Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista colombiana de Gastroenterología
versão impressa ISSN 0120-9957versão On-line ISSN 2500-7440
Rev. colomb. Gastroenterol. vol.35 no.4 Bogotá out./dez. 2020 Epub 12-Jul-2021
https://doi.org/10.22516/25007440.561
Review article
Current diagnosis and treatment of gastroparesis: A systematic literature review
1Médico internista y docente, Clínica Versalles, Universidad Javeriana. Cali, Colombia.
2Gastroenterología, Coordinador de área, Clínica Universitaria Colombia. Bogotá, Colombia.
3Gastroenterólogo, Clínica Universitaria Colombia. Bogotá, Colombia.
4Médico general, Universidad Javeriana. Clínica Versalles. Cali, Colombia.
Normal gastric emptying reflects a coordinated effort between different regions of the stomach and the duodenum, and also an extrinsic modulation by the central nervous system and distal bowel factors. The main events related to normal gastric emptying include relaxation of the fundus to accommodate food, antral contractions to triturate large food particles, the opening of the pyloric sphincter to allow the release of food from the stomach, and anthropyloroduodenal coordination for motor relaxation. Gastric dysmotility includes delayed emptying of the stomach (gastroparesis), accelerated gastric emptying (dumping syndrome), and other motor dysfunctions, e.g., deterioration of the distending fundus, most often found in functional dyspepsia. The symptoms of gastroparesis are nonspecific and may mimic other structural disorders.
Keywords: Gastric emptying; Gastroparesis; Gastric dysmotility
El vaciamiento gástrico normal refleja un esfuerzo coordinado entre diferentes regiones del estómago y el duodeno, y también una modulación extrínseca por parte del sistema nervioso central y factores del intestino distal. Los principales eventos relacionados con el vaciamiento gástrico normal incluyen el fondo de relajación para acomodar la comida, contracciones antrales para triturar partículas grandes de comida, contracción pilórica para permitir la liberación de comida del estómago y coordinación antropiloroduodenal de los fenómenos motores de relajación. La dismotilidad gástrica incluye el vaciamiento tardío del estómago (gastroparesia), vaciamiento gástrico acelerado (síndrome de dumping) y otras disfunciones motoras, como el deterioro del fondo de distensión, que se encuentra con mayor frecuencia en la dispepsia funcional. Los síntomas de la gastroparesia son inespecíficos y pueden simular otros trastornos estructurales.
Palabras clave: Vaciamiento gástrico; gastroparesia; dismotilidad gástrica
Introduction
Gastric emptying depends on the coordinated activity between different areas of the stomach and the duodenum. This process is extrinsically modulated by the central nervous system (CNS) and by factors occurring in the distal intestine. Gastroparesis is a chronic symptomatic disorder consisting of delayed gastric emptying without mechanical obstruction being present. Its main causes are of endocrine, neurological and metabolic origin, and the most frequent clinical entity is diabetic gastroparesis. Symptoms are variable and may overlap with other gastrointestinal diseases. Patients with gastroparesis suffer from nausea, vomiting, early satiety, anorexia, weight loss, and epigastric pain1. Different diagnostic techniques and therapeutic approaches have been proposed over time. Therefore, this systematic review aims to describe the current relevant literature on the different diagnostic options and therapeutic approaches to gastroparesis. A summary of what is known to date about gastroparesis, with an emphasis on its etiology, pathophysiology, and current definitions is performed prior to describing how the review was conducted and the results retrieved. With that in mind, the findings retrieved from the studies included in the review regarding contrast radiography techniques, gastric emptying scintigraphy, drugs affecting gastric emptying, gastroparesis breath test, electrogastrography, antroduodenal manometry, ultrasonography, magnetic resonance imaging and positron emission computed tomography are presented in this paper. Furthermore, dietary treatment, pharmacological treatment (anti-5-hydroxytryptamine3 [anti-5HT3], anti-D 2, peptide agonists associated with gastric emptying, antidepressants, and macrolides), gastric electrical stimulation and surgical approaches are also described.
What is known about gastroparesis?
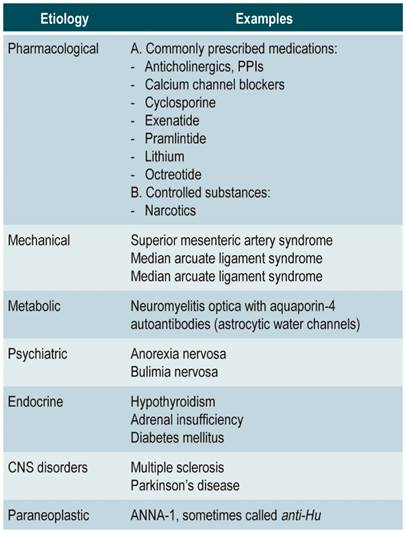
The main causes of gastroparesis are of endocrine, neurological and metabolic origin. It usually occurs in diabetic patients, after undergoing surgeries involving vagotomy and by unknown causes (idiopathic gastroparesis) (Table 1). Symptoms are variable and include nausea, vomiting, early satiety, anorexia, weight loss, and epigastric pain 1.
Pathophysiological alterations in patients with gastroparesis are associated with (Figure 1):
Impaired gastric accommodation caused by loss of gastric inhibitory peptides or vagus nerve damage.
Depletion of interstitial cells of Cajal in diabetes cases and post-infection lesions, resulting in arrhythmias (e.g., tachygastria and ectopic pacemakers, associated with nausea and vomiting).
Abnormal smooth muscle contractions due to altered function of the neurons of the enteric nervous system.
Atrophy or fibrosis of the smooth muscle.
Altered release of gastrointestinal peptides (motilin, ghrelin, and pancreatic polypeptide, which facilitate gastric motility).
Pyloric sphincter dysfunction and the concept of pyloric spasm (2) .
Table 1 Reversible causes of gastroparesis
ANNA-1: type 1 anti-neuronal nuclear antibodies; PPI: Proton pump inhibitors.
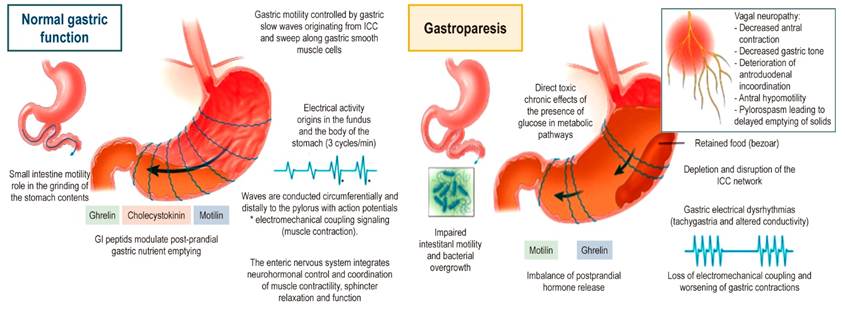
Figure 1 Gastroparesis pathophysiology. ICC: Interstitial cells of Cajal. Adapted from: Reddymasu SC, Sarosiek I, McCallum RW. Severe gastroparesis: medical therapy or gastric electrical stimulation. Clin Gastroenterol Hepatol. 2010;8(2):117-24.
Patients with gastroparesis have nonspecific symptoms that can simulate those of structural disorders such as ulceropeptic disease, partial gastric or intestinal obstruction, gastric cancer, and pancreatic biliary disorders. Symptoms of gastroparesis and functional dyspepsia also overlap; the latter is characterized by experiencing chronic or recurrent discomfort in the upper abdomen, although many people report dysmotility together with nausea, vomiting, and early satiety, and some subgroups of patients experience delayed gastric emptying3,4.
The correlation of symptoms with delayed gastric emptying is variable in diabetic gastropathy, idiopathic gastroparesis, and functional dyspepsia cases. Recent studies have reported that early satiety, postprandial fullness, and vomiting predicted delayed gastric emptying in patients with functional dyspepsia3,4. In addition, a feeling of fullness and meteorism have been found to predict delayed gastric emptying in patients with diabetes5,6.
