Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista MVZ Córdoba
versão impressa ISSN 0122-0268
Rev.MVZ Cordoba vol.19 no.2 Córdoba maio/ago. 2014
ORIGINAL
Follicular dynamics, corpus luteum growth and regression in multiparous buffalo cows and buffalo heifers
Dinámica folicular, crecimiento y regresión del cuerpo lúteo en búfalas multíparas y novillas
Alejandro Ojeda R,1* Esp, Ricardo Londoño O,1* MVZ, Carlos Gutierrez R,1 MV, Angela Gonella-Diaza,2 MSc.
1Fundacion Educativa Para La Equidad y el Desarrollo Rural. Carrera 1ª A No 11-130 Oficina 505 Torre 1. Chia, Cundinamarca, Colombia.
2Universidad Cooperativa de Colombia, Faculty of Veterinary Medicine and Animal Husbandry, Animal nutrition, toxicology and reproduction Research Group. Calle 30 No. 33 - 51. Bucaramanga, Colombia.
*Correspondence: alejojeda@hotmail.com
Received: May 2013; Accepted: December 2013.
ABSTRACT
Objective. Characterize the follicular dynamics and luteal growth and regression pattern of multiparous (MB) and heifer (BH) Murrah buffaloes in Colombian tropical conditions. Material and methods. Ten MB and ten BH were synchronized with a progesterone-releasing intravaginal device. No artificial insemination was performed during the estrous and daily ultrasound examinations were performed 15 days later to determine the number and diameter of the structures present in both ovaries in the subsequent natural estrous cycle. The Student’s T test was used to evaluate differences between MB and BH. All data are presented as mean ± standard deviation. Results. The length of the estrous cycle was 22.00±4.50 days for MB and 22.00±2.70 days for BH. Follicular growth occurs in one (n=1; 5.89%), two (n=14; 82.35%) or three waves (n=2; 11.76%). The first wave initiated the day after ovulation with the recruitment of 8.33±2.06 and 10.00±2.72 follicles in MB and BH, while the second wave started on day 11.00±2.00 and 10.50±2.82, presenting 8.37±2.26 and 8.00±1.51 follicles. The third wave began on day 16.21±3.10 showing 6.50±1.70 follicles, only BM had three waves. The maximum luteal diameter was 19.58±4.16 mm and 17.74±3.32 mm respectively. There were no significant differences between the groups for these variables. Conclusions. These results show that the follicular development in buffaloes occurs in waves, where two waves is the most common pattern, as previously reported by other authors.
Key words: Estrous cycle, ovarian follicles, tropical zones (Source: CAB).RESUMEN
Objetivo. Caracterizar la dinámica folicular y el patrón de crecimiento y regresión del cuerpo lúteo de búfalas multíparas (BM) y búfalas novillas (BN) de la raza Murrah en condiciones del trópico colombiano. Materiales y métodos. Diez BM y diez BN fueron sincronizadas con dispositivo intravaginal de liberación de progesterona. No se realizó la inseminación artificial al momento del celo y 15 días después se inició el seguimiento ultrasonográfico para determinar el número y diámetro de las estructuras presentes en ambos ovarios en el subsecuente ciclo estral natural. Las diferencias entre BM y BN se evaluaron con pruebas T. Los datos se presentan como media ± desviación estándar. Resultados. La duración del ciclo estral fue de 22.00±4.50 y 22.00±2.70 días en BM y BN. El crecimiento folicular ocurrió en una (n=1; 5.89%), dos (n=14; 82.35%) o tres (n=2; 11.76%) ondas. La primera onda inicio el día siguiente a la ovulación con el reclutamiento de 8.33±2.06 y 10.00±2.72 folículos en BM y BN, mientras que la segunda onda inicio el día 11.00±2.00 y 10.50±2.82 con 8.37±2.26 y 8.00±1.51 folículos. La tercera onda inicio el día 16.21±3.10 con 6.50±1.70 folículos, sólo BM presentaron tres ondas. El diámetro máximo luteal fue de 19.58±4.16 mm y 17.74±3.32 mm. No se observaron diferencias significativas entre los grupos para estas variables. Conclusiones. Los resultados muestran que el desarrollo folicular de las búfalas se dio en ondas, siendo dos ondas el patrón más común, similar a lo reportado por otros autores.
