Introduction
Subepithelial lesions (SELs), described as lumps or masses covered by healthy-appearing mucosa, are usually found incidentally during endoscopic studies; they are typically asymptomatic and are estimated to be identified in 1% of esophagogastroduodenoscopies (EGDs) performed1,2. Recently, their incidence has increased; however, this phenomenon may be due to the greater availability of endoscopic studies in our setting. The present research intends to characterize the endosonographic lesions in the upper GI tract in patients treated at a gastroenterology referral center in the Coffee Region between January 2020 and January 2022.
Materials and methods
A descriptive study was conducted with a retrospective collection of information. It included all patients treated at Unión de Cirujanos (gastroenterology referral center for the Coffee Region located in Manizales, Colombia) from February 2020 to January 2022 who underwent endoscopic ultrasound as part of the study of lesions with a subepithelial appearance located in the esophagus, stomach, or duodenum that were found incidentally in previous endoscopic studies.
A descriptive analysis of the recorded data was performed, and the median was calculated for the numerical variables, while the qualitative variables were described with frequencies. Endoscopic ultrasound (EUS) was performed with a high-resolution Fujinon radial scanning endoscopic ultrasound machine, SU-1 processor, with variable frequencies of 7.5, 12, and 20 MHz, by three experienced endosonographers, and all procedures were performed under intravenous sedation. Endosonographic characteristics were prospectively recorded for all lesions: location, maximum diameter, growth pattern, echolayer of origin, and echogenicity.
Results
One hundred fifty-two EUSs were performed; mucosal lesions were found in 44 cases, extrinsic compressions in nine, and a diverticular formation in one. The remaining 108 cases indeed corresponded to SELs (Figure 1).
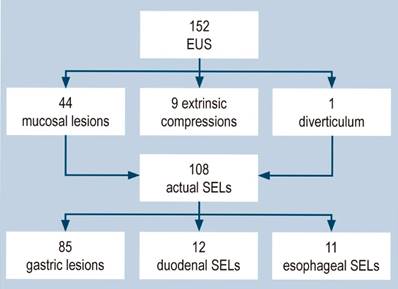
Figure 1 Studies performed, patients excluded, and the number of studies by anatomical location. SELs: subepithelial lesions; EUS: endoscopic ultrasound. Image owned by the authors.
Of the 108 patients with SEL, 72 (66.6%) were female, and the average age was 58. Most of the SELs evaluated were located in the stomach (78.7%); of these, the most frequent location was the antrum. The most common presumptive diagnosis based on endosonographic findings was gastrointestinal stromal tumor (GIST; 60.1%), followed by ectopic pancreas (14.8%) and lipoma (12%).
The average age, sex, echolayer of origin, and presumptive diagnoses of the lesions according to their location are described in Tables 1, 2, and 3.
Table 1 Endosonographic characteristics and presumptive diagnoses of gastric subepithelial lesions
| Gastric subepithelial lesions | Presumptive diagnosis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Echolayer | GIST | EP | LEI | LIP | NET | Total | |||
| Average age | 59.3 | ||||||||
| Sex | Male | 29 | |||||||
| Female | 56 | ||||||||
| Average diameter | 14.6 mm | 2 | 16 | 1 | 17 | ||||
| Location | Antrum | 38 | 2 and 3 | 1 | 2 | 3 | |||
| Body | 35 | 3 | 11 | 10 | 25 | ||||
| Fundus | 11 | 4 | 39 | 1 | 4 | 40 | |||
| Cardia | 1 | Total | 56 | 14 | 1 | 10 | 4 | 85 | |
GIST: gastrointestinal stromal tumors; LEI: leiomyoma; LIP: lipoma; EP: ectopic pancreas; NET: neuroendocrine tumor. Table prepared by the authors.
