboratory and histopathological criteria. (12)
RECURRENT DISEASE
Diagnosis of listed potentially recurrent hepatic diseases through the use of biopsies is controversial because histopathological changes overlap with, or may be modified by, acute or chronic rejection or obstruction of a bile duct. Nevertheless, histopathology is part of the diagnostic criteria of all of these conditions.
Viral Hepatitis C (HVC)
Chronic infection with the hepatitis C virus (HCV) is one of the main conditions that leads to liver transplantation in adults, and recurrence of HCV infection after liver transplantation is universally considered to be one of the most important causes of morbidity and mortality in the post-transplant period. The diagnosis of recurrence requires demonstration of the presence of HCV RNA levels in serum followed by histological confirmation.
Reinfection occurs as the result of viremia that persists into the post-transplant period since almost all patients are viremic at the time of transplantation. In addition, reinfection can occur during reperfusion of the graft in the operating room, and viral titers can even reach pre-transplant levels in the first 12 to 72 hours. The highest peaks of viral RNA are reached between 3 and 4 months after transplant, even higher than those presented in the time prior to transplantation. This is almost certainly a result of immunosuppression. In addition, peripheral monocytes may also harbor viruses and act as a source for reinfection of the transplanted liver. De novo infections in previously HCV-negative patients is totally unusual. (13, 14)
Clinical signs, symptoms and severity of recurrence vary: one third of patients remain stable or even totally asymptomatic, 25% -30% of patients whose initial condition is good evolve rapidly with deterioration of liver function and development of fibrosis three to seven years after transplantation, and more than half develop fibrosis in the next 10 years. Chronic and progressive liver disease is most common which is similar to what is observed in the immunocompetent population although the viral load is greater and progression to fibrosis is more rapid very likely due to early activation of stellate cells in progressive and chronic liver disease. Less than 10% developed aggressive cholestasis which is highly fatal. This is usually related to excessive immunosuppression and extremely high levels of viral RNA which produces a direct cytopathic effect on hepatocytes. There is also an increased risk of death in patients undergoing retransplantation a result of graft failure due to recurrence of HVC. (14-17)
Recurrent HVC is termed stable when protocol biopsies show no fibrosis (F0) or mild portal fibrosis (F1). Progression is defined as occurrence of one of the following criteria within the first year after transplantation: significant higher stage fibrosis (F2), signs and symptoms of portal hypertension, cholestatic hepatitis, liver function tests such as gamma glutamyl transpeptidase (GGT) or aminotransferases at least 5 times normal, elevated serum levels of RNA -HVC with typical histology, always in the absence of other complications. (18)
The most important variables that determine the progression of the disease are the use of organs from elderly donors, inadequate immunosuppression, comorbid conditions such as biliary complications that affect the quality of the graft, metabolic syndrome, diabetes, hyperlipidemia and obesity, NASH, and the use of drugs such as renin-angiotensin inhibitors and ursodeoxycholic acid. The only factor that can modify the natural history of recurrent disease is antiviral therapy to obtain a sustained viral response (SVR). A genome-wide association study can show relations among genetic factors that influence this response. Factors that influence SVR include the presence of genotype IL28 B, viral genotypes, proper adherence to immunosuppression and antiviral treatment and the absence of significant fibrosis at baseline and other factors related to both the donor and recipient. (16, 19)
Histological characteristics have been classified into three groups. (20)
1. Typical patterns of acute or chronic hepatitis C
2. Fibrosing cholestatic hepatitis C
3. Plasma-cell-rich hepatitis C
Typical Early-Stage Pattern of Recurrent Acute Hepatitis C
Histopathological features of early-stage recurrent HCV infections can be modified by immunosuppressive therapy. Frequently, the allograft is subject to concurrent processes which make it relatively difficulty to diagnose a recurrence of HCV, other causes of graft dysfunction, or the very unusual event of de novo hepatitis C. In the first weeks following transplantation, the main cause of dysfunction is acute rejection. After three to seven weeks, dysfunction is most likely due to a recurrence of HVC. The greatest difficulty for diagnosis is graft dysfunctions which occur between the third and fourth weeks after transplantation. (16)
In 75% of recipients, recurrence of acute hepatitis C occurs after an average of 86 days sometime between the between the fourth and twelfth weeks after transplantation. (21) Diagnosis in this very early period requires quantification of viral RNA and clinical and/or histopathological exclusion of other causes such as acute rejection, ischemic injuries, reperfusion changes and surgical complications all of which occur more frequently than does recurrence of acute HCV.