Palabras clave: Ciclo estral, folículos ováricos, zonas tropicales (Fuente: CAB).
INTRODUCTION
Buffaloes have become a species of economic importance in developing countries in tropical and subtropical regions. They show an efficient conversion, are resistant and require relatively low maintenance costs in the tropics, where the constant availability of food is not always ideal (1). In addition, buffalo milk is of high quality and better paid by dairy processing plants; whereas for meat production, weight gains similar to and in some cases higher than those reported for bovine animals in the same conditions have been observed (1,2). This means that buffaloes are a good source of animal protein, both milk and meat (2, 3).
In the past 30 years, the world’s buffalo population (172,263,305) has increased 34%, while during this same period the world cattle inventory has only grown 12%. In addition, since the 1970s the world production of buffalo milk has increased 200% (4). In Colombia, the presence of large expanses of floodable lands with poor soil drainage, high rain seasonality and low fertility, where bovine production fails to be efficient, buffaloes have become in a promising species since it adapts to these conditions and manages efficient production parameters (5). Therefore, the interest in Buffalo production has notably increased in the last 20 years, with an increase in the number of heads and the establishment of an Association of Buffalo Producers (Aso Bufalos de Colombia; 1).
The productivity of the buffalo system is largely limited by the reproductive efficiency of female buffaloes. When compared to female bovines, the reproductive efficiency of the buffalo is affected by characteristics such as: the late onset of puberty (6-8); the poor presentation of signs of estrous that hinder the use of artificial insemination techniques when the estrous is detected (6.8); the long intervals of postpartum anestrus in females that give birth not during the mating station, mainly due to their condition of seasonally polyestrous and short-day breeders (7-9); a lower population of preantral follicles, approximately 10 times less than that in cows, affecting superovulation programs (7,9,10) and a longer gestation (7). However, it is worth highlighting that buffaloes have greater longevity and hardiness than cows and some authors have found that, using genotypes suitable for a specific production, handling and environment system, reproductive parameters can be satisfactory and be deemed a viable production alternative (11,12).
The knowledge and understanding of the physiological phenomena that occur in the bovine ovary allowed the development of reproductive biotechnologies that potentiate the exploitation of female gametes and the reproductive efficiency of cows (13,14). Despite the importance of buffaloes in the global and regional economy, there are still very few studies that deepen in the ovarian physiology of female buffaloes and to date no other experiment has been found where it has been proposed to determine the follicular dynamics of female buffaloes under the conditions of the Colombian tropics; further, comparing the follicular dynamic among multiparous buffaloes (BM) and buffaloes heifers (BN). The objective of this study was to determine the follicular dynamics and the growth and regression pattern of the corpus luteum in Murrah BM and BN subjected to Colombian tropical conditions.
MATERIALS AND METHODS
Study site. The experimental phase took place in the facilities of the Centro Internacional de Formacion Agropecuaria (CIFA; north latitude 5°39.075” and west longitude 74°34.843”), located in the municipality of Puerto Salgar (Cundinamarca, Colombia). This municipality is located 195 km from Bogota D.C., 117 meters above sea level and has an average temperature of 27°C and a relative humidity between 75 and 80%.
Animals and grazing. Two experimental groups of 10 each were randomly selected from a population of 500 female buffalo cows. A group with virgin BN and another group with BM. The experimental units selection criteria was: at the time of starting the experiment all animals had regular estrous cycles and were clinically healthy with a body condition of 3 to 3.5 (scale of 1-5) and that all individuals belong to the Murrah breed. The BN cows had an age of 24.72±1.45 months and BM of 81.97±31.75 months with 2.7±0.8 deliveries. To ensure that all animals were under the same conditions they were kept grazing in a experimentation pasture, where they remained until the end of the study. The pasture had Brachiaria mutica established and mineralized salt and water ad libitum.