Table 2 Endosonographic characteristics and presumptive diagnoses of esophageal subepithelial lesions
| Esophageal subepithelial lesions | Presumptive diagnosis | ||||||
| Echolayer | GIST | LEI | CYST | Total | |||
| Average age | 53.9 | ||||||
| Sex | Male | 3 | 1 and 2 | 1 | 1 | ||
| Female | 8 | 2 | 1 | 4 | 1 | 6 | |
| Average diameter | 18.1 mm | 3 | 1 | 1 | |||
| Location | Proximal | 0 | 3 and 4 | 1 | 1 | ||
| Middle | 3 | 4 | 2 | 2 | |||
| Distal | 8 | Total | 3 | 5 | 3 | 11 | |
GIST: gastrointestinal stromal tumors; LEI: leiomyoma; CYST: intestinal duplication cyst. Table prepared by the authors.
Table 3 Endosonographic characteristics and presumptive diagnoses of duodenal subepithelial lesions
| Duodenal subepithelial lesions | Presumptive diagnosis | |||||||
| Echolayer | GIST | EP | LIP | CYST | Total | |||
| Average age | 56.4 | |||||||
| Sex | Male | 4 | 2 | 2 | 2 | |||
| Female | 8 | 3 | 2 | 3 | 1 | 6 | ||
| Average diameter | 13 mm | 3 and 4 | 1 | 1 | ||||
| Location | Bulb | 11 | 4 | 3 | 3 | |||
| D2 | 1 | Total | 6 | 2 | 3 | 1 | 12 | |
GIST: gastrointestinal stromal tumors; LEI: leiomyoma; CYST: intestinal duplication cyst; Ep: ectopic pancreas. Table prepared by the authors.
Discussion
Most SELs are detected incidentally during procedures requested for another indication. They are found with the same frequency in men and women, generally after the fifth decade of life; these data are consistent with those in this work. It has been reported that gastric SEL is found in up to 0.3% of middle-aged adults3.
SEL can be classified as non-neoplastic, which includes inflammatory changes, cysts, ectopic pancreatic tissue, and varicose veins, and neoplastic, with a low malignant potential such as lipomas, leiomyomas or with a high malignant potential such as GIST, neuroendocrine tumors or lymphomas4. The majority are asymptomatic since they usually do not involve the mucosa; however, in some cases, they can manifest with digestive bleeding, iron deficiency anemia, abdominal pain, or signs of intestinal obstruction depending on the location and size of the lesion, mainly when they are close to the pylorus, cardia, ileocecal valve, or rectum5.
Diagnosis is usually challenging since the endoscopic appearance is usually insufficient, and the diagnostic yield of conventional biopsies is usually meager6. Most are identified in the endoscopic evaluation as small lumps (less than 20 mm) covered with normal-looking mucosa, data consistent with those in our paper, although some have endoscopic characteristics that may suggest their etiology, such as lipomas. They are usually identified as yellowish in color and soft in consistency when pressed with forceps; this sign is known as the pillow sign and has a reported sensitivity of 98% for this diagnosis2,7. On the other hand, ectopic pancreatic tissue is usually identified as a nodular lesion with central umbilication in the antrum (Figure 2)8.