Patients with functional dyspepsia show exaggerated sensitivity to gastric distension, which suggests the contribution of afferent neuron dysfunction to the pathogenesis of the symptom. Also, in diabetic patients with symptoms of dyspepsia, gastric distension causes nausea, meteorism and marked abdominal discomfort. It is believed that these symptoms may be caused by sensory nerve dysfunction in these patients7,8.
In up to half of patients undergoing routine examinations such as upper gastrointestinal (GI) endoscopy or gastrointestinal endoscopy (GE) and laboratory tests, the identification of organic or biochemical abnormalities that easily explain gastroparesis symptoms is not possible. In most adults, gastroparesis is idiopathic. Complications associated with this disease may include Mallory-Weiss tears, bezoar formation, malnutrition, aspiration pneumonia, and electrolyte disorders3,4.
These so-called functional gastrointestinal disorders are highly prevalent, misunderstood, and have a great health care and socioeconomic impact. Therefore, it is important to understand their possible diagnostic and therapeutic options9.
Gastrointestinal and non-gastrointestinal disorders may be accompanied by gastroparesis, such as gastroparesis associated with gastroesophageal reflux disease (GERD) and generalized disorders of gastrointestinal motility.
Gastroparesis associated with GERD
Rates of up to 40% of gastroparesis prevalence have been described in patients with GERD, although other studies have reported occurrence rates of only 10%10. The pathophysiology of delayed gastric emptying in patients with GERD is not clear. It is believed that distended gastric stasis may promote transient lower esophageal sphincter relaxation with subsequent gastroesophageal acid reflux10. Recent studies suggest that delayed emptying of the proximal stomach, but not of the entire stomach, can be correlated with esophageal acid exposure, so performing gastric scintigraphy in patients with GERD symptoms refractory to acid suppressor therapy is considered appropriate11.
Generalized disorders of gastrointestinal motility
Chronic intestinal pseudo-obstruction
It is a syndrome with recurrent symptoms that suggest intestinal obstruction without mechanical cause. Imaging findings in patients with chronic intestinal pseudo-obstruction include luminal dilatation with air-fluid levels throughout the small bowel. This disorder can be caused by several systemic diseases such as scleroderma, amyloidosis, myxedema, long-term diabetes mellitus, and the paraneoplastic complications most commonly observed in small cell lung carcinoma.
Myopathic and neuropathic chronic intestinal pseudo-obstruction are the two main types of this disease, and they can be differentiated by means of small intestine manometry. In myopathic form, contractions of low amplitude with normal coordination are observed. In neuropathic type, the amplitude of contractions is normal, but their morphology is disorganized, including disruption of phase III activity, bursts of non-propagating activity during fasting, and failure to achieve conversion from the fasting to the postprandial fed motor pattern. In the case of pseudo-obstruction of paraneoplastic origin, a variety of autoantibodies may be detected, such as ANNA-1 or anti-Hu antibodies, among others12.
Constipation
A study found that 19% of patients with primary constipation had delayed gastric emptying. Another study conducted in patients with irritable bowel syndrome reported that delayed emptying of solids occurred in 64% of them, especially in those with predominating constipation. This fact has major implications for treatment, as patients with chronic severe constipation and proximal colonic dysmotility do not respond satisfactorily to surgical treatment (subtotal colectomy)13.
Nongastrointestinal disorders
The most relevant nongastrointestinal disorders associated with delayed gastric emptying are ischemic gastroparesis, gastroparesis associated with a malignancy, chronic pancreatitis, kidney failure, and infectious causes of gastroparesis.
Ischemic gastroparesis
Patients with chronic atherosclerosis may experience episodes of gastric ischemia that may manifest as gastritis, ulcers, or gastroparesis. Diagnosis is made by angiography, and revascularization is considered the treatment of choice because it usually improves gastric emptying and corrects gastric dysrhythmias14.
Gastroparesis associated with a malignancy
Gastroparesis has been described in patients with esophageal, gastric, pancreatic, breast and lung carcinoma. Although its pathophysiology is unknown, it has been attributed to paraneoplastic effects, neural invasion, or side effects of chemotherapy. In gastric cancer cases, tumor infiltration of the wall may alter the coordination of the neuromuscular function.
Cases of gastroparesis have been reported after radiation therapy to the abdomen and during chemotherapy15, as well as after bone marrow transplantation and celiac plexus blockage to treat chronic pain in pancreatic cancer16.
Chronic pancreatitis
A study found that 44% of patients with chronic pancreatitis of the accessory pancreatic duct had delayed gastric emptying. It was also considered that some of the abdominal pain, nausea and vomiting episodes in these patients may be due to gastroparesis17.
Kidney failure
The intensity of symptoms such as nausea, vomiting, anorexia, and early satiety observed in patients on hemodialysis patients has been correlated with the degree of delayed gastric emptying18. Other studies have also reported that delayed gastric emptying of solids in patients with kidney failure is correlated with changes in nutritional status biochemical indicators19.
Infectious causes of gastroparesis
Delayed gastric emptying may occur in patients with acute viral infection caused by herpes zoster virus, Epstein-Barr virus, cytomegalovirus (CMV), rotavirus, and parvovirus-like agents such as norovirus.
In almost all cases, the delay in gastric emptying is transient and resolves over time after recovering from the viral infection. Although reports are largely anecdotal, a small number of these patients develop chronic symptoms20.
CMV gastroenteritis occurs most commonly in immunocompromised people, particularly in transplant patients. In these cases, GI endoscopy usually shows large gastric folds and gastric inflammation, including acute superficial gastritis, ulcers, and duodenal erosions. Viral cultures of gastric biopsy specimens and histological evidence of CMV inclusions in the gastric mucosa usually allow confirming the diagnosis.
Delayed gastric emptying occurs in one third of HIV-positive people, particularly in those with advanced HIV disease, defined by a low CD4 cell count, significant weight loss, and enteric infections21.
The effects of Helicobacter pylori on gastric motor function have been controversial. Few works suggest an association with gastroparesis22, and in almost all studies, an association between active H. pylori infection and delayed gastric emptying or functional dyspepsia has not been found. Only one study reported the low prevalence of H. pylori infection and its relationship with reactive gastropathy23.
Gastrointestinal motility and sensitivity disorders can be identified in tests such as esophageal manometry, acid perfusion test, gastric emptying scan, sphincter of Oddi manometry, colonic transit measurements, and anorectal manometry.
The clinical practice guidelines for the management of gastroparesis in adults published in 2013 recommend restoring fluids and electrolytes and providing nutritional support, preferably through the oral route1. Pharmacological treatment is used in conjunction with diet therapy to improve gastric emptying and achieve relief of symptoms of associated gastroparesis. Prokinetic agents are usually the first-line pharmacological treatment and work by increasing gastrointestinal motility. In contrast, metoclopramide oral solution at the lowest effective dose is the drug of choice in patients who do not respond to treatment with prokinetics. Other pharmacological recommendations include oral administration of erythromycin to improve gastric emptying and antiemetics, and the use of agents alleviating symptoms associated with gastroparesis, or tricyclic antidepressants for the treatment of refractory nausea and vomiting. Neither antiemetics nor tricyclic antidepressants improve gastric emptying time and are only recommended, under certain conditions, as pharmacological treatment of gastroparesis in adults2.