Synchronization protocol. A transrectal, palpation and ultrasound examination was carried out prior to the start of the synchronization protocol to evaluate the structures present in the ovaries and select the animals that would enter the experiment. A fixed-time artificial insemination protocol was used (Sincrogest, Ourofino, Saude Animal, Brazil) with a progesterone-releasing intravaginal device on day zero, together with the application of 2 mg of estradiol benzoate (Sincrodiol, Ourofino Saude Animal, Brazil). Later on day 8, the intravaginal device was removed and PGF2 alfa was applied (Sincrocio, Ourofino Saude Animal, Brazil). Finally, 1 mg of estradiol benzoate was applied on day 9 (Sincrodiol, Ourofino Saude Animal, Brazil). Fifty-two hours after removing the intravaginal device a new transrectal examination was conducted to determine the response to the treatment by the presence of a preovulatory follicle. No artificial insemination was performed after the detection of estrous. Due to the low intensity of estrous behavior of buffaloes (6.8), all animals with a follicle larger than 10 mm, the presence of uterine tone and the presence of mucous vaginal discharge were considered in estrus (15).
Ultrasound examinations. Fifteen days after observing synchronized estrus, a new estrus detection began and daily ultrasound examinations was performed with an ultrasound device attached to a linear transducer of 7.5 MHz (Mindray DP 2200 Vet). The day of estrus was taken as day zero of the estrous cycle and thereafter, daily ultrasound monitoring was performed in order to make ovarian maps and assessing the number and diameter of the antral follicles and the diameter of the CL (16). After viewing the image of the ovary in the monitor of the ultrasound device, each ovary was scanned at various levels to ensure and capture the greatest amount of structures and determine their correct size, the data from the measurement of each structure were stored for further analysis. These ultrasound exam were carried out until a second estrus was evident and a second ovulation occurred in each one of the buffaloes.
A follicular wave was considered when finding the growth of a dominant follicle and its cohort and the day of emergence of the follicular wave was defined as the day when the first follicle reached a diameter of 4 mm (17). The deviation of the dominant follicle was determined as the moment in which the diameter of the largest follicle was at least two standard deviations above the mean of the follicles of its cohort (18). The dominant follicle was defined as that which grew at least 10 mm and its diameter was greater than that of the other members of its cohort (17,19).
Data processing and statistical analysis. The information from the ovarian map was entered in Excel 2010 spreadsheets, in order to organize the information corresponding to the following variables: length of the estrous cycle, number of follicular waves during the estrous cycle, day of emergence and number of follicles recruited in each wave, day and diameter of the deviation of the dominant follicle (18), maximum diameter reached by the preovulatory follicle and corpus luteum, day and diameter in which the luteal regression began (determined as the second consecutive day in which the diameter of the structure reduces; 18). Each of the variables analyzed was subject to descriptive statistics and t-Student tests were performed to compare the data from BM and BN and those from different follicular growth waves. A significant difference was considered when p<0.05. The data were analyzed in Excel (Microsoft Office) and SAS 9.0 (The SAS Institute Inc). All data were presented as mean ± standard deviation.
RESULTS
Response to the synchronization protocol. Of the total number of females that initiated the protocol (10 BM and 10 BN) a preovulatory follicle was found in 85% (9 BM and 8 BN). The daily ultrasound exam began after 15 days.
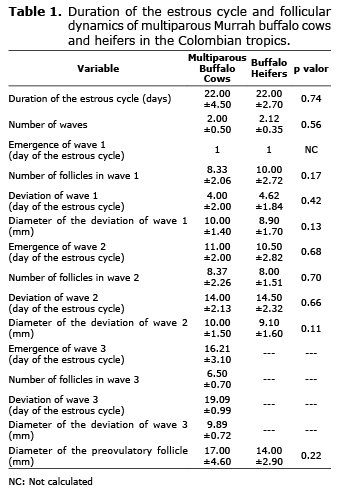
Length of the estrous cycle and follicular dynamics. No significant differences were found for the length of the estrus cycle between BM and BN (p>0.05), it being 22.00±4.50 and 22.00±2.70 days, respectively. The follicular growth pattern occurred in one (n=1; 5.89%), two (n=14; 82.35%) or three waves (n=2; 11.76%; (Table 1).