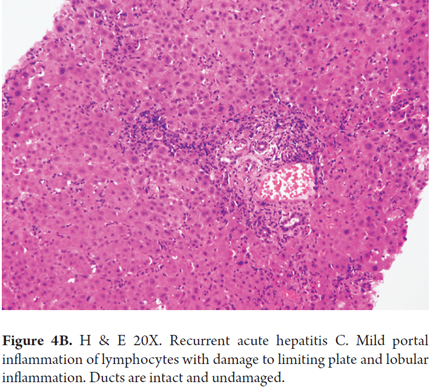
In the first weeks or months after transplantation the most useful histopathological features for distinguishing between recurrent hepatitis C and other causes of graft dysfunction are the combination of necrosis and inflammation, lobular hepatitis with sinusoidal inflammation of lymphocytes, the presence of occasional apoptotic hepatocytes, Councilman bodies, Kupffer cell activation and absence of significant portal inflammation (Figures 4A and 4B). The presence of more than 50 acidophilic or Councilman bodies is a b indication of recurrence of hepatitis C. These findings may or may not be associated with increased levels of aminotransferases. Most of the time serial biopsies are needed to demonstrate histopathological progression of a recurrence of hepatitis C. (22)
In some cases recurrence coincides with severe acute rejection. The key to distinguishing between the two is the paucity of panlobular necroinflammatory activity in acute rejection, although inflammation around the central vein may be present. Inflammatory damage of the bile duct (ductulitis) is greater in acute rejection than in recurrent hepatitis. Also, the absence of interface hepatitis and heterogeneous infiltration are associated with acute rejection. The patient's clinical history and time elapsed following transplantation can aid in making the differential diagnosis. (20)
Typical Late-Stage Pattern of Recurrent Chronic Hepatitis C
Generally, the morphology of typical late-stage recurrences of chronic hepatitis is very similar to that observed in any liver that has not been transplanted. Changes typical of chronic hepatitis C can be demonstrated in 70% to 90% of recipients after one year and in 90% to 95% after 5 years. Recurrent chronic hepatitis C is characterized by varying degrees of portal mononuclear infiltrate which forms lymphoid clumps or true follicles. This is sometimes the only finding in the first months of recurrence. Portal inflammation is located more peripherally toward the periportal region and is accompanied by interface hepatitis. A ductal reaction may develop, but it is usually not as obvious or severe as in cases of advanced fibrosis (Figures 5 and 6). (19, 20)
Steatosis is found in up to 30% of biopsies from patients after transplantation (Figure 7), especially in genotype 3 recipients. HVC can induce steatosis by direct alteration of mitochondrial functions or through interference with the pathways of the lipid metabolism. (23) While authors do not completely agree, it seems that steatosis and metabolic syndrome after transplantation increase the risk of fibrosis following the first year after transplantation but that if fibrosis does not develop within this period, thereafter patients are less likely to develop it. (24)
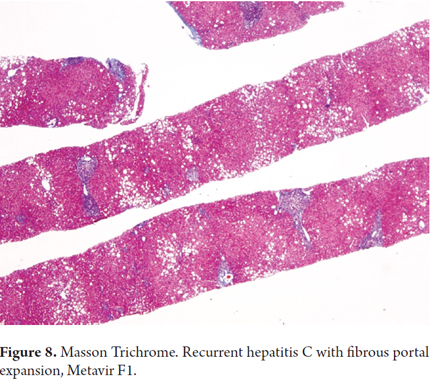
Additional immunohistochemical studies have shown that those with CK19 and vimentin immunoreactivity in stage F0-F1 biopsies rapidly progress to fibrosis. Since this is also related to a higher numbers of apoptotic cells, these findings may be predictors of early fibrosis and may require closer monitoring (Figure 8). (25)
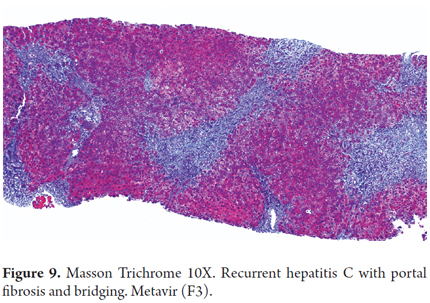
The progression of fibrosis is accelerated in recurrent hepatitis C in 20% to 54% of liver transplant recipients. The development of bridging fibrosis and even cirrhosis occurs within the first 5 years after transplantation (Figure 9). Although it is unusual, there may also be ductal damage and even destruction of the interlobular bile duct by granulomas. This implies differential diagnosis between recurrent primary biliary cirrhosis and primary sclerosing cholangitis. This is especially true if there has been a previous infection but is less likely if the infection was acquired following transplantation. Stabilization or regression of fibrosis in patients with SVR is usually only seen in serial and long term biopsies. A liver biopsy remains the gold standard despite possible sampling variability, and it is the benchmark against which the various non-invasive tests are compared for measurement of fibrosis. (25, 26)
Fibrosing Cholestatic Hepatitis C