Data collection
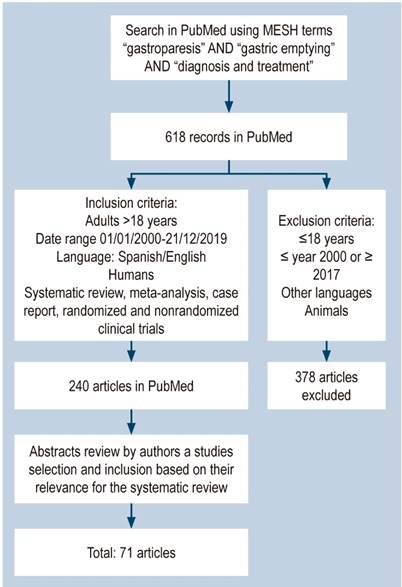
A search was conducted in the PubMed database using the MESH terms “gastroparesis” AND “gastric empathy” AND “diagnosis and treatment”. The search yielded 618 results. After duplicates were removed, the following inclusion criteria were applied as search filters: systemic review articles, meta-analysis, case reports, and randomized and non-randomized clinical trials conducted in humans older than 18 years published in Spanish or English between January 2000 and December 31, 2016. Studies conducted in animals, or in participants under 18 years, written in languages, and published in different dates were excluded.
In total, 240 articles were retrieved from the search conducted in PubMed after applying the search filters. Then, we carried out a systematic reading of their abstracts, and those considered to be the most relevant for the purposes of this review were selected for full analysis (71 articles) (Figure 2).
Results
Based on the above, the following information regarding the current diagnosis and treatment of gastroparesis in adults was obtained.
Diagnosis
Gastroparesis is diagnosed in symptomatic patients in whom delayed gastric emptying is confirmed and after ruling out other causes.
Upper digestive tract endoscopy and imaging studies of the upper digestive tract must be performed to rule out pyloric stenosis, neoplasms, or active ulcerative disease in the antrum, pylorus, or duodenum. Endoscopy is more sensitive than barium x-ray to detect mucosal lesions, although double-contrast techniques improve the sensitivity of imaging studies24.
A contrast x-ray of the small intestine is performed in patients with resistant symptoms and which the origin of symptoms seems to be the small intestine (e.g., extensive distension, steatorrhea, and fecal emesis), or in patients with evidence of dilated small bowel loops on a simple x-ray. When an upper gastrointestinal tract x-ray is requested, a gastrointestinal transit test may be requested; in this test, barium contrast medium is administered orally to dilute lesions in the small intestine. This imaging study allows the accurate detection of a high-grade obstruction of the small intestine, it usually provides adequate assessment of the terminal ileum, and may rarely suggest superior mesenteric artery syndrome. On the other hand, enteroclysis (small bowel enema), which is performed after a nasoduodenal or orogastric tube is placed, provides double-contrast imaging and is more accurate for detecting small lesions of the intestinal mucosa, medium to intermediate-grade obstructions, and small bowel neoplasms. Finally, a contrast (oral and intravenpus) computed tomography may also be useful for the detection and location of an intestinal obstruction.
Once a mechanical condition of the stomach and small intestine is ruled out, the gastric emptying rate of solids is usually determined by scintigraphy. An abnormal gastric emptying test result suggests, but does not confirm, that symptoms are associated with gastroparesis. When gastric emptying is normal, other causes should be considered. However, in symptomatic patients with normal gastric emptying, it is not possible to rule out a disorder of motor gastric function because regional gastric abnormalities, which include impaired fundic accommodation or gastric and electrical dysrhythmias, may be accompanied by symptoms25.
Other complementary tests to confirm delayed gastric emptying include thyroid function tests to rule out hypothyroidism, and hemoglobin A1c test (HbA1c) to estimate long-term blood glucose regulation in diabetic patients. Moreover, based on the findings related to the current disease, the patient’s history, and physical examination, autoimmune tests, neuromuscular disease studies, or paraneoplastic phenomena will be considered. Once other causes are ruled out, idiopathic gastroparesis is diagnosed26,27.
Several methods have been proposed for quantifying gastric emptying, gastric motor function and myoelectrical activity, including contrast imaging techniques, gastric emptying scintigraphy, gastroparesis breath test, electrogastrography, antroduodenal manometry, ultrasonography, magnetic resonance imaging, and positron emission tomography.
Contrast imaging techniques
Upper gastrointestinal series with barium contrast is not a sensitive method for measuring gastric emptying, because it is difficult to quantify the relative fraction of contrast passing through the intestine and because barium is not a physiological test meal. However, it may suggest gastroparesis due to poor gastric emptying, gastric dilation, and presence of retained food or a bezoar. Lack of, or very little, barium emptying at 30 minutes and gastric barium retention at 6 hours suggest gastroparesis28.
The relevance of barium X-ray is based on the exclusion of mucosal lesions and mechanical gastric outlet obstruction.
Gastric emptying scintigraphy
Gastric emptying scintigraphy of a solid-phase meal is considered the gold standard for diagnosing gastroparesis because it quantifies the emptying of a physiological caloric meal. The measurement of solid-meal gastric emptying is more sensitive to detect gastroparesis because fluid emptying may remain normal even in patients with advanced delayed gastric emptying. Liquid-phase emptying studies are most commonly performed after gastric surgery in cases where rapid emptying syndrome is suspected. The usefulness of gastric scintigraphy to guide treatment and predict clinical response has been discussed29. In this regard, some clinicians have proposed performing a dual-phase study of solid and liquid emptying in patients who underwent gastric surgery to establish whether symptoms are caused by delayed solid emptying or by rapid fluid emptying30.
For solid-phase gastric emptying scintigraphy studies, almost all imaging studies centers use an egg sandwich labeled as having sulfide colloid and technetium-99m (99mTc) as a test meal24. More recently, a technique consisting of using egg whites as a meal and performing scans immediately after meal ingestion and at 1, 2, and 4 hours has been proposed to provide a degree of standardization among imaging centers31. This test meal has a very low-fat content and, in theory, could provide results that differ from conventional foods. Regardless of the food being used, cooking the radiotracer with it is necessary to ensure the attachment of the radioisotope during the solid-phase study. Gastric emptying scintigraphy should be prolonged at least two hours after eating.
However, even if scintigraphy is prolonged for this period, there may be significant day-to-day variability (up to 20 %) in gastric emptying rhythms. The test is less safe for shorter times due to the larger variations of normal gastric emptying. Some researchers recommend prolonging the scan for 4 hours to improve accuracy to establish the presence of gastroparesis32,33.
The simplest method for interpreting this gastric emptying imaging study is to report the percentage of retention at defined times after eating (usually 2-4 hours). It is also possible to estimate half gastric emptying times; however, extrapolation of the emptying curve of an individual in which 50% of the food consumed is not emptied during the actual imaging time may provide an inaccurate estimation of gastric emptying half-time34.
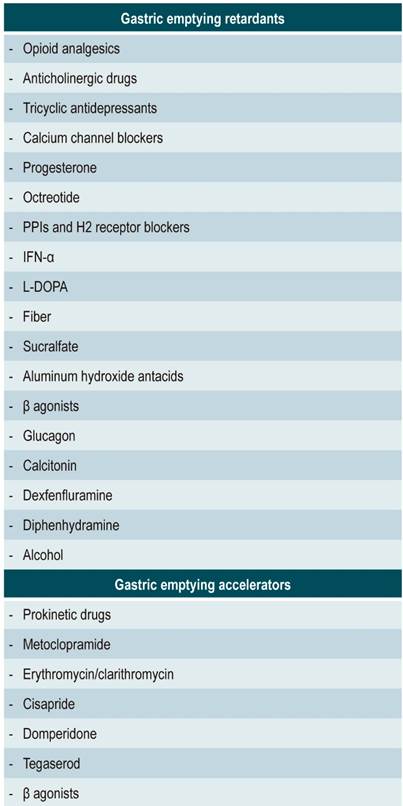
Patients should discontinue medications that may affect gastric emptying for an appropriate period based on the biological half-life of the drugs prior to undergoing the scitigrapgy (Table 2). Most medications will take 48 to 72 hours to be eliminated from the body.
When symptoms are severe, serotonin receptor antagonists, such as ondansetron, may be administered before performing gastric scintigraphy, since they have little effect on gastric emptying32. Also, hyperglycemia (glucose > 270 mg/dL) delays gastric emptying in diabetic patients. Thus, gastric emptying scintigraphy may be postponed until relative euglycemia is achieved, so that a safe estimation of the emptying parameters without the presence of an acute metabolic alteration is obtained35,36. Premenopausal women have slower gastric emptying than men.