For all the experimental units, the emergence of the first follicular growth wave occurred the day after ovulation, and an average of 8.33±2.06 and 10.00±2.72 follicles for BM and BN, respectively were observed on the ultrasound. Follicular deviation in the first wave took place at 4.00±2.00 days for BM and 4.62±1.84 days for BN, with a diameter of the dominant follicle of 10.00±1.4 mm and 8.9±1.7 mm, respectively. There were no significant differences between the groups for these variables (Table 1). The second wave of follicular growth began at 11.00±2.00 days for BM and 10.50±2.82 days for BN. An average of 8.37±2.26 and 8.00±1.51 follicular structures were ultrasonographically observed for BM and BN, respectively. On day 14.00±2.13 and 14.50±2.32 the deviation of the dominant follicle started in BM and BV, finding a diameter of 10.00±1.50 and 9.10±1.60 mm, respectively. The third wave of follicular growth started on day 16.21±3.10 with 6.50±1.70 follicles recruited. Only two multiparous BM showed three waves of follicular growth. The maximum diameter reached by the preovulatory follicle was 17.00±4.60 mm for BM and 14.00±2.90 mm for BN. No significant differences were found between the groups for these variables (Table 1).
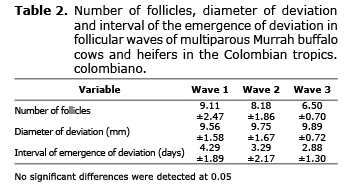
When comparing the waves of follicular growth among themselves, regardless of the group, it was found that the interval since the emergence of the wave to the deviation of the dominant follicle was 4.29±1.89, 3.29±2.17 and 2.88±1.30 days for the first, second and third wave, respectively; likewise, the number of follicles was 9.11±2.47, 8.18±1.86 and 6.50±0.70. Finally, the diameter of the dominant follicle in the deviation was 9.56±1.58 mm for wave one, 9.75±1.67 mm for wave two and 9.89±0.72 mm for wave three. There was no statistical difference between the waves of follicular growth (Table 2).
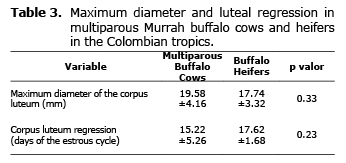
Corpus luteum diameter and growth. Through daily ultrasound exams it was to observe that the maximum luleal diameter was 19.58±4.16 mm for BM and 17.74±3.32 mm for BN. Luteal regression took place at 15.22±5.26 and 17.62±1.68 days of the estrous cycle for BM and BN, respectively. No significant differences were observed between the groups for these variables (Table 3).
DISCUSSION
The duration of the estrous cycle in this study showed no significant differences when comparing BN to BM nor when comparing the number of waves of follicular growth. Similar results have been obtained by other authors such as Presicce et al (16). They worked with Mediterranean BM and BN and found that for animals with two waves of follicular growth, the duration of the estrous cycle ranged from 20 to 26 days. However, they found a higher proportion of animals with a single wave of follicular growth, which had a shorter cycle duration (8 to 12 days). Baruselli et al (20), working with Murrah animals, also found 3.33, 66.66 and 33.33% of animals with one, two and three waves respectively. In this study, the duration of the estrous cycle of animals with one wave was 13 days. Awasthi et al (17) found a high proportion of animals with one wave (62.5%) in which the estrous cycle lasted 20.8±0.58 days. It is possible that the absence of significant differences in the duration of the estrous cycle in this study is due to the low proportion of animals with a single wave (n=1) and to the duration of their cycle that was 18 days. The variables that influence the number of waves during the estrous cycle have not been accurately determined yet; however, it has been proposed that the breed, physiological and nutritional conditions, environmental conditions, among others, may affect this variable (19,21,22).
The results obtained in this and other studies are consistent with the fact that the first follicular wave emerges on day one of the estrous cycle (17.23). As to the emergence of the second wave, it occurred at 11±2.00 days for BM and 10.5±2.82 days for BN. These data are similar to those found by other authors who have determined the emergence of the second wave around the day 10 for animals with two waves and around day 8 for animals with three waves (20,23,24). This study evidenced that the number of follicles recruited in the first wave was 8.33±2.06 and 10±2.72 and in the second wave 8.37±2.26 and 8±1.51, for BM and BN respectively, while for the third wave 6.5±0.7 follicles were recruited, which showed no statistical differences when making a comparison between groups or waves. Similar results were found by Baruselli et al (20), who, working with Murrah BM, concluded that the number of follicles recruited in the first wave was 7.72±4.64 and 7.50±2.75 for animals with estrous cycles of two and three waves respectively, where there are no significant differences.