Fibrosing cholestatic hepatitis (FCH) was initially described as a severe, fulminant, form of recurrent hepatitis B in the livers of transplant recipients. Nevertheless, it also occurs in 1% to 5% of patients with recurrent HVC. It is considered an enigmatic event whose pathogenesis is poorly understood. It is characterized by onset within the first year after transplantation, rapid deterioration of liver function, and high mortality. Type 1b genotype has been associated with the most severe disease with poor prognosis, rapid progression of fibrosis and resistance to conventional therapies. Increased risk of fibrosing cholestatic hepatitis has also been shown in patients transplanted for chronic HVC whose donor had genotype IL28B. Clinically, FCH is characterized by rapid and progressive deterioration of graft function evidenced by severe jaundice, coagulopathy and encephalopathy. Usually, patients with FCH have elevated serum bilirubin and prothrombin times with mild to moderate increases in transaminases. Some studies have shown an association with high levels of viral RNA. (27-30)
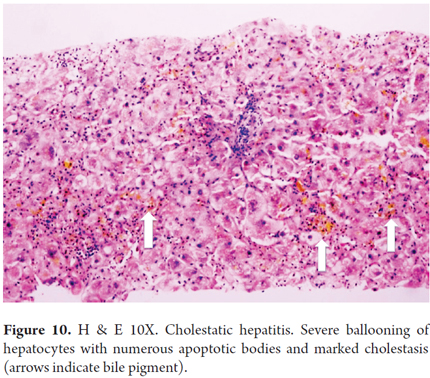
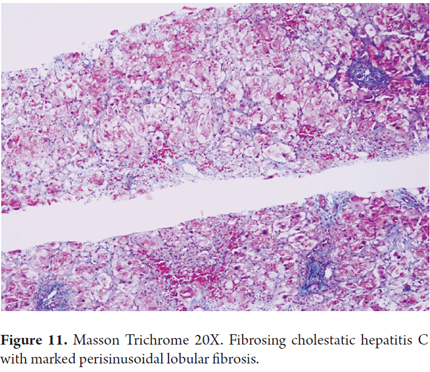
Histologically, FCH presents itself as severe hepatocyte ballooning with numerous apoptotic bodies and irregular necrosis with marked, predominantly canalicular, cholestasis in the centrilobular area. There is also serious ductal reaction with infiltration of polymorphonuclear neutrophils, periportal fibrosis (zone 1) and perisinusoidal lobular fibrosis (Figures 10 and 11). Many cases show a marked collapse of the parenchyma, with confluent necrosis and accelerated progression of fibrosis, and sometimes there is nodular regenerative hyperplasia or even established cirrhosis. (27-29)
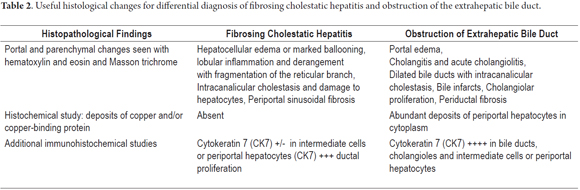
Differential diagnosis should consider other causes of cholestasis such as biliary complications, including anastomotic strictures, obstruction of the bile duct, bile duct damage induced by ischemia and cholestasis due to toxic drugs. (27-28) Table 2 shows the most significant histopathological changes that help differentiate a diagnosis between FCH and obstruction of a bile duct.
Recurrent Plasma-Cell-Rich Hepatitis C
Plasma-cell-rich hepatitis C is a rare form of graft dysfunction that develops in patients transplanted for reasons other than autoimmune hepatitis, especially described in patients with recurrent hepatitis C. It is characterized by a very rich infiltrate of plasma cells. For some authors it is a type of post-transplant de novo autoimmune hepatitis or plasma cell hepatitis or even part of the spectrum of acute cellular rejection that is rich in plasma cells. (31, 32) It is associated with graft failure and a poor prognosis. The mechanism by which it is produced is not yet clear, but it has been suggested that impaired immune status induced by interferon treatment or completion of antiviral treatment may be the cause. (33) Regardless of the nomenclature, little is known about its pathogenesis. Several mechanisms have been implicated in the loss or deterioration of self-tolerance. They include regulation of the thymus, altered activity of regulatory T cells, molecular mimicry, the use of calcineurin inhibitors, the presence of autoantibodies and several genetic polymorphisms. (34, 35)
Clinically, plasma-cell-rich hepatitis C is characterized by changes in biochemistry. Typical serum antibodies including antinuclear antibodies (ANAs) and antimitochondrial antibodies (AMAs) are present together with plus atypical serum antibodies such as those directed against S-transferase and T1 glutathione (anti-GSTT1). Plasma-cell-rich hepatitis C has the histological features of classic autoimmune hepatitis with portal inflammation in which plasmocytes predominate, interface hepatitis, and periportal (sometimes central perivenular) necroinflammatory commitment (Figure 12). The main problems for differential diagnosis are distinguishing plasma-cell-rich hepatitis C from plasma-cell-rich acute rejection and determining whether the two events are present simultaneously. When they are, it is recommended that the predominant morphological picture be emphasized. The diagnosis is confirmed by a liver biopsy, but the best treatment for this condition has yet to be determined. (35, 36)
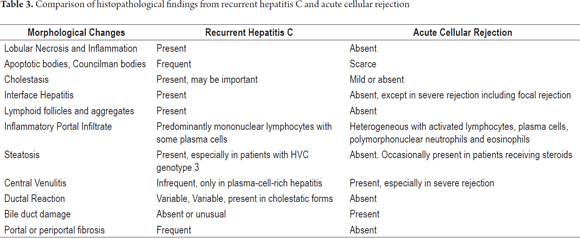
Table 3 shows a comparison of the major histopathological changes in recurrent hepatitis C and acute cellular rejection.