Gastroparesis breath test
To measure gastric emptying, breath tests using a non-radioactive carbon isotope (13C) have been validated; this isotope is administered together with a solid food meal attached to a medium-chain triglyceride called octanoate37. In other studies, this isotope has been bound to acetate or algal protein38. After ingestion and through gastric emptying, these substances are absorbed into the small intestine and metabolized to 13CO2, which is expelled by the lungs during breathing. Measuring 13CO2 levels allows the assessment of solid-phase gastric emptying. The octanoate breath test has yielded reproducible results that correlate with gastric emptying scintigraphy findings37; however, it is necessary to validate these tests in patients with emphysema, cirrhosis, celiac disease, or pancreatic insufficiency since octanoate metabolism may be altered in these patients38.
Electrogastrography
This study is carried out by attaching skin electrodes to the abdominal wall at the level of the stomach. It records gastric myoelectrical activity, known as slow wave, which is responsible for controlling the maximum frequency and amplitude of distal gastric contractions. Consumption of any meal increases the amplitude of the electrogastrography signal, which has been associated with an increase in antral contractility or mechanical distension of the stomach39.
Electrogastrography test quantifies the dominant frequency and regularity of gastric myoelectric activity, the percentage of time during which abnormal slow wave rhythms exist while fasting and after ingestion and assesses the increase in signal amplitude after a meal40.
Electrogastrography is considered abnormal when dysrhythmias occur for more than 30% of the recording time, or when consumption of a meal does not cause an increase in signal amplitude39.
This test is considered complementary to gastric emptying scintigraphy in the management of patients with resistant symptoms, suggestive of an upper gastrointestinal motility disorder39.
Antroduodenal manometry
Gastric motor activity depends on the state of fasting or ingestion and is specific to each of them. The interdigestive pattern (fasting) consists of three cyclical phases known as the migratory motor complex (MMC), which occurs every two hours unless interrupted by an intake. Phase I is a period of motor latency, followed by a period of intermittent phasic contractions (phase II). Phase III consists of a burst of regular rhythmic contractions that spread from the antrum to the proximal small intestine; during this phase, dietary fibers and non-digestible solids are removed from the proximal intestine.
Antroduodenal manometry evaluates gastric and duodenal motor function in both fasting and postprandial periods. It can be performed for short periods of 5 to 8 hours but is usually carried out on an outpatient basis for a period of 24 hours, in which symptoms are correlated with abnormal motor patterns. Its use is especially indicated in patients with motor dysfunction with unexplained nausea and vomiting, patients with gastric or small bowel stasis, and patients with chronic intestinal pseudo-obstruction41,42.
In cases of gastroparesis, antroduodenal manometry may show a decrease in the frequency or strength of antral contractions and detect most phase III complexes in the duodenum. In some people, it is possible to observe an increase in tonic and phasic activity of the pylorus (pylorospasm) or irregular bursts of small intestine contractions.
Myopathic disorders, such as scleroderma or amyloidosis, produce low amplitude rhythmic contractions, while neuropathic conditions are characterized by normal-amplitude contractions with abnormal propagation, including loss of intestinal phase III, random bursts of activity and lack of conversion to the fed pattern after consuming a meal. Therefore, antroduodenal manometry makes it possible to differentiate the cause of gastroparesis between these two etiologies.
Also, antroduodenal manometry is a useful test for diagnosing rumination syndrome or hidden mechanical intestinal obstructions, where two characteristic motor patterns are observed: cluster postprandial contractions lasting more than 30 minutes and separated by a period of quiescence (> 8 seconds), or simultaneous summation suggesting a common cavity phenomenon of a dilated segment of the intestine42.
Some studies suggest that findings in antroduodenal manometry influence less than 20% of therapeutic decisions in patients with dysmotility syndromes43.
Other motor gastric function tests
Ultrasonography
Transabdominal ultrasonography measures various parameters of motor gastric function. Serial changes in the antral cross-sectional area may provide a gastric emptying rate, which is considered complete when the antral area returns to the baseline level when fasting. Ultrasonography has also been used to measure the accommodation of the proximal stomach. Duplex ultrasonography can quantify the transpyloric flow of the liquid gastric content. Unfortunately, determining gastric emptying through ultrasound depends on the operator of the test and this test has been proved to be safe only to measure fluid emptying rates44.
Magnetic resonance imaging
Stomach emptying and accommodation can be measured through magnetic resonance imaging using transaxial abdominal studies every 15 minutes. Magnetic resonance imaging allows differentiating between the gastric volume of a meal and the total gastric volume and determining gastric secretion rates. This is a non-invasive study that does not cause irradiation, but it is limited by the availability of equipment, the time required for its interpretation, and cost-related issues44.
Proton computed tomography
Radionuclide imaging of the stomach wall has been used as a non-invasive measurement of gastric adaptation after intravenous injection of 99mTc pertechnetate, which is located in the gastric mucosa, and the subsequent performance of imaging studies using single photon emission computed tomography45.
General measures
The treatment of gastroparesis has two main objectives: to identify and treat its cause, and to treat associated symptoms. This requires correcting hydroelectrolytic and nutritional deficiencies, modifying the diet and using prokinetic drugs that stimulate gastric motor activity, antiemetics to treat nausea and vomiting, and psychotropic agents to minimize symptoms. Although narcotic analgesics can improve abdominal pain rapidly, their chronic use can cause delayed gastric emptying, nausea, and vomiting, as well as dependence, which should be avoided. Total parenteral nutrition, although used in some refractory patients, has been associated with infections and thrombosis.
Similarly, lifestyle habits should be modified, and comorbidities should be monitored. This is the case of diabetic patients with gastroparesis, who usually have labile diabetes with long periods of significant hyperglycemia, since the latter delays gastric emptying, even when there are no fixed gastric motor deficits, and is likely to be mediated by reduced phasic antral contractility and induction of pyloric pressure waves34,46. It should also be noted that the intake of high amounts of alcohol tends to decrease antral contractility and impair gastric emptying47, as well as smoking48.
Dietary aspects
Gastroparesis can cause food aversion, poor oral intake, and consequent malnutrition. A study conducted in patients with diabetic gastroparesis and idiopathic gastroparesis found that 194 of them (64%) had calorie deficit diets, and only 5 patients (2%) followed the diet suggested for patients with gastroparesis. Deficiencies were evident in several vitamins and minerals. Patients with idiopathic disorders were more likely to have diets deficient in vitamins A, B6, C and K, iron, potassium, and zinc than those with diabetes49.
Patients who had a calorie deficit diet were characterized by having the most severe symptoms (abdominal distension and constipation). In addition, according to a multivariate logistic regression analysis, patients attending a nutritional consultation increased the chances of meeting total daily energy needs (Odds ratio [OR]: 1.51; p = 0.08)46.
Pharmacological treatment
Prokinetics are considered the first-choice medication to treat patients with gastroparesis, although evidence of its efficacy is limited. A meta-regression analysis of the association between symptom improvement and frequency through performed based multiple studies about gastroparesis did not find a significant correlation between these two aspects50.
Metoclopramide
Metoclopramide was approved to treat gastroparesis by the United States Food and Drug Administration (FDA) in 1979 and remains the first-line medication for the treatment of these patients. This drug acts as a prokinetic due to its antagonistic effect on dopamine receptor 2 (D2R), promoting gastric emptying and binding to the 5-Hydroxytryptamine receptor 4 (serotonin 5-HT4) to stimulate the cholinergic nerve pathways in the stomach51. Physiologically, it accelerates intestinal transit by increasing the tone and amplitude of gastric contractions, it increases the pressure of the lower esophageal sphincter, and it improves antro-pyloro-duodenal coordination. Moreover, this antiemetic agent provides relief through central and peripheral dopamine receptors antagonism52.