These results contrast with the experiment carried out in Egypt by Barkawi et al (23), who found a significant difference (p>0.05) in the number of follicles recruited in the first wave when comparing animals with two (6.3±0.3) and three waves (7.8±0.4). All the previous results contrast when compared with the number of follicles recruited in a wave of follicular growth in cows, where it has been estimated that: “the emergence of the follicular wave is characterized by the sudden growth of 8-41 small follicles” (25). It is likely that the marked difference between these species is a consequence of the lower number of primordial follicles of buffalo females with respect to bovine females (7,9,10).
The results obtained for the deviation of the dominant follicle showed that it occurs approximately four days after the emergence of the wave and when it has a diameter of 8-10 mm. Again, no differences were found when comparing between groups or waves. Sartori et al (26), working with Holstein cows, determined that the dominant follicle deviated when it reached 9.80±0.30 mm in lactating cows and 8.30±0.20 mm in heifers. In another study, Sartori et al (27) found that the diameter at which the dominant follicle deviate was 9.10±0.40 mm in Holstein cows. Working with BN, Gimenes et al (28) found that the dominant follicle is diverted when it reached 7.20±0.20 mm. This result is lower than that found in this study and could be explained by the method used to determine follicular deviation in both cases. In the present study, it was determined that follicular deviation was the moment when the diameter of the largest follicle was at least two standard deviations above the average of the follicles of its cohort (18), while, Gimenes et al (28) defined the beginning of the deviation as “the end of the common growth phase, when differences between the diameters of the two largest follicles were detected.”
Gimenes et al (28,29) conducted experiments to characterize follicular deviation in bovine heifers and BN, respectively. They determined that the deviation in heifers occurs between 1.5 - 4 days after ovulation, when the follicle has a size of 5 to 7 mm (29), while in BN it was determined that the deviation occurred 2.6±0.2 days post-ovulation at a size of 7.2±0.2 (28).
The maximum diameter reached by the preovulatory follicle in the animals under study was 17±4.6 and 14±2.9 mm for BM and BN. Other studies report similar diameters when working with buffaloes and larger when working with cows. Baruselli et al (20) found that the preovulatory diameter of BM was 1.57, 1.55±0.16 and 1.34±0.13 cm for animals with one, two or three waves of follicular growth, respectively. Similarly, Awasthi et al (17) working with Mehsana buffaloes, found that the maximum diameter of the preovulatory follicle was 12.94±0.59 and 16.03±3.30 for animals with one or two waves. However, in a study comparing the pattern of follicular growth between Mediterranean BM and BN carried out in Italy by Presicce et al (16), significant differences were found (p>0.05) between the maximum diameter of the ovulatory follicle with 13.8±0.6 and 11.0±0.7. It is likely that the marked differences in the conditions under which the experiment of Presicce et al (16) was carried out and this study, are responsible for this discrepancy between results.
When assessing the characteristics of the CL, it was determined that the maximum diameter was 19.58±4.16 mm for BM and 17.74±3.32 mm for BN. Despite the apparent numerical difference in these results, no significant differences were found. In the study of Barkawi et al (23), the maximum diameter for the CL was found to be 15.00±0.40 for BN in Egypt, while Di Francesco et al (30) working with Mediterranean buffaloes during the mating season, found that the CL at day 10 post insemination reached 18.6±0.9 mm and 20.2±0.6 mm in empty and pregnant buffaloes, respectively. They found no statistical differences when comparing the growth pattern of the CL in animals in the reproductive season and in the transition period to the next stadium. However, differences were evidenced in the synthesis of P4, where animals in the reproductive season showed higher concentrations of this hormone. In this study the regression of the CL was found from day 15.22±5.26 for the BM group and 17.62±1.68 for the BN group, data consistent with those previously reported by other authors. Satheshkumar et al (31) found that in Murrah BM the luteal regression began on day 16.20±50.76; while Barkawi et al (23) determined that the half-life of the CL was 17.1±0.8 days in buffaloes with normal estrous cycles, while in females with follicular cysts and persistent CL, this half-life increased to 24.80±4.30 and 28.30±6.10 days, respectively. In conclusion, the follicular dynamics and growth pattern and regression of the CL in BM and BN under Colombian tropical conditions are similar to what has been previously reported by other authors. However, it is necessary to conduct new studies with a larger number of animals involved.