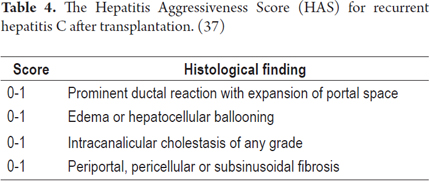
Various systems for measuring how aggressive recurrent hepatitis C is have been used. Regardless of the observed variation, these systems can be used to identify individuals who are at increased risk of progression to fibrosis which is the best predictor of graft loss. Table 4 provides an overview of the parameters evaluated in this semi-quantitative measurement. If the biopsy score is 0, it is considered to be non-aggressive hepatitis C. A score of 1 or 2 corresponds to aggressive hepatitis C, and a score of 3 or 4 suggests fibrosing cholestatic hepatitis C. (37)
Recurrent Viral Hepatitis B (HVB)
The post-transplantation recurrence rate of hepatitis B is over 80% if proper preventive treatment is not administered. However, in more than 90% of transplant recipients it is possible to achieve clinical control with combination therapy using immunoglobulin and nucleotide analogs. Recurrent viral hepatitis B is diagnosed when the recurrence of surface antigen (HBsAg) is detected with or without detection of HBV DNA by PCR. PCR can sometimes detect HBV DNA years before surface antigen can be detected. Similar to HCV, HBV has a wide spectrum of histopathological expressions including acute hepatitis, chronic hepatitis, cirrhosis, minimal changes in the carrier, viral fibrosing cholestatic hepatitis B. Before the introduction of prophylactic treatment, the recurrence rates of HVB infections after transplantation were very high. Risk factors favoring recurrence include HBeAg, high levels of viral DNA at the time of transplantation (even when HBeAg cannot be detected), hepatocellular carcinoma associated with cirrhosis, patients with histories of resistance to antiviral drugs prior to transplant, and post-transplant absence of prophylaxis. It must be added that patients are considered to be low-risk when they have low viral loads, are HBeAg negative, and when they have acute liver failure due to HBV infections at the time of transplantation. Hepatitis D coinfections have the lowest recurrence rates of chronic hepatitis which is explained by the fact that HDV inhibits replication of HBV even in immunocompromised patients. The exact mechanism of recurrence is not well understood but is probably related to the requirement for immunosuppression, especially the use of steroids, or to extrahepatic infections. (38-40)
Histopathological changes are very similar to those described for recurrence of HCV. The cells with ground glass patterns may be present in large numbers, especially in fibrosing cholestatic hepatitis. This pattern of cytopathic damage in ballooning cells, severe cholestasis, hepatocanalicular fibrosis and periportal fibrosis is associated with high rates of liver failure. (41)
Recurrent Autoimmune Hepatitis (AIH) and de Novo Hepatitis
Autoimmune hepatitis (AIH) is a progressive chronic inflammatory liver disease which responds to immunosuppressive therapy. Despite the good post-transplant results, and in the absence of HCV infection, recurrence in the allograft occurs in nearly a third of the cases: up to 12% in the first year and from 36% to 68 % within 5 years. Percentages vary from study to study depending on the diagnostic criteria used, the protocols for immunosuppression and the use of protocol biopsies. Though not yet universally confirmed, factors that have been considered to be risk factors for recurrence include the presence of human leukocyte antigen DR3 (HLA-DR3) or HLA-DR4 in the recipient, early discontinuation of corticosteroids, severe necroinflammatory activity in the explant or recipient at the time of transplantation, and patients with chronic AIH that is resistant to steroids. Patients transplanted for fulminant autoimmune hepatitis also have lower risks of recurrence. (42-44)
Successful treatment depends on early diagnosis of recurrence, the criteria for which include:
- History of AIH as etiology of transplant
- Dependence and response to steroids
- Presence of specific autoantibodies (ANA, SMA and/or LKM1)
- Elevated serum aminotransferases
- Hypergammaglobulinemia G
- Histological characteristics of interface hepatitis and portal inflammation with predominance of plasma cells.
