Introduction
Paracoccidioidomycosis (PCM) is an infectious fungal disease endemic in South America with greater prevalence in Brazil, Venezuela, Colombia, and Argentina1-3. It is caused by two types of fungi that inhabit the soil in the mycelial form: Paracoccidioides brasiliensis and Paracoccidioides lutzii4,5. Upon inhalation, they enter the pulmonary alveoli, whose body temperature allows their conversion to yeast, which favors their asexual reproduction and generates acute infection of the disease, mainly in children, or chronic infection, primarily in adults6, which sometimes progresses to its secular form and causes pulmonary fibrosis7,8. Since there are few reports in the literature of esophageal involvement caused by PCM, our objective is to bring the case of a farmer who debuts with constitutional syndrome associated with dysphagia, with biopsy findings of lesions evidenced in the upper gastrointestinal endoscopy (UGIE) which were compatible with PCM and confirmed esophageal and pulmonary involvement by chest CT findings.
Case presentation
A 57-year-old male Colombian farmer patient with no pathological history. He presented a clinical picture of two-month evolution, initially characterized by retrosternal foreign-body sensation. Subsequently, progressive dysphagia from solids to liquids, regurgitating 20 minutes after food intake, without nausea and without gagging, accompanied by sialorrhea in the last week of the clinical picture.
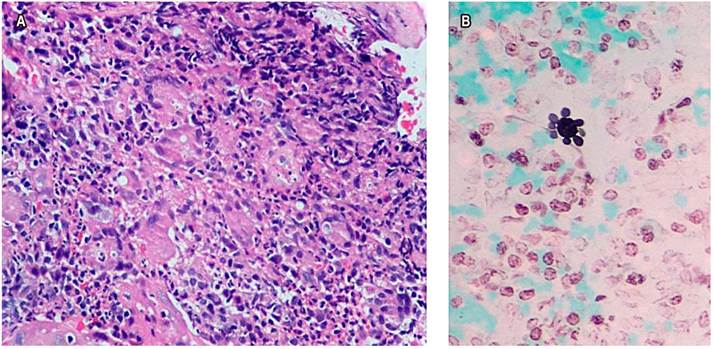
Upon admission, he reported occasional non-productive cough without dyspnea and a 10 kg weight loss in the last month. The patient showed a stable condition, systemic inflammatory response absence, vital signs unaltered, a 17 kg/m² body mass index (BMI), and signs of grade I dehydration. Constitutional syndrome secondary to neoplasia of gastrointestinal origin was initially suspected, so he was taken to UGIE (Figure 1), biopsies were taken, and extension studies such as chest tomography were requested (Figure 2A) showing lesions in the tree-in-bud and esophageal obstruction in the middle and distal third of the esophagus (Figure 2B). Tuberculosis infection was ruled out with 3 serial sputum smear tests and culture-negatives, a negative enzyme-linked immunosorbent assay (ELISA) test for human immunodeficiency virus (HIV), and blood count within normal limits. The histopathological study (Figure 3) of the esophageal biopsy showed a chronic non-necrotizing granulomatous inflammation disease secondary to fungal infection. The Grocott-Gomori’s Methenamine Silver (GMS) stain confirmed extracellular yeasts with narrow-based gemmation and “steering wheels” appearance compatible with PCM. He underwent esophageal balloon dilatation and stent placement and was started on treatment with itraconazole capsules of 100 mg every 24 hours a day for 6 months, improving dysphagia and respiratory symptoms. However, there is no post-treatment imaging follow-up record.
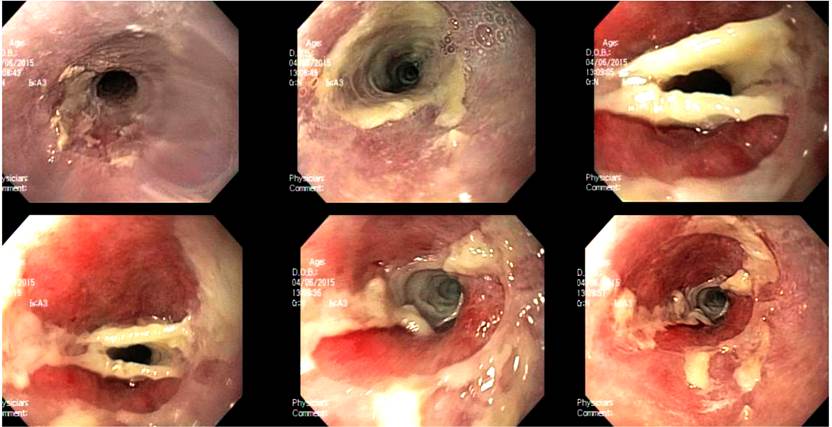
Figure 1 Endoscopic findings, esophageal stricture obstruction, and white endoluminal membranes without ulceration or bleeding.
