Introduction
Worldwide, chronic liver diseases such as metabolic dysfunction-associated steatotic liver disease (MASLD), viral hepatitis, alcohol-related liver disease, and autoimmune liver disorders are significant causes of morbidity and mortality. Early identification of hepatic fibrosis at any stage can lead to treatments that significantly improve outcomes.
Hepatic fibrosis, a common structural alteration in many chronic liver diseases, is a key prognostic factor. Its extent correlates with the risk of developing cirrhosis, which ranks as one of the top twenty global causes of death. The mortality rate one year after diagnosis can range from 1% to 57%1.
The most common causes of chronic liver disease include metabolic hepatic steatosis, alcohol consumption, and viral hepatitis. Progressive liver injury may lead to severe complications such as hepatocellular carcinoma, cholangiocarcinoma, coagulopathy, hepatorenal and hepatopulmonary syndromes, and pulmonary portal hypertension. Risk factors encompass obesity, bariatric surgery, transfusion of blood products, use of intravenous drugs, risky sexual behaviors, congenital heart diseases, and a family history of autoimmune or liver diseases2.
Patients with liver damage at risk of developing cirrhosis may remain asymptomatic for years, only to present suddenly with complications such as ascites, anasarca, variceal bleeding, jaundice, and encephalopathy. This highlights the importance of early-stage identification through paraclinical tests in the diagnostic efforts for fibrosis2.
The clinical history, liver biochemistry profile, and ultrasound are fundamental in the diagnostic process of liver diseases. For staging fibrosis, which is crucial in decision-making for treatment and follow-up, non-invasive, easily accessible tests have been developed to avoid biopsy2.
Although liver biopsy remains the gold standard for diagnosis, assessing activity, fibrosis status, or therapeutic response, it is an invasive procedure with complication risks. It is reserved for patients when the diagnosis is uncertain and understanding the etiology could influence treatment decisions and prognosis. Biopsy has limitations ranging from technical difficulties to potential complications such as pain, infections, and bleeding3. Furthermore, the biopsy sample represents only a small portion of the liver and may not accurately reflect the extent of fibrosis due to its irregular distribution. Additionally, biopsy does not assess the dynamic process of fibrogenesis, where disease and treatment evolution may lead to regression or progression. These limitations have spurred the development of non-invasive serological and imaging methods for evaluating hepatic fibrosis4.
Multivariate tools such as the APRI Platelet Ratio Index and the FIB-4 Index for Liver Fibrosis are based on transaminase measurements and platelet count. However, FIB-4 and APRI index calculations, while useful in general practice, are not sufficient for determining the degree of fibrosis in early and intermediate stages. Consequently, guidelines recommend non-invasive tests like transient elastography, magnetic resonance elastography, or acoustic radiation force impulse imaging, among others5.
Hepatic fibrosis increases tissue stiffness and decreases elasticity, which can be assessed through liver elastography. Currently, it is the preferred non-invasive imaging technique for evaluating fibrosis, measuring liver stiffness as a quantitative biomarker of fibrosis magnitude in patients with chronic liver disease. It is even sufficient for suspecting liver disease in asymptomatic individuals6.
No diagnostic test is perfect, and a test might have high sensitivity but low specificity, or vice versa7. Therefore, these tests should be evaluated alongside clinical findings. Although studies have identified the diagnostic performance of these tests8, and some exist in Colombia, it is crucial to continue providing information about these tests over time to observe their behavior. The aim of this study was to describe the diagnostic performance of elastography in detecting hepatic fibrosis compared to biopsy in patients enrolled in a liver disease care center in Bogotá.
Methods
This study was designed as a retrospective cohort and cross-sectional investigation. It entailed the review of 3,066 patient records who were treated for various liver diseases between 2019 and 2022 at two private care centers in Bogotá. The diseases covered included hepatitis C, metabolic hepatic steatosis, alcohol-induced liver disease, and autoimmune hepatitis.
The inclusion criteria specified that participants should be patients over 18 years old who received care in private consultation at two gastroenterology centers, Gastromedicall and Unidad de Gastroenterología Integral in Bogotá, Colombia. These patients were diagnosed with conditions such as metabolic hepatic steatosis, autoimmune hepatitis, alcohol-induced liver disease, primary biliary cholangitis, sclerosing cholangitis, hepatitis B, non-cirrhotic portal hypertension, and congestive hepatopathy, all confirmed by liver biopsy results. Additionally, these patients must have undergone a FibroScan within six months before or after their biopsy date, with the report available.
The study excluded patients with incomplete APRI, FIB-4, biopsy, or FibroScan data. Specifically, five patients were excluded due to missing descriptions of fibrosis stage in their biopsy results.