- Exclusion of other causes of graft dysfunction, including typical, late, and atypical acute cellular rejection. (43)
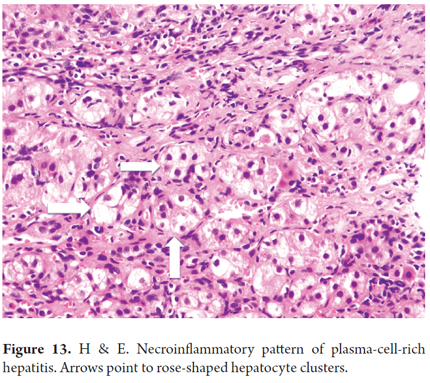
Between 5% and 10% of children and 1% to 2% of adults transplanted for any etiology other than AIH or HVC develop de Novo autoimmune hepatitis which can occur several years after transplantation. The presence of specific antigens histocompatibility complex, HLA-DR3 in the recipient and HLA-DR4 in the donor, have been described in both children and adults. Up to 50% of patients have experienced one or more previous episodes of acute cellular rejection and steroid dependency, so these are considered to be risk factors for the development of de novo AIH in children and adults. In adults, the use of allografts from elderly and female donors is also considered to be a risk factor. De Novo autoimmune hepatitis has the same clinical, serological and histological features as AIH: it is plasma-cell-rich and has a necroinflammatory pattern with interface hepatitis and rose shaped formations (Figure 13). Especially in the pediatric population, de Novo autoimmune hepatitis can have central-lobular necrosis, inflammation, central plasma-cell-rich perivenulitis, prominent lobular hepatitis, and minimal or no interface hepatitis. As in recurrent AIH, early diagnosis and treatment lead to success and survival of the transplanted organ and the patient. The differential diagnosis must consider acute cellular rejection, mainly in plasma-cell-rich hepatitis, and recurrence of other autoimmune diseases. (45, 46)
Recurrent Primary Biliary Cirrhosis (PBC)
PBC is one of the most common indications for liver transplantation. It may have a high recurrence rate between 21% and 37% at 10 years after transplantation and between 40% and 50% at 10 years after transplantation. Some authors have asserted that recurrence occurs in 100% of patients, with 3 to 5.5 years median time to recurrence. Histopathology, and therefore biopsies, is indispensable for diagnosis during follow-up. (43, 47)
The diagnosis of recurrence is usually very difficult partly because the diagnostic criteria used prior to transplantation cannot be used. Antimitochondrial antibodies (AMA) may persist or recur rapidly in the receiver, and liver function tests may continue to show activity of alkaline phosphatase or elevated serum immunoglobulin M and GGT from prior to transplantation. Symptoms of recurrence are usually not specific and can be found in many other conditions, including acute or chronic rejection, viral infections, injury to the bile duct and hepatic artery pathologies. Because they lose value for diagnosis, histopathology becomes essential. (47, 48)
Histopathological diagnostic criteria for recurrence of PBC are:
- Confirmation of PBC prior to transplantation and in the explant.
- Graft biopsy with predominantly lymphocytic portal infiltrates, formation of lymphoid aggregates or epithelioid granulomas, and evidence of biliary injury with lymphocytic ductulitis (Figure14).
- Persistence of AMA or AMA-M2
- Complete exclusion of other causes of graft dysfunction including acute rejection, chronic rejection, graft disease, host disease, vascular complications, biliary complications, cholangitis, viral hepatitis and drugs. (43, 49)
Parietal cells antibodies have been detected in 41% of pre-transplant PBC cases, in 47% of PBC cases immediately after transplantation, and in 100% of patients who develop recurrences which makes these antibodies potentially useful as markers of recurrence. Many other factors have been described as having probable associations with recurrence. These include advanced age of the recipient, large differences in ages between donor and recipient, prolonged warm ischemia time, donor-recipient HLA compatibility, male recipients, immunosuppression based on tacrolimus, suspension of steroids, and acute rejection episodes. Recurrence does not seem to affect the long-term outcome, and the need for retransplantation is very small. (50).
Primary Sclerosing Cholangitis (PSC)
There recurrence rate ranges from 20% to 30% and occurs between six months and ten years after transplantation with an average of 5 years. Ulcerative colitis, elderly donors and prolonged INR at the time of transplantation are considered to be independent risk factors that identify patients with more aggressive disease and increased risk of graft failure and death and higher rates higher retransplantation. A history of poor immunosuppression and rejection episodes also contribute to the recurrence of the underlying disease. Pretransplant colectomy for the management of chronic inflammatory bowel disease factor that mitigates against recurrence. (51, 52)
The criteria for the diagnosis of recurrent PSC are:
- Confirmation of diagnosis of primary sclerosing cholangitis before transplantation and in the explant.
- Cholangiography or magnetic resonance cholangiopancreatography (MRCP) showing non-anastomotic hepatic biliary stenosis and/or extrahepatic stenosis with irregularities and characteristic rose shape 90 days or more after transplantation.