Parkman et al.53 evaluated the efficacy of using metoclopramide nasal spray (10 or 20 mg) and tablets (20 mg) 4 times a day in 89 diabetic patients with symptoms suggestive of gastroparesis. Symptoms improved with the three nasal spray therapy modalities, showing better tolerance and similar or higher efficacy than the tablet-based treatment. Furthermore, in a multicenter study conducted in the United States, 285 diabetic patients with gastroparesis (82.5% with type 2 diabetes) were randomly assigned to a 4-week placebo or metoclopramide therapy consisting of oral administration (nasal spray) of 10 mg or 14 mg, 3 times a day 30 minutes before meals54. In the subgroup analysis, females undergoing spray-based metoclopramide therapy showed a significantly greater relief of symptoms52. It should be noted that nasal spray administration does not eliminate the possibility of neurological adverse effects.
Domperidone
This drug exerts its prokinetic effect by acting as a dopamine D2 receptor antagonist, thus improving antrum and duodenum contractions and, in turn, peristalsis. It also has antiemetic properties because it crosses the blood-brain barrier and acts on chemoreceptors located in the fourth ventricle55.
In a double-blind, randomized, multicenter clinical trial comparing the use of domperidone 20 mg with metoclopramide 10 mg in patients with diabetic gastroparesis, a similar efficacy was found between both groups in terms of reducing the onset and severity of symptoms such as nausea, vomiting, early satiety, swelling, and distension. However, there was a significantly greater reduction in mental acuity in patients who were administered metoclopramide for 4 weeks. Moreover, fewer central neurological adverse effects (drowsiness, anxiety, depression, and acathisia) were observed after 2 and 4 weeks of follow-up in the domperidone group. In addition, domperione has no cholinergic activity56.
Cisapride
Cisapride promotes the release of acetylcholine into the myenteric plexus of the intestine and indirectly stimulates gastrointestinal motility. It acts as a 5-HT4 receptor agonist and a 5-HT3 receptor antagonist, which contributes to the release of acetylcholine and its subsequent prokinetic effects. Unlike metoclopramide, it has no CNS effects due to its lack of antidopaminergic activity57.
Although it was approved by the FDA for treating nighttime heartburn in patients with gastroesophageal reflux, a few studies have reported improved gastric emptying of both solids and liquids after single or repeated doses of cisapride58. Some studies have shown that improvement in gastric emptying does not necessarily translate into a relief of symptoms58, while others report that using this drug has not benefits in the treatment of gastroparesis. On the contrary, pharmacological surveillance after its commercialization has identified several cases of cardiac arrhythmia and sudden death, as this drug acts directly on the potassium channels of the heart, prolonging the QT interval and predisposing patients to the development of ventricular arrhythmias59.
Other drug options
There are other types of prokinetic medications that are being used as attractive options for treating gastroparesis, including 5-HT receptor agonists (tegaserod and mosapride), dopamine receptor antagonists (levosulpiride), cholecystokinin receptor antagonists (dexloxiglumide), and motilin receptor agonists (mitemcinal [GM-611]. For example, levosulpiride, which has both antiemetic and prokinetic effects, can relieve symptoms and accelerate gastric emptying in diabetic patients with gastroparesis60.
Ghrelin receptor agonists are a new class of prokinetics61,62. Oral administration of TZP-102, which was initially used in Phase IIb controlled trials conducted in in patients with type 1 or type 2 diabetes with clinical signs of gastroparesis and gastric emptying retardation, has been evaluated61. Gastric emptying at baseline and at week 12 showed differences between the groups, but this study had some limitations including the permission of concomitant use of antiemetics and opioid analgesics, and the mismatch between symptom responses62.
Relamorelin is another ghrelin receptor agonist that is administered subcutaneously. It has been reported that it improved gastric emptying and reduced symptoms severity in a pilot trial conducted in 10 patients with type 1 diabetes. It is currently in Phase II testing63.
Psychotropic agents have also been used to treat patients with gastroparesis. Based on the assumption that visceral hypersensitivity contributes to the onset of symptoms, psychotropic drugs, especially tricyclic antidepressants, are often used in this setting, although evidence of their efficacy is unconvincing64.
The efficacy of antidepressants to treat patients with dyspeptic symptoms, including those with delayed emptying, was also addressed in a placebo-controlled multicenter trial using amitriptyline and escitalopram65. Said study included 292 patients with functional dyspepsia treated at 8 centers in the United States and who were randomly assigned to 12 weeks of treatment at night with placebo or with amitriptyline 50 mg or escitalopram 10 mg. Gastric emptying rate was obtained at baseline and relief of symptoms of functional dyspepsia was assessed weekly. Response rates, defined as adequate symptoms relief for at least 5 of the last 10 weeks in the trial, were 40%, 53% and 38% for placebo, 53% for amitriptyline, and 38% for escitalopram65.
Gastric electrical stimulation (GES)
The GES system has been approved by the FDA to treat patients who fail to respond or cannot tolerate medical therapy. This device consists of two electrodes that deliver low-energy, high-frequency pulses and are implanted in the muscle layer of the greater curvature, 9 or 10 cm from the pylorus, through laparotomy or laparoscopy. Leads are connected to a pulse generator, which is implanted subcutaneously into the abdominal wall (Figure 3).
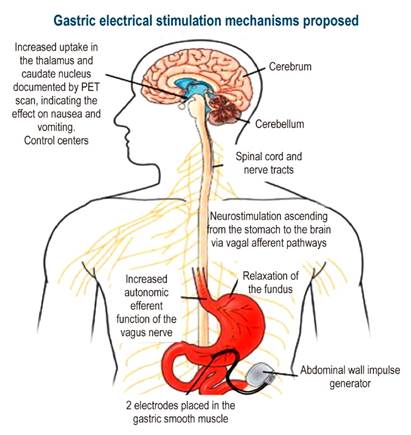
Figure 3 Gastric electrical stimulation device. PET: Positron emission tomography. Adapted from: Reddymasu SC, Sarosiek I, McCallum RW. Severe gastroparesis: medical therapy or gastric electrical stimulation. Clin Gastroenterol Hepatol. 2010;8(2):117-24.
Electrical stimulation at a 10% higher rate than that of the intrinsic slow wave initiates and establishes the passage of gastric myoelectric activity with long-lasting high-energy pulses66.
The main effect of GES is increasing vagal activity based on the sympathetic-vagal relationship, which causes better fundal accommodation and improves food intake and storage capacity.
PET scans show increased activity in the thalamus and caudate nuclei after chronic therapy with GES. The device stimulates the projection of vagal afferent pathways to the nucleus of the solitary tract in the dorsal spine and the thalamus through reticular formation, achieving better control of symptoms.
A study showed efficacy of GES in 20 of 26 patients, with decreased nausea and vomiting at 3 and 6 months67. In this research, gastric neurostimulation promoted gastric emptying of fluids but not of solids. Another study, a randomized, double-blind clinical trial conducted in 33 patients with chronic gastroparesis that was first controlled with made-up stimulation for two months and followed by an open-label phase in which the device was activated for one year, reported that Improvement was observed mainly in patients with diabetic gastroparesis, being higher than the improvement observed in the idiopathic gastroparesis group68. Long-term follow-up over one year showed a decrease in mean vomiting frequency (25 to 6 times per week), with a concurrent improvement in quality of life. Subsequent studies have described improvements in nutritional parameters and reduced supplementary feeding requirements69.
If GES is combined with pyloroplasty to accelerate gastric emptying, better results are achieved.
Surgery and endoscopic interventions for the management of gastroparesis
Surgery is often considered the last resort treatment in severe, drug-resistant gastroparesis, and few studies reporting results in this regard are available70.