Ultimately, 65 patients were included in the final analysis. The study recorded all necessary clinical and paraclinical variables required for the construction of indices, such as age, transaminase levels, and platelet count, treating sex as an independent variable. Data was systematically input into an MS Excel spreadsheet and analyzed using Stata 17.0 statistical software. Qualitative variables were summarized using frequencies and percentages, while quantitative variables were described using mean and standard deviation. The bivariate analysis employed parametric (Student’s t-test) or non-parametric (Wilcoxon rank-sum test) tests based on the normality of the distribution of quantitative variables, and the χ2 test for qualitative variables, with a p-value of < 0.05 considered statistically significant.
Further, APRI and FIB-4 indices were developed and their results compared with FibroScan outcomes against the biopsy-confirmed diagnosis of hepatic fibrosis. The study delineated the diagnostic accuracy measures, including sensitivity, specificity, and positive and negative likelihood ratios of the APRI, FIB-4, and FibroScan indices. Additionally, an area under the receiver operating characteristic curve (AUROC) analysis was performed, with a 95% confidence interval (CI). The study did not seek approval from an institutional ethics committee due to the private nature of the consultation centers involved. However, it adhered to the ethical guidelines outlined in the Declaration of Helsinki and the 1993 Resolution 8430 for human research, ensuring data confidentiality and protection through an anonymized MS Excel spreadsheet.
Results
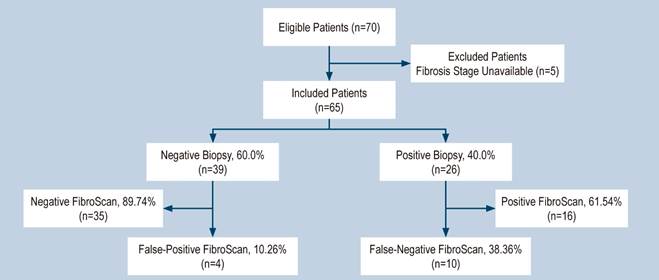
Of the 70 patients initially considered eligible, five were excluded due to the absence of fibrosis state reporting in their biopsy results, leading to a final cohort of 65 patients for analysis. Among these, 26 patients (40%) showed a biopsy positive for any degree of fibrosis. For the FibroScan evaluation, a threshold of 7 kPa was established, with readings greater than or equal to this value considered positive, and those below it deemed negative. Of the patients with biopsy-confirmed fibrosis, 16 (61.54%) had positive FibroScan results, while 10 (38.36%) were negative.
In total, 39 patients exhibited no fibrosis upon biopsy examination. Among this group, four (10.26%) had positive FibroScan findings, and 35 (89.74%) had negative outcomes. This resulted in four false positives (10.26%) and 10 false negatives (38.36%) when comparing FibroScan results to biopsy findings, as depicted in the participant flow diagram (Figure 1).
In terms of demographic characteristics, males constituted 24.62% of the study population, with an average age of 53.53 years, ranging from a minimum of 23 to a maximum of 77 years. In regards to fibrosis status, biopsy results classified eight patients as having advanced fibrosis stages F3 and F4, accounting for 12.8% of the cases. The remaining general characteristics are presented in Table 1.
Table 1 Basic Demographic and Clinical Characteristics of Participants
ALT: Alanine Aminotransferase; APRI: AST to Platelet Ratio Index; AST: Aspartate Aminotransferase; FIB-4: Index for Liver Fibrosis. Author’s own research.
Table 2 displays the characteristics adjusted for biopsy-confirmed fibrosis and the statistical outcomes for patients categorized by the presence or absence of fibrosis along with their p-values. For qualitative variables with a normal distribution, the difference was assessed using the mean difference, while for those with a non-normal distribution, the median rank test statistic was reported. The sole variable that exhibited a statistically significant difference between the two groups was the elastography measure. Upon performing a Spearman’s correlation test, it was found that there was neither a positive nor a negative correlation between the elastography values and the APRI and FIB-4 indices.
Table 2 Comparison According to the Biopsy Report Indicating Any Degree of Fibrosis*
| Characteristic | No Fibrosis χ2/Rank sum | With Fibrosis χ2/Rank sum | p-Value |
|---|---|---|---|
| Male | 10 (62.50) | 6 (37.50) | 0.781 |
| Age (%) | 52.74 (52.74) | 56.42 (56.42) | 0.271 |
| Platelets (103/u/L) | 258.121 | 267.578 | 0.715 |
| AST (U/L) | 1.222 | 923 | 0.383 |
| ALT (U/L) | 1.369.5 | 775.5 | 0.269 |
| FIB-4 | 1.215.5 | 929.5 | 0.338 |
| APRI | 1.285.5 | 859.5 | 0.984 |
| Elastography (kPa) | 874.5 | 1.270.5 | < 0.001 |
*Statistics were reported. ALT: Alanine Aminotransferase; APRI: AST to Platelet Ratio Index; AST: Aspartate Aminotransferase; FIB-4: Index for Liver Fibrosis. Author’s own research.