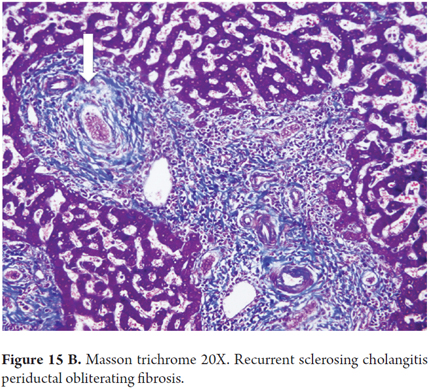
- Histopathological findings of the typical PSC image of fibrous cholangitis or fibro-obliterating cholangitis, portal fibrosis, portal inflammation and ductal reaction which may be focal (Figures 15A and B).
- Exclusion of all causes of non-anastomotic strictures of the bile duct. (43)
A diagnosis of recurrent PSC is often very difficult to make and requires differentiating from other conditions that can produce stenosis of the bile duct. These conditions include surgical causes, mechanical causes, by ischemic events of the bile ducts, thrombosis of the hepatic artery, stenosis of the hepatic artery, chronic ductopenic rejection and infectious cholangitis.
CONCLUSION
Liver transplantation is an effective treatment for the vast majority of terminal liver diseases and acute fulminant kidney failure of any etiology. Survival rates are excellent for both grafts and patients with liver disease. For diagnosis, monitoring and understanding of the pathology prior to, during and after transplantation, a liver biopsy is still considered to be a fundamental tool that requires proper correlation with a complete medical history, diagnostic imaging and morphological study.
REFERENCES
1. Banff Working Group, Demetris AJ et al. Liver biopsy interpretation for causes of late liver allograft dysfunction. Hepatology. 2006;44(2):489-501. [ Links ]
2. Petrovic LM. Recurrent diseases following liver transplantation: Current concepts. Curr Opin Organ Transplant. 2012;17(3):293-302. [ Links ]
3. Berenguer M, Aguilera V, Prieto M, Carrasco D, Rayón M, San Juan F, Landaverde C, Mir J, Berenguer J. Delayed onset of severe hepatitis C-related liver damage following liver transplantation: a matter of concern? Liver Transpl. 2003;9(11):1152-1158. [ Links ]
4. Mylene Sebagh, Kinan Rifai, Cyrille Feray, Funda Yilmaz, Bruno Falissard, Bruno Roche, Henri Bismuth, Didier Samuel, and Michel Reynes All Liver Recipients Benefit From the Protocol 10-Year Liver Biopsies Hepatology. 2003;37(6):1293-1301. [ Links ]
5. Kim H, Lee KW, et al. Response-Guided Therapy for Hepatitis C Virus Recurrence Based on Early Protocol Biopsy after Liver Transplantation. J Korean Med Sci. 2015;30(11):1577-83. [ Links ]
6. Gutiérrez JA, Carrión AF, Avalos D, OBrien C, Martin P, Bhamidimarri KR, Peyton A. Sofosbuvir and simeprevir for treatment of hepatitis C virus infection in liver transplant recipients. Liver Transpl. 2015;21(6):823-30. [ Links ]
7. Adeyi O, Fischer SE, Guindi M. Liver allograft pathology: approach to interpretation of needle biopsies with clinicopathological correlation. J Clin Pathol. 2010;63(1):47-74. [ Links ]
8. Kanodia KV, Vanikar AV, Modi PR, Patel RD, Suthar KS, Nigam LK, Trivedi HL Histological and Clinicopathological Evaluation of Liver Allograft Biopsy: An Initial Experience of Fifty Six Biopsies. J Clin Diagn Res. 2015;9(11):EC17-20. [ Links ]
9. Mylene Sebagh, Karin Blakolmer, Bruno Falissard, Bruno Roche, Jean-Francois Emile, Henri Bismuth, Didier Samuel, and Michel Reynes. Accuracy of Bile Duct Changes for the Diagnosis of Chronic Liver Allograft Rejection: Reliability of the 1999 Banff Schema. Hepatology. 2002;35(1):117-125. [ Links ]
10. Inomata Y, Tanaka K. Pathogenesis and treatment of bile duct loss after liver transplantation. J Hepatobiliary Pancreat Surg. 2001;8(4):316-22. [ Links ]
11. Nakanuma Y, Tsuneyama K, Harada K. Pathology and pathogenesis of intrahepatic bile duct loss. J Hepatobiliary Pancreat Surg. 2001;8(4):303-15. [ Links ]
12. Demetris A et al. Update of the International Banff Schema for Liver Allograft Rejection: working recommendations for the histopathologic staging and reporting of chronic rejection. An International Panel. Hepatology. 2000;31(3):792-9. [ Links ]
13. Kim WR, Stock PG, Smith JM, et al. OPTN/SRTR 2011 Annual Data Report liver. Am Transplant. 2013;13(suppl 1):73-102. [ Links ]