In a prospective study conducted in 35 patients (86% women) who underwent total or near-total laparoscopic gastrectomy for treating gastroparesis symptoms that did not respond to prokinetic and antiemetic therapies, being the most common reflux, nausea, and abdominal pain, surprisingly, 46% of them had previously undergone pyloromyotomy, 54% had undergone fundoplication, and 23% had undergone GES treatment. Follow-up at 6 months showed that surgery significantly improved nausea, abdominal distension, and burping, while no significant effect was observed in relation to pain71. These studies on surgical management of gastroparesis report favorable results, but they have been conducted in an uncontrolled environment, with a relatively short follow-up time.
Taking into account the existing relevant literature, surgical management of gastroparesis must be considered with caution and temporary nasointestinal tube feeding can be used to evaluate the tolerance to nutrients that enter the small intestine rapidly70.
Discussion
The concept of gastroparesis still deals with the lack of association of symptoms with delayed gastric emptying. Based on recent studies, factors involved in its pathophysiology include pyloric resistance and increased duodenal contractility alteration. Studies on its pathophysiology confirm the importance of neuropathy and inadequate glycemic control as a long-term risk factor in the pathogenesis of gastroparesis in patients with type 1 diabetes. Loss of interstitial cells of Cajal, perhaps mediated through a M2 macrophage-deficiency, can be a key event at the cellular level.
The role of dietary therapy is under study, and a series of cases reporting favorable outcomes with surgical or endoscopic interventions have been published.
This paper reviews the available evidence on gastroparesis to provide clear and updated information about its diagnosis and treatment options. As stated above, there are several tests to assess patients with suspected gastroparesis. Gastric dysmotility treatments are based on dietary, pharmacological, and surgical therapies that relieve symptoms and maintain adequate nutrition
REFERENCES
1. Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L; American College of Gastroenterology. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108(1):18-37; quiz 38. https://doi.org/10.1038/ajg.2012.373 [ Links ]
2. Tack J, Carbone F, Rotondo A. Gastroparesis. Curr Opin Gastroenterol. 2015;31(6):499-505. https://doi.org/10.1097/MOG.0000000000000220 [ Links ]
3. Farre R, Tack J. Food and symptom generation in functional gastrointestinal disorders: physiological aspects. Am J Gastroenterol. 2013;108(5):698-706. https://doi.org/10.1038/ajg.2013.24 [ Links ]
4. Sarnelli G, Caenepeel P, Geypens B, Janssens J, Tack J. Symptoms associated with impaired gastric emptying of solids and liquids in functional dyspepsia. Am J Gastroenterol. 2003;98(4):783-8. https://doi.org/10.1111/j.1572-0241.2003.07389.x [ Links ]
5. Jones KL, Russo A, Stevens JE, Wishart JM, Berry MK, Horowitz M. Predictors of delayed gastric emptying in diabetes. Diabetes Care 2001;24(7):1264-9. https://doi.org/10.2337/diacare.24.7.1264 [ Links ]
6. Hoogerwerf WA, Pasricha PJ, Kalloo AN, Schuster MM. Pain: the overlooked symptom in gastroparesis. Am J Gastroenterol 1999;94(4):1029-33. https://doi.org/10.1111/j.1572-0241.1999.01008.x [ Links ]
7. Søfteland E, Brock C, Frøkjær JB, Simrén M, Drewes AM, Dimcevski G. Rectal sensitivity in diabetes patients with symptoms of gastroparesis. J Diabetes Res 2014; 2014:784841. https://doi.org/10.1155/2014/784841 [ Links ]
8. Bharucha AE, Batey-Schaefer B, Cleary PA, Murray JA, Cowie C, Lorenzi G, Driscoll M, Harth J, Larkin M, Christofi M, Bayless M, Wimmergren N, Herman W, Whitehouse F, Jones K, Kruger D, Martin C, Ziegler G, Zinsmeister AR, Nathan DM; Diabetes Control and Complications Trial-Epidemiology of Diabetes Interventions and Complications Research Group. Delayed Gastric Emptying Is Associated With Early and Long-term Hyperglycemia in Type 1 Diabetes Mellitus. Gastroenterology. 2015;149(2):330-9. https://doi.org/10.1053/j.gastro.2015.05.007 [ Links ]
9. Stanghellini V, Tack J. Gastroparesis: separate entity or just a part of dyspepsia? Gut. 2014;63(12):1972-1978. https://doi.org/10.1136/gutjnl-2013-306084 [ Links ]
10. Stacher G, Lenglinger J, Bergmann H, Schneider C, Hoffmann W, Wol-fl G, Stacher-Janotta G. Gastric emptying: a contributory factor in gastrooesophageal reflux activity? Gut 2000;47(5):661-6. https://doi.org/10.1136/gut.47.5.661 [ Links ]
11. Barnert JRA, Dumitrascu DL, Wienbeck M. Gastroesophageal reflux disease: emptying of the proximal and the distal stomach measured by ultrasonography. Gastroenterology. 2001;120(5 Suppl 1):A460. https://doi.org/10.1016/S0016-5085(08)82281-0 [ Links ]
12. Lee H-R, Lennon VA, Camilleri M, Prather CM. Paraneoplastic gastrointestinal motor dysfunction: clinical and laboratory characteristics. Am J Gastroenterol. 2001;96(2):373-9. https://doi.org/10.1111/j.1572-0241.2001.03454.x [ Links ]
13. Bonapace ES, Davidoff S, Krevsky B, Fisher RS, Parkman HP. Whole gut transit scintigraphy in the clinical evaluation of patients with upper and lower gastrointestinal symptoms. Am J Gastroenterol. 2000;95(10):2838-47. https://doi.org/10.1111/j.1572-0241.2000.03195.x [ Links ]
14. Casey KM, Quigley TM, Kozarek RA, Raker EJ. Lethal nature of ischemic gastropathy. Am J Surg. 1993;165 (5):646-9. https://doi.org/10.1016/S0002-9610(05)80453-2 [ Links ]
15. Brand RE, DiBaise JK, Quigley EM, Gobar LS, Harmon KS, Lynch JC, Bierman PJ, Bishop MR, Tarantolo SR. Gastroparesis as a cause of nausea and vomiting after high-dose chemotherapy and haemopoietic stem-cell transplantation. Lancet. 1998;352(9145):1985. https://doi.org/10.1016/S0140-6736(05)61330-X [ Links ]
16. Eagle DA, Gian V, Lauwers GY, Manivel JC, Moreb JS, Mastin S, Wingard JR. Gastroparesis following bone marrow transplantation. Bone Marrow Transplant 2001;28(1):59-62. https://doi.org/10.1038/sj.bmt.1703084 [ Links ]
17. Chowdhury RS, Forsmark CE, Davis RH, Toskes PP, Verne GN. Prevalence of gastroparesis in patients with small duct chronic pancreatitis. Pancreas. 2003;26(3):235-8. https://doi.org/10.1097/00006676-200304000-00005 [ Links ]
18. Van Vlem B, Schoonjans R, Vanholder R, De Vos M, Vandamme W, Van Laecke S, Lameire N. Delayed gastric emptying in dyspeptic chronic hemodialysis patients. Am J Kidney Dis. 2000;36(5):962-8. https://doi.org/10.1053/ajkd.2000.19094 [ Links ]