Acknowledgements
To the Centro Internacional de Formación Agropecuaria (CIFA) and its entire staff for their invaluable cooperation in the preparation of this study.
REFERENCES
1. Cervantes E, Espitia A, Prieto E. Viabilidad de los sistemas bufalinos en Colombia. Rev Colombiana Cienc Anim 2010. 2(1):215-24. [ Links ]
2. De Rensis F, Lopez-Gatius F. Protocols for synchronizing estrus and ovulation in buffalo (Bubalus bubalis): A review. Theriogenology 2007; 67(2):209-16. [ Links ]
3. Singh J, Nanda AS, Adams GP. The reproductive pattern and efficiency of female buffaloes. Anim Reprod Sci 2000; 60:593-604. [ Links ]
4. Almaguer-Pérez Y. El búfalo, una opción de la ganadería. REDVET Revista Electronica Veterinaria. 2007; 8(8):1-23. [ Links ]
5. Angulo RR, LF. Berdugo, JA. Características de calidad de las canales bufalinas y vacunas comercializadas en Medellín, Colombia. [en linea]. Livestock Research for Rural Development 2005; 17(9): Artículo 103. URL Disponible en: http://www.lrrd.org/lrrd17/9/angu17103.htm. [ Links ]
6. Drost M. Bubaline versus bovine reproduction. Theriogenology 2007; 68(3):447-9. [ Links ]
7. Mondadori RG, Luque MCA, Santin TR, Bao SN. Ultrastructural and morphometric characterization of buffalo (Bubalus bubalis) ovarian preantral follicles. Anim Reprod Sci 2007; 97(3-4):323-33. [ Links ]
8. Perera B. Reproductive cycles of buffalo. Anim Reprod Sci 2011; 124(3-4):194-9. [ Links ]
9. Mondadori RG, Santin TR, Fidelis AAG, Porfirio EP, Bao SN. Buffalo (Bubalus bubalis) Pre-antral follicle population and ultrastructural characterization of antral follicle oocyte. Reprod Domest Anim 2010; 45(1):33-7. [ Links ]
10. Kumar A, Solanki VS, Jindal SK, Tripathi VN, Jain GC. Oocyte retrieval and histological studies of follicular population in buffalo ovaries. Anim Reprod Sci 1997; 47(3):189-95. [ Links ]
11. Perera B. A review of experiences with oestrous synchronization in buffaloes in Sri Lanka. Buffalo J1987. p. 105-14. [ Links ]
12. Usmani RH, Dailey RA, Inskeep EK. Effects of limited suckling and varying prepartum nutrition on postpartum reproductive traits of milked buffalos. J Dairy Sci 1990;73(6):1564-70. [ Links ]
13. Azawi OI, Ali AJ, Lazim EH. Pathological and anatomical abnormalities affecting buffalo cows reproductive tracts in Mosul. Iraqi J Vet Sci 2008; 22(2): 59-67. [ Links ]
14. Campanile G, Baruselli PS, Neglia G, Vecchio D, Gasparrini B, Gimenes LU, et al. Ovarian function in the buffalo and implications for embryo development and assisted reproduction. Anim Reprod Sci 2010; 121(1-2):1-11. [ Links ]
15. Neglia G, Natale A, Esposito G, Salzillo F, Adinolfi L, Zicarelli L, et al. Follicular dynamics in synchronized Italian Mediterranean buffalo cows. Italian Ital J Anim Sci 2007;6:611-4. [ Links ]
16. Presicce GA, Senatore EM, Bella A, De Santis G, Barile VL, De Mauro GJ, et al. Ovarian follicular dynamics and hormonal profiles in heifer and mixed-parity Mediterranean Italian buffaloes (Bubalus bubalis) following an estrus synchronization protocol. Theriogenology 2004; 61(7-8):1343-55. [ Links ]