14. García-Retortillo M, Forns X, Feliu A, et al. Hepatitis C virus kinetics during and immediately after liver transplantation. Hepatology 2002;35(3):680-7. [ Links ]
15. Pelletier SJ, Schaubel DE, Punch JD, Wolfe RA, Port FK, Merion RM. Hepatitis C is a risk factor for death after liver retransplantation. Liver Transpl. 2005;11(4):434-440. [ Links ]
16. Vasuri F, Malvi D, Gruppioni E,Grigioni WF, DErrico-Grigioni A. Histopathological evaluation of recurrent hepatitis C after liver transplantation: a review. World J Gastroenterol. 2014;20(11):2810-24. [ Links ]
17. Berenguer M, López-Labrador FX, Wright TL. Hepatitis C and liver transplantation. J Hepatol. 2001;35:666-678. [ Links ]
18. Gambato M, Crespo G, Torres F, Llovet L, Carrión J, Londoño M, Lens S, Mariño Z, Bartres C, Miquel R, Navasa M, Forns X Simple prediction of long-term clinical outcomes in patients with mild hepatitis C recurrence after liver transplantation. Transpl Int. 2016;29(6):698-706. [ Links ]
19. Carmen Vinaixa, Ángel Rubín, Victoria Aguilera, and Marina Berenguer. Recurrence of hepatitis C after liver transplantation. Ann Gastroenterol. 2013;26(4):304–313. [ Links ]
20. Demetris AJ. Evolution of hepatitis C virus in liver allografts. Liver Transpl. 2009;15 (Suppl 2):S35-S41. [ Links ]
21. Vasuri F, Morelli MC, Gruppioni E, Fiorentino M, Ercolani G, Cescon M, Pinna AD, Grigioni WF, DErrico-Grigioni A. The meaning of tissue and serum HCV RNA quantitation in hepatitis C recurrence after liver transplantation: a retrospective study. Dig Liver Dis. 2013;45(6):505-509. [ Links ]
22. Saxena R, Crawford JM, Navarro VJ, Friedman AL, Robert ME. Utilization of acidophil bodies in the diagnosis of recurrent hepatitis C infection after orthotopic liver transplantation. Mod Pathol. 2002;15(9):897-903. [ Links ]
23. Gordon FD, Pomfret EA, Pomposelli JJ, Lewis WD, Jenkins RL, Khettry U. Severe steatosis as the initial histologic manifestation of recurrent hepatitis C genotype 3. Hum Pathol. 2004;35(5):636-638. [ Links ]
24. Brandman D, Pingitore A, Lai JC, Roberts JP, Ferrell L, Bass NM, Terrault NA. Hepatic steatosis at 1 year is an additional predictor of subsequent fibrosis severity in liver transplant recipients with recurrent hepatitis C virus. Liver Transpl. 2011;17(12):1380-1386. [ Links ]
25. Meriden Z, Forde KA, Pasha TL, Hui JJ, Reddy KR, Furth EE, Wells RG. Histologic predictors of fibrosis progression in liver allografts in patients with hepatitis C virus infection. Clin Gastroenterol Hepatol. 2010;8(3):289-296. [ Links ]
26. Berenguer M, Schuppan D. Progression of liver fibrosis in post-transplant hepatitis C: Mechanisms, assessment and treatment. Journal of Hepatology. 2013;58(5):1028–1041. [ Links ]
27. Satapathy SK, Sclair S, Fiel MI, Del Río Martin J, Schiano T. Clinical characterization of patients developing histologically-proven fibrosing cholestatic hepatitis C post-liver transplantation. Hepatol Res. 2011;41(4):328-39. [ Links ]
28. Salomao M, Verna EC, Lefkowitch JH, Moreira RK. Histopathologic distinction between fibrosing cholestatic hepatitis C and biliary obstruction. Am J Surg Pathol. 2013;37(12):1837-44. [ Links ]
29. Shu-Yuan Xiao, Liang Lu and Hanlin L. Wang Fibrosing Cholestatic Hepatitis: Clinicopathologic Spectrum, Diagnosis and Pathogenesis. Int J Clin Exp Pathol. 2008;1(5): 396-402. [ Links ]
30. Hanouneh IA, Zein NN, Askar M, Lopez R, John B. Interleukin-28B polymorphisms are associated with fibrosing cholestatic hepatitis in recurrent hepatitis C after liver transplantation. ClinTransplant. 2012;26(4):E335-6. [ Links ]
31. Ueda Y, Yoshizawa A, Ogura Y, Miyagawa-Hayashino A, Haga H, Chiba T, Uemoto S. Plasma cell hepatitis induced by the termination of antiviral therapy for recurrent hepatitis C after living donor liver transplantation. Hepatol Res. 2014;44(10):E279-83. [ Links ]