19. De Schoenmakere G, Vanholder R, Rottey S, Duym P, Lameire N. Relationship between gastric emptying and clinical and biochemical fac- tors in chronic haemodialysis patients. Nephrol Dial Transplant 2001;16(9):1850-5. https://doi.org/10.1093/ndt/16.9.1850 [ Links ]
20. Nowak TV, Goddard M, Batteiger B, Cummings OW. Evolution of acute cytomegalovirus gastritis to chronic gastrointestinal dysmotility in a nonimmunocompromised adult. Gastroenterology 1999;116(4):953-8. https://doi.org/10.1016/S0016-5085(99)70079-X [ Links ]
21. Neild PJ, Nijran KS, Yazaki E, Evans DF, Wingate DL, Jewkes R, Gazzard BG. Delayed gastric emptying in human immunodeficiency virus infection. Dig Dis Sci. 2000;45(8):1491-9. https://doi.org/10.1023/A:1005587922517 [ Links ]
22. Mearin F, de Ribot X, Balboa A, Salas A, Varas MJ, Cucala M, Bartolome R, Armengol JR, Malagelada JR. Does Helicobacter pylori infection increase gastric sensitivity in functional dyspepsia? Gut 1995;37(1):47-51. https://doi.org/10.1136/gut.37.1.47 [ Links ]
23. Salicru M, Juarez D, Genta RM. Low prevalence of H. pylori infection in patients with gastroparesis. Dig Liver Dis. 2013;45(11):905-8. https://doi.org/10.1016/j.dld.2013.05.001 [ Links ]
24. Quigley EMM, Hasler W, Parkman HP. AGA technical review on nausea and vomiting. Gastroenterology. 2001;120(1):263-86. https://doi.org/10.1053/gast.2001.20516 [ Links ]
25. Hornbuckle K, Barnett JL. The diagnosis and work-up of the patient with gastroparesis. J Clin Gastroenterol. 2000;30(2):117-24. https://doi.org/10.1097/00004836-200003000-00004 [ Links ]
26. Parkman HP. Idiopathic gastroparesis. Gastroenterol Clin North Am. 2015;44(1):59-68. https://doi.org/10.1016/j.gtc.2014.11.015 [ Links ]
27. Pasricha PJ, Parkman HP. Gastroparesis: definitions and diagnosis. Gastroenterol Clin North Am. 2015;44(1):1-7. https://doi.org/10.1016/j.gtc.2014.11.001 [ Links ]
28. Parkman HP, Harris AD, Krevsky B, Urbain JL, Maurer AH, Fisher RS. Gastroduodenal motility and dysmotility: update on techniques available for evaluation. Am J Gastroenterol. 1995;90(6):869-92. [ Links ]
29. Galil MA, Critchley M, Mackie CR. Isotope gastric emptying tests in clinical practice: expectation, outcome, and utility. Gut. 1993;34(7): 916-19. https://doi.org/10.1136/gut.34.7.916 [ Links ]
30. Rahim MK, Durr-E Sabih, Mateen A, Najam Uddin, Yousaf M. Studies of gastric emptying time in patients with non-ulcer dyspepsia. Nucl Med Commun. 2007;28(11):852-8. https://doi.org/10.1097/MNM.0b013e3282f0d167 [ Links ]
31. Tougas GH, Eaker EY, Abell TL, Abrahamsson H, Boivin PL, Chen J, Hocking MP, Quigley EM, Koch KL, Tokayer AZ, Stanghellini V, Chen Y, Huizinga JD, Ryden J, Bourgeois I, McCallum RW. Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol 2000;95(6):1456-62. https://doi.org/10.1111/j.1572-0241.2000.02076.x [ Links ]
32. Guo JP, Maurer AH, Fisher RS, Parkman HP. Extending gastric emptying scintigraphy from two to four hours detects more patients with gastroparesis. Dig Dis Sci. 2001;46(1):24-9. https://doi.org/10.1023/a:1005697422454 [ Links ]
33. Tomita T, Okugawa T, Yamasaki T, Kondo T, Toyoshima F, Sakurai J, Oshima T, Fukui H, Daimon T, Watari J, Kashiwagi T, Matsumoto T, Miwa H. Use of scintigraphy to evaluate gastric accommodation and emptying: comparison with barostat. J Gastroenterol Hepatol. 2013;28(1):106-11. https://doi.org/ 10.1111/j.1440-1746.2012.07261.x [ Links ]
34. Camilleri M, Hasler W, Parkman HP, Quigley EM, Soffer E. Measurement of gastroduodenal motility in the GI laboratory. Gastroenterology. 1998;115(3):747-62. https://doi.org/10.1016/s0016-5085(98)70155-6 [ Links ]
35. Singla R, Homko C, Schey R, Parkman HP. Diabetes-related autoantibodies in diabetic gastroparesis. Dig Dis Sci. 2015;60(6):1733-1737. https://doi.org/10.1007/s10620-015-3690-0 [ Links ]
36. Bharucha AE, Kudva Y, Basu A, Camilleri M, Low PA, Vella A, Zinsmeister AR. Relationship between glycemic control & and gastric emptying in poorly controlled type 2 diabetes. Clin Gastroenterol Hepatol. 2015;13(3):466-476; e1. https://doi.org/10.1016/j.cgh.2014.06.034 [ Links ]
37. Bromer MQ, Kantor SN, Wagner DA, Knight LC, Maurer AH, Parkman HP. Simultaneous measurement of gastric emptying with a simple muffin meal using 13C-octanoate breath test and scintigraphy in normal subjects and patients with in dyspeptic symptoms. Dig Dis Sci. 2002;47(7):1657-63. https://doi.org/10.1023/A:1015856211261 [ Links ]
38. Kim D-Y, Myung S-J, Camilleri M. Novel testing of human gastric motor and sensory functions: Rationale, methods, and potential applications in clinical practice. Am J Gastroenterol 2000;95(12):3365-73. https://doi.org/10.1111/j.1572-0241.2000.03346.x [ Links ]
39. Parkman HP, Hasler WL, Barnett JL, Eaker EY; American Motility Society Clinical GI Motility Testing Task Force. Electrogastrography: a document prepared by the gastric section of the American Motility Society Clinical GI Motility Testing Task Force. Neurogastroenterol Motil. 2003;15(2):89-102. https://doi.org/10.1046/j.1365-2982.2003.00396.x [ Links ]
40. Chen JZ, McCallum Dis RW. Clinical applications of electrogastrography. Am J Gastroenterol 1993;88(9):1324-36. [ Links ]
41. Barshop K, Staller K, Semler J, Kuo B. Duodenal rather than antral motility contractile parameters correlate with symptom severity in gastroparesis patients. Neurogastroenterol Motil. 2015;27(3):339-346. https://doi.org/10.1111/nmo.12496 [ Links ]
42. Chen JD, Lin Z, Pan J, McCallum RW. Abnormal gastric myoelectrical activity and delayed gastric emptying in patients with symptoms sug- gestive of gastroparesis. Dig Dis Sci. 1996;41(8):1538-45. https://doi.org/10.1007/BF02087897 [ Links ]
43. Parkman HP, Miller MA, Trate DM, Knight LC, Brown KL, Maurer AH, Fisher RS. Electrogastrography and gastric emptying scintigraphy are complementary for assessment of dyspepsia. J Clin Gastroenterol 1997;24(4):214-9. https://doi.org/10.1097/00004836-199706000-00006 [ Links ]
44. Szarka LA, Camilleri M. Methods for measurement of gastric motility. Am J Physiol Gastrointest Liver Physiol. 2009;296(3):G461-75. https://doi.org/10.1152/ajpgi.90467.2008 [ Links ]
45. Kim D-Y, Delgado-Aros S, Camilleri M, Samsom M, Murray JA, O’Connor MK, Brinkman BH, Stephens DH, Lighuani SS, Burton DD. Noninvasive measurement of gastric accommodation in patients with idiopathic nonulcer dyspepsia. Am J Gastroenterol 2001;96(11):3099-105. https://doi.org/10.1111/j.1572-0241.2001.05264.x [ Links ]
46. Olausson EA, Störsrud S, Grundin H, Isaksson M, Attvall S, Simrén M. A small particle size diet reduces upper gastrointestinal symptoms in patients with diabetic gastroparesis: a randomized controlled trial. Am J Gastroenterol. 2014;109(3):375-85. https://doi.org/10.1038/ajg.2013.453 [ Links ]