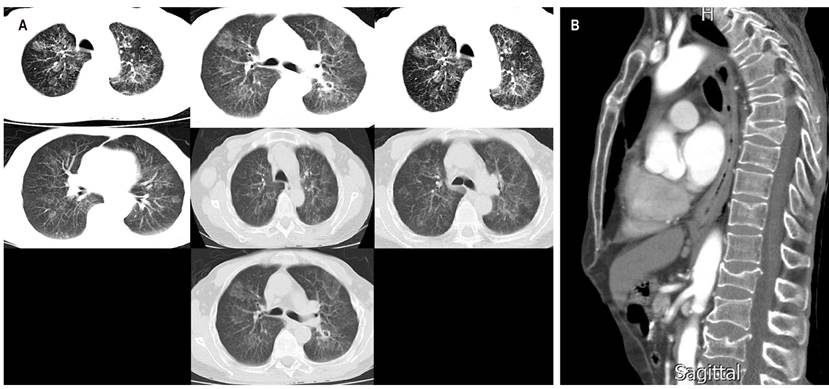
Figure 2 A. High-resolution chest tomography showing generalized interstitial parenchymal involvement with hyperdensities in the tree-in-bud. B. Sagittal chest tomography showing esophageal obstruction in the esophagus in the middle and distal third.
Discussion
Paracoccidioidomycosis is a systemic disease endemic in South America, especially in Santander, Colombia. This disease predominantly affects men between 30-50 years old, but it most often affects farmers, whose primary infection is pulmonary, where the fungus is previously inhaled in mycelial form. The systemic involvement will depend on the host’s immune response. This disease has three clinical presentations: acute, subacute (symptoms occur approximately 45 days after exposure), and chronic (more frequent in adults and characterized by a reactivation of the primary infection that could have been acquired months or even years earlier, and its symptoms depend on the affected organ). Disseminated manifestation, especially esophageal, is rare in immunocompetent individuals. In these patients, the mucous membranes with greater involvement are found in the oral and the laryngeal cavity. However, very few reports exist in the literature9-12. Diagnosis methods range from histological studies and fungal culture to antibody detection6,13-16).
Several risk factors for contracting the infectious agent were considered in this case, including the geographic area where the patient resides or works and gender. Its age group predisposed him to debut with reactivated chronic disease. In addition to the clinical evolution chronicity, he presented constitutional syndrome evidenced by involuntary weight loss; pulmonary involvement, manifested by cough without expectoration and occasional dyspnea, possibly explained by pulmonary parenchymal involvement (Figure 2), and esophageal involvement, characterized by progressive dysphagia, which in turn was confirmed through imaging findings showing endoluminal involvement and endoluminal stenosis (Figures 1 and 2B). The treatment for this disease consists of azoles, especially itraconazole, which has better tolerance, absorption, and fewer adverse effects. This is the treatment that was administered to the patient, showing a subsequent clinical picture improvement. Sulfonamides and amphotericin B, the latter, may be prescribed for severe cases17. Studies have shown that the presence of comorbidities and pulmonary fibrosis is associated with high morbimortality due to exacerbation of the underlying disease and intrinsic complications of the infection, which is why the patient’s prognosis is good since there is no pathological history and there is no evidence of pulmonary fibrosis in the requested lung tests18,19.
Conclusion
In conclusion, it is necessary to consider the geographic area, gender, occupation, and the time of evolution of the signs and symptoms of the patient’s clinical picture must be considered for a PCM diagnostic approach. All of the above must be supported by imaging tests (in our case, chest tomography and UGIE with a histological biopsy study) and antifungal pharmacological management, which consists of azoles. Itraconazole is an excellent option due to its tolerance, absorption, and reduction of adverse drug effects. Sulfonamides and amphotericin B can improve the patient’s clinical evolution. This entity must be considered within the differential diagnoses in people presenting the same symptomatology, whose final prognosis will depend on the degree of pulmonary fibrosis and associated pathological history.











 texto em
texto em