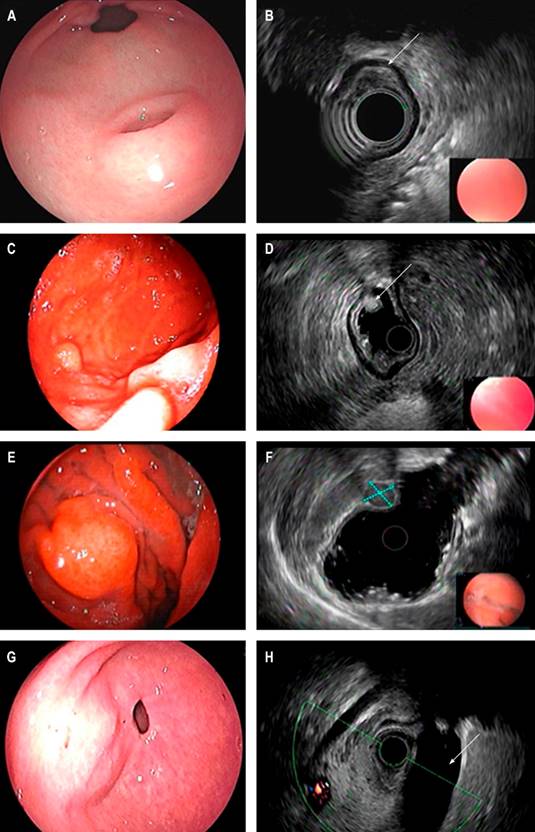
Figure 2 Endoscopic view of SEL of the antrum suggestive of an ectopic pancreas. A. Endosonographic appearance. B. Lesion involving the third echolayer (arrow); endoscopic view of the distal gastric body SEL. C. Endosonographic appearance. D. Third echolayer lesion consistent with a lipoma (arrow); endoscopic view of the gastric body SEL. E. Endosonographic appearance. F. Fourth echolayer lesion suggestive of GIST (dotted lines); endoscopic view of an elevation of a subepithelial appearance of the gastric antrum. G. Endosonographic image. H. Endosonographic image with extrinsic compression of the gallbladder (arrow). Image owned by the authors.
In order of frequency, they are located in the stomach, esophagus, duodenum, and colon, data consistent with the results described in our population2. Leiomyomas are the subepithelial lesions most frequently found in the distal two-thirds of the esophagus, and GISTs are the most frequent in the stomach (Table 4)5.
Table 4 Endoscopic and endosonographic characteristics of the most common subepithelial lesions
| Subepithelial lesion | Endoscopic appearance | Endosonographic appearance | Compromised echolayer | Most frequent location |
|---|---|---|---|---|
| Lipoma | Yellowish, positive pillow sign | Hyperechogenic, homogeneous | 3.rd | Any location of the GIT |
| Leiomyoma | No specific features | Hypoechoic, well-defined edges | 2.nd, 3.rd, 4.th | Esophagus, stomach |
| Duplication cyst | Smooth, regular surface, compressible | Anechoic, negative Doppler signal | 3.rd | Any location of the GIT |
| Ectopic pancreas | Central umbilication | Hypoechoic, heterogeneous | 3.rd, 4.th | Antrum, gastric body, duodenum |
| Varicose veins | Bluish hue, tortuous | Anechoic, positive Doppler signal | 3.rd | Any location of the GIT |
| Low-risk GIST | Subepithelial elevation without mucosal ulceration or bleeding, <30 mm | Hypoechoic, heterogeneous, hypervascular | 2.nd or 4.th | Esophagus, stomach, small intestine, rectum |
| High-risk GIST | Areas of ulceration or bleeding, >30 mm | Hypoechoic, heterogeneous areas of cystic degeneration or hyperechoic foci | 2.nd or 4.th | Esophagus, stomach, small intestine, rectum |
| Schwannoma | No specific features | Hypoechoic, homogeneous | 4.th | Gastric body |
| Lymphoma | No specific features | Hypoechoic | 2.nd, 3.rd, 4.th | Stomach, small intestine |
| Neuroendocrine tumors | Rounded, yellowish or reddish hue | Hypoechoic or hyperechoic | 2.nd, 3.rd | Stomach, small intestine, rectum |
GIT: gastrointestinal tract. Table prepared by the authors.
EUS is the gold standard in the evaluation of SELs of the gastrointestinal tract9, as it allows differentiating extrinsic compressions from intramural lesions, establishing the echolayer of origin, size, echogenicity, and margins, as well as identifying regional lymphadenopathy and obtain tissue samples if necessary10. There are pathognomonic endosonographic features for lipomas and varicose veins; however, for other types of lesions, its diagnostic accuracy is reported between 43% and 67%11.
We recommend taking a biopsy of SEL suggestive of GIST, those with high-risk endosonographic characteristics (irregular edges, cystic degeneration, ulceration, echogenic foci, heterogeneity), or those greater than 20 mm12.