47. Bujanda L. The effects of alcohol consumption upon the gastrointestinal tract. Am J Gastroenterol. 2000;95(12):3374-82. https://doi.org/10.1111/j.1572-0241.2000.03347.x [ Links ]
48. Miller G, Palmer KR, Smith B, Ferrington C, Merrick MV. Smoking delays gastric emptying of solids. Gut. 1989;30(1):50-3. https://doi.org/10.1136/gut.30.1.50 [ Links ]
49. Homko CJ, Duffy F, Friedenberg FK, Boden G, Parkman HP. Effect of dietary fat and food consistency on gastroparesis symptoms in patients with gastroparesis. Neurogastroenterol Motil. 2015;27(4):501-8. https://doi.org/10.1111/nmo.12519 [ Links ]
50. Janssen P, Harris MS, Jones M, Masaoka T, Farré R, Törnblom H, Van Oudenhove L, Simrén M, Tack J. The relation between symptom improvement and gastric emptying in the treatment of diabetic and idiopathic gastroparesis. Am J Gastroenterol. 2013;108(9):1382-91. https://doi.org/10.1038/ajg.2013.118 [ Links ]
51. Hoogerwerf WA, Pasricha PJ, Kalloo AN, Schuster MM. Pain: the overlooked symptom in gastroparesis. Am J Gastroenterol. 1999;94(4):1029-33. https://doi.org/10.1111/j.1572-0241.1999.01008.x [ Links ]
52. Lata PF, Pigarelli DL. Chronic metoclopramide therapy for diabetic gastroparesis. Ann Pharmacother. 2003;37(1):122-6. https://doi.org/10.1345/aph.1C118 [ Links ]
53. Parkman HP, Carlson MR, Gonyer D. Metoclopramide nasal spray is effective in symptoms of gastroparesis in diabetics compared to conventional oral tablet. Neurogastroenterol Motil. 2014;26(4):521-528. https://doi.org/10.1111/nmo.12296 [ Links ]
54. Parkman HP, Carlson MR, Gonyer D. Metoclopramide nasal spray reduces symptoms of gastroparesis in women, but not men, with diabetes: results of a phase 2B randomized study. Clin Gastroenterol Hepatol. 2015;13(7):1256-1263; e1. https://doi.org/10.1016/j.cgh.2014.12.030 [ Links ]
55. Reddymasu SC, Soykan I, McCallum RW. Domperidone: review of pharmacology and clinical applications in gastroenterology. Am J Gastroenterol. 2007;102(9):2036- 2045. https://doi.org/10.1111/j.1572-0241.2007.01255.x [ Links ]
56. Sugumar A, Singh A, Pasricha PJ. A systematic review of the efficacy of domperidone for the treatment of diabetic gastroparesis. Clin Gastroenterol Hepatol. 2008;6(7):726-733. https://doi.org/10.1016/j.cgh.2008.02.065 [ Links ]
57. Quigley, EM. Cisapride: what wan we learn from the rise and fall of a prokinetic? J Dig Dis. 2011;12(3):147-156. https://doi.org/10.1111/j.1751-2980.2011.00491.x [ Links ]
58. Richards RD, Valenzuela GA, Davenport KG, Fisher KL, McCallum RW. Objective and subjective results of a randomized, double- blind, placebo-controlled trial using cisapride to treat gastroparesis. Dig Dis Sci. 43. 1993;38(5):811-816. https://doi.org/10.1007/BF01295905 [ Links ]
59. Rabine JC, Barnett JL. Management of the patient with gastroparesis. J Clin Gastroenterol. 2001;32(1): 11-18. https://doi.org/10.1097/00004836-200101000-00005 [ Links ]
60. Mansi C, Borro P, Giacomini M, Biagini R, Mele MR, Pandolfo N, Savarino V. Comparative effects of levosulpiride and cisapride on gastric emptying and symptoms in patients with functional dyspepsia and gastroparesis. Aliment Pharmacol Ther. 2000;14(5):561-9. https://doi.org/10.1046/j.1365-2036.2000.00742.x [ Links ]
61. McCallum RW, Lembo A, Esfandyari T, et al. TZP-102 Phase 2b Study Group. Phase 2b, randomized, double-blind 12-week studies of TZP-102, a ghrelin receptor agonist for diabetic gastroparesis. Neurogastroenterol Motil. 2013;25(11):e705-17. https://doi.org/10.1111/nmo.12184 [ Links ]
62. Camilleri M, Acosta A. A ghrelin agonist fails to show benefit in patients with diabetic gastroparesis: let’s not throw the baby out with the bath water. Neurogastroenterol Motil 2013;25(11):859-63. https://doi.org/10.1111/nmo.12226 [ Links ]
63. Shin A, Camilleri M, Busciglio I, Burton D, Smith SA, Vella A, Ryks M, Rhoten D, Zinsmeister AR. The ghrelin agonist RM-131 accelerates gastric emptying of solids and reduces symptoms in patients with type 1 diabetes mellitus. Clin Gastroenterol Hepatol. 2013;11(11):1453-1459.e4. https://doi.org/10.1016/j.cgh.2013.04.019 [ Links ]
64. Vanormelingen C, Tack J, Andrews CN. Diabetic gastroparesis. Br Med Bull. 2013;105:213-230. https://doi.org/10.1093/bmb/ldt003 [ Links ]
65. Talley NJ, Locke GR, Saito YA, Almazar AE, Bouras EP, Howden CW, Lacy BE, DiBaise JK, Prather CM, Abraham BP, El-Serag HB, Moayyedi P, Herrick LM, Szarka LA, Camilleri M, Hamilton FA, Schleck CD, Tilkes KE, Zinsmeister AR. Effect of Amitriptyline and Escitalopram on Functional Dyspepsia: A Multicenter, Randomized Controlled Study. Gastroenterology. 2015;149(2):340-9.e2. https://doi.org/10.1053/j.gastro.2015.04.020 [ Links ]
66. Skole KS, Panganamamula KV, Bromer MQ, Thomas P, Meilahn JE, Fisher RS, Parkman HP. Efficacy of gastric electrical stimulation for gastroparesis refractory to medical therapy: a single center experience. Am J Gastroenterol 2002;97(9):S48. https://doi.org/10.1016/S0002-9270(02)04617-8 [ Links ]
67. Abell TL, Van Cutsem E, Abrahamsson H, Huizinga JD, Konturek JW, Galmiche JP, Voeller G, Filez L, Everts B, Waterfall WE, Domschke W, Bruley des Varannes S, Familoni BO, Bourgeois IM, Janssens J, Tougas G. Gastric electrical stimulation in intractable symptomatic gastroparesis. Digestion. 2002;66(4):204-12. https://doi.org/10.1159/000068359 [ Links ]
68. Abell T, McCallum R, Hocking M, Koch K, Abrahamsson H, Leblanc I, Lindberg G, Konturek J, Nowak T, Quigley EM, Tougas G, Starkebaum W. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125(2):421-8. https://doi.org/10.1016/S0016-5085(03)00878-3 [ Links ]
69. Abell T, Lou J, Tabbaa M, Batista O, Malinowski S, Al-Juburi A. Gastric electric stimulation for gastroparesis improves nutritional parame- ters at short, intermediate, and long-term follow-up. J Parenter Enteral Nutr. 2003;27(4):277-81. https://doi.org/10.1177/0148607103027004277 [ Links ]
70. Tack J. The difficult patient with gastroparesis. Best Pract Res Clin Gastroenterol. 2007;21(3):379-91. https://doi.org/10.1016/j.bpg.2007.01.002 [ Links ]
71. Bhayani NH, Sharata AM, Dunst CM, Kurian AA, Reavis KM, Swanstrom LL. End of the road for a dysfunctional end organ: laparoscopic gastrectomy for refractory gastroparesis. J Gastrointest Surg. 2015;19(3):411-7. https://doi.org/10.1007/s11605-014-2609-y [ Links ]
Citation: Mayor V, Aponte D, Prieto R, Orjuela E. Current diagnosis and treatment of gastroparesis: A systematic literature review. Rev. Colomb. Gastroenterol. 2020;35(4):471-484. https://doi.org/10.22516/25007440.561
Received: May 07, 2020; Accepted: September 16, 2020











 texto em
texto em