Table 3 encapsulates the diagnostic precision metrics for various elastography cutoff points ranging from >7 to 13.1 kPa, which are the thresholds recommended in literature for differentiating fibrosis. The highest diagnostic yield was noted at the cutoff point of ≥7 kPa, which accurately classified 78.46% of patients and demonstrated a positive likelihood ratio of 6.00.
Table 3 Sensitivity, Specificity, Positive Likelihood Ratio (LR+), Negative Likelihood Ratio (LR-), and Percent Correctly Classified According to Elastography Cutoff Points
| Cutoff Point | Sensitivity | Specificity | Correctly Classified | LR+ | LR- |
|---|---|---|---|---|---|
| > 7 | 61.54% | 89.74% | 78.46% | 6.00 | 0.42 |
| > 7.4 | 57.69% | 89.74% | 76.92% | 5.62 | 0.47 |
| > 7.5 | 53.85% | 89.74% | 75.38% | 5.25 | 0.51 |
| > 7.7 | 42.31% | 94.87% | 73.85% | 8.25 | 0.60 |
| > 7.9 | 38.46% | 94.87% | 72.31% | 7.50 | 0.64 |
| > 8.5 | 30.77% | 94.87% | 69.23% | 6.00 | 0.72 |
| > 8.8 | 26.92% | 94.87% | 67.69% | 5.25 | 0.77 |
| > 10 | 26.08% | 94.87% | 66.15% | 4.50 | 0.81 |
| > 10.6 | 19.23% | 94.87% | 64.62% | 3.75 | 0.85 |
| > 11.5 | 19.23% | 97.44% | 66.15% | 7.50 | 0.82 |
| > 12 | 19.23% | 100.00% | 67.69% | - | 0.80 |
| > 12.2 | 15.38% | 100.00% | 66.15% | - | 0.84 |
| > 13.1 | 11.54% | 100.00% | 64.62% | - | 0.88 |
LR: Likelihood Ratio. Author’s own research.
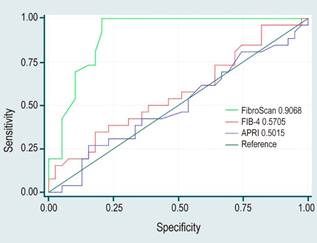
Figure 2 illustrates the comparative analysis between ROC curves for elastography and the FIB-4 and APRI indices correlated with biopsy outcomes. It is clearly shown that elastography outperforms with an AUC of 0.57 for the FIB-4 index and 0.50 for the APRI index.
Author’s own research.
Figure 2. Comparison of ROC Curves for Elastography and FIB-4 and APRI Indices Relative to Biopsy.
Table 4 collates the areas under the curve of the AUROC tests for the tested parameters along with their respective confidence intervals. Elastography emerges as the most effective measure.
Discussion
Our study assessed the diagnostic accuracy of FibroScan for detecting fibrosis by comparing its AUROC curve with biopsy diagnosis, revealing an AUROC of 0.90%. This result is considered excellent. Furthermore, the study reviewed the performance of the APRI and FIB-4 indices, which yielded AUROCs of 0.50% and 0.57%, respectively, indicative of moderate performance, thereby establishing elastography as a superior diagnostic tool.
Key performance metrics of a diagnostic test include sensitivity, or the proportion of truly diseased individuals as per the reference test who are correctly identified by the test under scrutiny; specificity, or the proportion of truly non-diseased individuals who are correctly identified by the test; positive predictive value, which is the proportion of individuals with a positive test result who are truly diseased; and negative predictive value, which is the proportion of individuals with a negative test result who are truly disease-free. These metrics should be interpreted against the backdrop of the disease’s prevalence, and when combined, they yield positive or negative likelihood ratios (LR). These LRs describe the probability of a test result occurring in diseased individuals compared to healthy ones9.
As of now, there are no studies reporting the prevalence of liver disease in Colombia. The recent literature on liver disease and the application of elastography stems from descriptive accounts from clinical settings10, which limit the interpretation of predictive values, hence these are not reported in our study.
A ROC curve, which is constructed to synthesize the sensitivity and specificity of a test, facilitates the graphical comparison across various cutoff points. It plots sensitivity against 1 - specificity across a range of values. The axis values range from 0 to 1, equating to 0%-100%. Tests with high diagnostic efficacy are characterized by a curve that approaches the top-left corner of the ROC plot. An increase in sensitivity, resulting from lowering the cutoff point, leads to a decrease in specificity. Conversely, tests with poor diagnostic efficacy are closer to the bottom-left or top-right corners of the curve9.