17. Awasthi MK, Khare A, Kavani FS, Siddiquee GM, Panchal MT, Shah RR. Is one-wave follicular growth during the estrous cycle a usual phenomenon in water buffaloes (Bubalus bubalis)? Anim Reprod Sci 2006; 92(3-4):241-53. [ Links ]
18. Taylor C, Rajamahendran R. Follicular dynamics, corpus-luteum growth and regression in lactating dairy-cattle. Canadian Ital J Anim Sci 1991;71(1):61-8. [ Links ]
19. Ginther OJ, Kastelic JP, Knopf L. Composition and characteristics of follicular waves during the bovine ESTROUS-CYCLE. Anim Reprod Sci 1989; 20(3):187-200. [ Links ]
20. Baruselli PS, Mucciolo RG, Visintin JA, Viana WG, Arruda RP, Madureira EH, et al. Ovarian follicular dynamics during the estrous cycle in buffalo (Bubalus bubalis). Theriogenology 1997; 47(8):1531-47. [ Links ]
21. Lucy MC, Savio JD, Badinga L, Delasota RL, Thatcher WW. Factors that affect ovarian follicular dynamics in cattle. Ital J Anim Sci 1992;70(11):3615-26. [ Links ]
22. Celik HA, Aydin I, Sendag S, Dinc DA. Number of follicular waves and their effect on pregnancy rate in the cow. Reprod Domest Anim 2005; 40(2):87-92. [ Links ]
23. Barkawi AH, Hafez YM, Ibrahim SA, Ashour G, El-Asheeri AK, Ghanem N. Characteristics of ovarian follicular dynamics throughout the estrous cycle of Egyptian buffaloes. Anim Reprod Sci 2009; 110(3-4):326-34. [ Links ]
24. Manik RS, Palta P, Singla SK, Sharma V. Folliculogenesis in buffalo (Bubalus bubalis): a review. Reprod Fertil Dev 2002;14(5):315-25. [ Links ]
25. Adams GP, Jaiswal R, Singh J, Malhi P. Progress in understanding ovarian follicular dynamics in cattle. Theriogenology 2008; 69(1):72-80. [ Links ]
26. Sartori R, Haughian JM, Shaver RD, Rosa GJM, Wiltbank MC. Comparison of ovarian function and circulating steroids in estrous cycles of Holstein heifers and lactating cows. J Dairy Sci 2004; 87(4):905-20. [ Links ]
27. Sartori R, Fricke PM, Ferreira JCP, Ginther OJ, Wiltbank MC. Follicular deviation and acquisition of ovulatory capacity in bovine follicles. Biol Reprod 2001; 65(5):1403-9. [ Links ]
28. Gimenes LU, Carvalho NAT, Sa Filho MF, Vannucci FS, Torres-Junior JRS, Ayres H, et al. Ultrasonographic and endocrine aspects of follicle deviation, and acquisition of ovulatory capacity in buffalo (Bubalus bubalis) heifers. Anim Reprod Sci 2011; 123(3-4):175-9. [ Links ]
29. Gimenes LU, Sa MF, Carvalho NAT, Torres JRS, Souza AH, Madureira EH, et al. Follicle deviation and ovulatory capacity in Bos indicus heifers. Theriogenology 2008; 69(7):852-8. [ Links ]
30. Di Francesco S, Neglia G, Vecchio D, Rossi P, Russo M, Zicarelli L, et al. Influence of season on corpus luteum structure and function and AI outcome in the Italian Mediterranean buffalo (Bubalus bubalis). Theriogenology 2012; 78(8):1839-45. [ Links ]
31. Satheshkumar S, Palanisamy A, Rangasamy S, Kathiresan D, Kumanan K. Comparative analysis of follicular and luteal dynamics in oestrous cycles of buffaloes and crossbred cattle. Buffalo Bulletin 2011; 30(2):148-56. [ Links ]