Due to the low yield of conventional biopsies, multiple strategies have been designed to perform them, such as biopsy-on-biopsy and using jumbo forceps or loops. It can also be performed under endosonographic guidance or with a mucosal incision that allows the lesion to be exposed and the biopsy to be taken13. This last technique is recommended for lesions smaller than 20 mm, and its general diagnostic yield is 89%, given the technical difficulty in taking needle samples in lesions of this size14. When comparing the diagnostic performance of fine needle aspiration (FNA) with fine needle biopsy (FNB), there is evidence in favor of the use of FNB without a recommendation of a specific type of needle15,16. The diagnostic accuracy with FNB has been reported between 83% and 100%17.
On the one hand, asymptomatic lesions with endoscopic and endosonographic characteristics compatible with varicose veins, ectopic pancreatic tissue, or lipomas do not require resection or additional follow-up. On the other hand, lesions diagnosed histologically as benign, such as leiomyomas, lipomas, ectopic pancreas, granular cell tumors, schwannomas, and glomus tumors, among others, do not require any follow-up or additional treatment since the risk of malignancy is very high low. Lesions with malignant confirmation require individualized treatment5. In the case of neuroendocrine tumors, type 1 tumors smaller than 10 mm could be candidates for annual endoscopic follow-up since the risk of malignancy is low18.
Managing GISTs smaller than 20 mm without suspicious endosonographic characteristics is controversial since the lesions’ follow-up and resection are accepted in the literature, given that they have a low risk of malignancy12,19. If follow-up is chosen, the method of choice is EUS, and even though there is no consensus on the best follow-up strategy, EUS has been recommended every 1-2 years for lesions between 10 and 20 mm and every 2-3 years for lesions smaller than 10 mm. Surgical resection of GISTs diagnosed in organs other than the stomach is advisable20.
When a histological diagnosis is not available, as is frequently the case in our environment, the symptoms, the location of the lesion, and the endosonographic characteristics must be taken into account. Lesions smaller than 20 mm in an asymptomatic gastric or esophageal area have a low risk of malignancy; therefore, their follow-up could be considered the best option. Although the follow-up interval and method are not standardized, esophagogastroduodenoscopy (EGD) and EUS have been suggested at 3-6 months to verify the stability of the lesion and, subsequently, EGD or EUS every 1-3 years21.
Given the retrospective nature of the study and that long-term follow-up of the lesions was not carried out, it is impossible to establish the incidence of malignancy or changes in lesions that would allow suggesting follow-up intervals in the study population.
Subepithelial lesions of the colon and rectum always require histological evaluation to establish the appropriate treatment since there is no evidence to support their follow-up as an adequate management strategy5.
Endoscopic resection aims to obliterate the lesions; the indication exists for lesions with the potential for malignancy that cause symptoms or patients who are candidates for bariatric surgery. The type of resection depends on the involved echolayer and its anatomical location.
Mucosal resection (MR) or endoscopic submucosal dissection (ESD) are proposed strategies for esophageal lesions. In the former, the use of bands has reported a technical success of up to 100% in lesions smaller than 20 mm22; for more extensive lesions up to 40 mm, endoscopic full-thickness resection (EFTR) could be considered23.
In gastric SEL with an indication for resection, the endoscopic route using MRI, ESD, or EFTR for lesions smaller than 40 mm or laparoscopic wedge resection is an option5.
Conclusion
SELs of the gastrointestinal tract originate in the muscularis mucosa, submucosa, or muscularis propria. They are most frequently located in the stomach, and their characterization usually requires endoscopic ultrasound and, in some cases, histopathological study.
The results are relevant since it is the first study in our environment that characterizes this type of lesion. However, given that the treatment described in the literature remains controversial (due to its low frequency, histological variety, and low malignant potential), it would be beneficial to conduct prospective studies in which long-term endosonographic follow-up is performed to establish the incidence of malignant transformation in our population and, thus, propose follow-up intervals or the need for additional interventions such as biopsies or resection of the lesions.











 texto em
texto em 