Our findings indicated an AUROC of 0.90 for FibroScan, denoting excellent performance and aligning with results by Mozés and colleagues, who reported an AUROC of 0.854. These outcomes are also supported by findings in Colombia from Prieto and colleagues in 2021, which involved 654 subjects at a referral center for liver disease. They underwent both elastography (SuperSonic) and biopsy, and the studies concluded that elastography is a useful tool for fibrosis assessment9,10.
The study revealed that the APRI and FIB-4 tests had modest performances. This contrasts with Zhong’s meta-analysis, which assessed APRI’s efficacy in diagnosing significant fibrosis, severe fibrosis, and cirrhosis, with AUROCs of 0.77, 0.80, and 0.83, respectively11. However, it is worth noting that these results pertained to patients with hepatitis C-related fibrosis, a data set not present in our current research.
With respect to FIB-4, commonly used for patients with hepatitis C virus and HIV, a 2022 meta-analysis of 37 primary studies by Mozés and colleagues in 2022 reported AUROC performances of 0.76 for predicting advanced fibrosis4. However, it must be highlighted that only eight of our study’s participants had advanced fibrosis, potentially accounting for the indices’ observed performance.
The meta-analyses cited earlier4,11 advocate for the sequential integration of biomarkers derived from biochemical profiles and elastography scores, especially with adjusted cutoffs for advanced fibrosis and cirrhosis. This recommendation takes into account the variable efficacy of such tests; hence, these indices must be interpreted with clinical circumspection within the broader clinical narrative when monitoring patients with hepatic fibrosis.
Correlation analyses failed to demonstrate any significant positive or negative correlation. However, given the reported efficacy in the literature, the employment of APRI and FIB-4 indices could enhance diagnostic acumen in scenarios where FibroScan is unavailable, particularly in regions with a high prevalence of liver pathology and associated risk factors.
Among the suite of non-invasive ultrasound-based imaging modalities, vibration-controlled transient elastography (VCTE) is globally recognized as the most thoroughly researched and validated. Esteemed organizations such as the American Gastroenterological Association (AGA), the European Association for the Study of Liver Diseases (EASL), and the Latin American Association for the Study of the Liver (ALEH) endorse its use for hepatitis B and C patients. Its diagnostic prowess in identifying cirrhosis and advanced hepatic fibrosis is well-documented, boasting a negative predictive value exceeding 90% in excluding cirrhosis3.
This technique hinges on the generation of a shear wave, with the speed of its hepatic traversal directly correlating with tissue rigidity-a faster wave signifies greater liver stiffness and thus, more advanced fibrosis. The VCTE (FibroScan, Echosens, Paris, France) induces a 50 Hz shear wave via a vibratory piston at the intercostal space, penetrating the liver 25 to 65 mm beneath the skin with the adult M probe or 25 to 75 mm with the XL probe. Kilopascals (kPa) serve as the unit of measurement, with a dynamic range from 2.5 to 75 kPa12.
For the test, patients lie supine with the right arm retracted behind the head to expose the intercostal space at the convergence of the mid-axillary line and a line extending from the xiphoid cartilage, or alternatively, at the first intercostal space below the upper margin of hepatic dullness. A physician, nurse, or a trained technician, with at least 100 supervised studies under their belt, can conduct the procedure. The study ensured adherence to all test quality criteria at the performing center.
Despite previous reports suggesting higher performance for APRI and FIB-4 scores, our study underscores FibroScan’s sustained excellence. Nevertheless, such findings warrant considered interpretation due to inherent study limitations, namely its retrospective nature, a relatively small cohort of 65 patients, and instances of incomplete fibrosis grading in biopsy reports. However, a notable strength of this work is the implementation of FibroScan at a highly experienced center, thus enriching the data from other larger-scale Colombian studies and providing a foundation for further research with enhanced statistical robustness for more substantial analyses.
Conclusions
FibroScan has proven to be a valuable instrument for the longitudinal assessment of liver disease, facilitating non-invasive fibrosis evaluation. It should, however, not be the solitary diagnostic modality but rather a component of a multifaceted diagnostic strategy that includes additional tests and clinical patient assessment. The FIB-4 and APRI tests yielded less robust performances than anticipated. This might be attributed to the limited patient sample size and the fact that the majority of patients were not at an advanced stage of fibrosis. Elastography demonstrated superior discrimination of hepatic fibrosis and seems to excel in detecting advanced stages of the condition. There is a call for prospective diagnostic studies with larger cohorts to increase statistical robustness.











 texto em
texto em