32. López Panqueva Rocío del Pilar. Biopsia hepática en la patología del trasplante, período postrasplante temprano, enfoque dirigido al diagnóstico histopatológico y su correlación clinicopatológica. Rev Col Gastroenterol. 2016;31(2):171-181. [ Links ]
33. Fiel MI, Schiano TD. Plasma cell hepatitis (de-novoautoimmune hepatitis) developing post liver transplantation. Curr Opin Organ Transplant. 2012;17(3):287-92. [ Links ]
34. Kerkar N, Yanni G. De novo and recurrent autoimmune hepatitis after liver transplantation: A comprehensive review. J Autoimmun. 2016;66:17-24 [ Links ]
35. Tanaka T, Sugawara Y, Kokudo N. Liver transplantation and autoimmune hepatitis. Intractable Rare Dis Res. 2015;4(1):33-8. [ Links ]
36. Salcedo M, Rodríguez-Mahou M, Rodríguez-Sainz C, Rincón D, Álvarez E, Vicario JL, Catalina MV, Matilla A, Ripoll C, Clemente G, Bañares R. Risk factors for developing de novo autoimmune hepatitis associated with anti-glutathione S-transferase T1 antibodies after liver transplantation. Liver Transpl. 2009;15(5):530-9. [ Links ]
37. Moreira RK, Salomao M, Verna EC, Brown RS Jr, Lefkowitch JH. The Hepatitis Aggressiveness Score (HAS): a novel classification system for post-liver transplantation recurrent hepatitis C. Am J Surg Pathol. 2013;37(1):104-13. [ Links ]
38. Manne V, Allen RM, Saab S. Strategies for the prevention of recurrent hepatitis B virus infection after liver transplantation. Gastroenterol Hepatol (N Y). 2014;10(3):175-9. [ Links ]
39. Maiwall R, Kumar M. Prevention and Treatment of Recurrent Hepatitis B after Liver Transplantation.J Clin Transl Hepatol. 2016;4(1):54-65. [ Links ]
40. Takaki A, Yasunaka T, Yagi T. Molecular Mechanisms to Control Post-transplantation hepatitis B Recurrence. Int J Mol Sci. 2015;16(8):17494-513. [ Links ]
41. Lefkowitch Jay H. Scheuer´s Liver biopsy interpretation. Ninth edition 2016 Elsevier. Cap 16, pag 366-374. [ Links ]
42. Liberal R, Zen Y, Mieli-Vergani G, Vergani D Liver transplantation and autoimmune liver diseases. Liver Transpl. 2013;19(10):1065-77. [ Links ]
43. Faisal N, Renner EL. Recurrence of autoimmune liver diseases after liver transplantation.World J Hepatol. 2015;7(29):2896-905. [ Links ]
44. Edmunds C, Ekong UD Autoimmune Liver Disease Post-Liver Transplantation: A Summary and Proposed Areas for Future Research.Transplantation. 2016;100(3):515-24. [ Links ]
45. Sebagh M, Castillo-Rama M, Azoulay D, Coilly A, Delvart V, Allard MA, Dos Santos A, Johanet C, Roque-Afonso AM, Saliba F, Duclos-Vallée JC, Samuel D, Demetris AJ. Histologic findings predictive of a diagnosis of de novo autoimmune hepatitis after liver transplantation in adults. Transplantation. 2013;96(7):670-8. [ Links ]
46. Ranka Vukotic, Giovanni Vitale, Antonia DErrico-Grigioni, Luigi Muratori, Pietro Andreone. De novo autoimmune hepatitis in liver transplant: State-of-the-art review. World J Gastroenterol 2016;22(10):2906-2914. [ Links ]
47. Raczyńska J, Habior A, Pączek L, Foroncewicz B, Pawełas A, Mucha K. Primary biliary cirrhosis in the era of liver transplantation. Ann Transplant. 2014;29(19):488-93. [ Links ]
48. Neuberger J. Recurrent primary biliary cirrhosis. Liver Transpl. 2003;9:539–46. [ Links ]
49. Lee J, Belanger A, Doucette JT, Stanca C, Friedman S, Bach N. Transplantation trends in primary biliary cirrhosis. Clin Gastroenterol Hepatol. 2007;5(11):1313-1315. [ Links ]
50. Ciesek S, Becker T, Manns MP, Strassburg CP: Anti-parietal cell autoantibodies (PCA) in primary biliary cirrhosis: a putative marker for recurrence after orthotopic liver transplantation? Ann Hepatol. 2010;9(2):181-85. [ Links ]
51. Hildebrand T et al; German PSC Study Group. Biliary strictures and recurrence after liver transplantation for primary sclerosing cholangitis: A retrospective multicenter analysis. Liver Transpl. 2016;22(1):42-52. [ Links ]
52. Ravikumar R, Tsochatzis E, et al. Risk factors for recurrent primary sclerosing cholangitis after liver transplantation. J Hepatol. 2015;63(5):1139-46. [ Links ]











 texto em
texto em