Introduction
Reduction of crude protein (CP) and metabolizableenergy (ME) inthe diet isacommon strategy to decrease metabolic disorders, such as ascites (Sahraei, 2012). However, reduction of these nutrients is also related with reduced performance (Jackson et al., 1982). Research studies conducted on the optimum level of CP and ME in broiler chicken diets regarding skeletal development and integrity are not conclusive, as different concentrations of CP and ME in the diet could modify the deposition rate of collagen and other important structural proteins (Rath et al., 2000). Bruno et al. (2007) showed that broilers fed 18.5% CP and 3,200 kcal ME kg-1 of diet had lower femur width and humerus weight compared to control broilers (22% CP and 2,950 kcal ME kg-1 of diet). Venäläinen et al. (2006) found that feeding broilers with low concentrations of ME (2,632 or 2,831 kcal kg-1) significantly decreased live weight but did not affect walking ability or tibia breaking strength. On the other hand, Banerjee et al. (2013) did not find differences in body weight gain and final live weight of chickens fed 16, 18, 20 or 21% CP for 13 weeks. Scarce studies related to gastrocnemius tendon and tibia breaking strength, gait score (indicator of walking ability) and valgus/varus angulation in broilers fed varying levels of CP and ME have been reported. Low dietary CP concentration may be used as an alternative to minimize production costs (Gardzielewska et al., 2005), reduce environmental pollution due to lower nitrogen excretion (Belloir et al., 2017), and reduce the risk of metabolic disorders (Sahraei, 2012). We hypothesize that chickens fed levels close to 16% CP and 3,000 kcal ME kg-1 of diet will have similar gastrocnemius tendon and tibia breaking strength, gait score, valgus/varus angulation, mortality and performance than those fed 21% CP and 3,000-3,025 kcal ME kg-1 of diet (common commercial starter diet levels). Therefore, the aim of this study was to evaluate the effect of varying dietary CP and ME levels on leg abnormalities and performance of starting broiler chickens.
Materials and Methods
Ethical Considerations
The experimental procedures followed the standards for ethics, biosafety, and animal wellbeing of the Official Mexican Standard (NOM-062-ZOO-1999; 2001) for the use of animals in research. Euthanasia procedures were performed according to the Mexican Official Norm (NOM-033-SAG/ZOO-2014, 2015).
Poultry facilities and management
Two experiments were carried out at the poultry research unit of Colegio de Postgraduados, Campus Montecillo, Estado de México, MX (98º 48' 27'' W and 19º 48' 23" N), located at an altitude of 2,278 m above sea level. In Experiment 1, the birds were housed in 3 × 1 m pens and in Experiment 2, birds were housed in 1.5 x 1 m pens. In both experiments, new wood-shavings were used as the bedding material. The lighting program was 23 h light, 1 h dark throughout the experimental period (8 to 51 d of age for Exp. 1 and 6 to 46 d of age for Exp. 2). At the beginning of the experiments, the house temperature was maintained at 32°C and then it was reduced (2°C per week) to 24°C. The chickens were housed under a curtain-ventilation system. Bell drinkers and hanging cylindrical feeders (111 and 121 cm in circumference, respectively) were used; water and feed were offered ad libitum.
Experimental design
At the time of arrival to the experimental facilities, the chickens were allocated in a common pen and offered a basal pre-starter corn-soy diet. After 8 or 6 days (Exp. 1 and 2, respectively), the chickens were weighed and average body weight (BW) was calculated. This average BW was then used to create groups of chicks with similar (p>0.05) starting weight and distributed in a completely randomized design. In Experiment 1, ninety Ross-308 chicks (8-d-old; straight-run; male and female) were randomly assigned to three treatments (3 replicates per treatment, 10 birds per replicate): 1) 13/2,900; 13% crude protein (CP) and 2,900 kcal metabolizable energy (ME) kg-1 of diet, 2) 17/3,000; 17% CP and 3,000 kcal ME kg-1 of diet and 3) 21/3,025 or control; 21% CP and 3,025 kcal ME kg-1 of diet. In Experiment 2, one hundred and ninety-two male Ross-308 chicks (6-d-old) were randomly distributed into 2 treatments (8 replicates per treatment, 12 birds per replicate): 1) 16/3,000; 16% CP and 3,000 kcal ME kg-1 of diet and 2) 21/3,000 or control; 21% CP and 3,000 kcal ME kg-1 of diet.
Dietary treatments
All diets were formulated to meet or exceed the NRC (1994) requirements for broilers, except for CP and ME which varied based on experimental treatment. In both trials, synthetic amino acids were added to treatments 13/2,900, 17/3,000 and 16/3,000 to balance their concentration to meet NRC (1994) nutrient recommendations.
Experiment 1. The starter phase lasted 21 d (from 8 to 29 d of age) during which the 3 experimental diets with different concentrations of CP and ME were offered. The finisher period followed at the end of the starter phase (30 to 51 d of age) where the chickens were fed a common diet containing 19% CP and 3,100 kcal ME kg-1 (Table 1).
Table 1 Composition of experimental diets (Experiment 1)1.
| Starter (8-29 d of age) | Finisher (30-51 d of age) | |||
|---|---|---|---|---|
| Ingredients (%) | 13/2,900 | 17/3,000 | 21/3,025; Control | Basal |
| Yellow Corn | 63.00 | 62.00 | 54.00 | 61.11 |
| Dehulled Soybean meal | 15.00 | 24.00 | 37.00 | 31.38 |
| Soybean oil | 4.85 | 3.84 | 3.31 | 2.98 |
| Calcium carbonate | 1.60 | 1.73 | 1.69 | 1.34 |
| Dicalcium phosphate | 1.58 | 1.75 | 1.67 | 1.57 |
| L-Arginine | 0.71 | 0.38 | 0.08 | 0.00 |
| L-Lysine-HCI | 1.75 | 1.22 | 0.51 | 0.11 |
| L-Threonine | 0.53 | 0.37 | 0.16 | 0.09 |
| DL-Methionine | 0.53 | 0.49 | 0.31 | 0.09 |
| L-Tryptophan | 0.15 | 0.09 | 0.02 | 0.00 |
| 2Mineral premix | 0.15 | 0.15 | 0.15 | 0.20 |
| 3Vitamin premix | 0.15 | 0.15 | 0.15 | 0.20 |
| Choline chloride (75%) | 0.21 | 0.21 | 0.21 | 0.21 |
| Coccidiostat¥ | 0.05 | 0.05 | 0.05 | 0.05 |
| Xanthophylls | 0.00 | 0.00 | 0.00 | 0.37 |
| Salt (NaCl) | 0.30 | 0.30 | 0.30 | 0.30 |
| Sand | 9.00 | 2.95 | 0.00 | 0.00 |
1In the starting period, the calculated diet composition was as follows: CP content: 13, 17 and 21%. Energy: 2,900, 3,000 and 3,025 kcal of ME kg-1, respectively. The finishing diet contained: 19% CP and 3,100 kcal of ME kg-1. Synthetic amino acids added to meet or exceed NRC requirements (1994).
2Mineral premix (per kg of premix): Zn 100 g; Fe 50 g; Cu 10 g; Mn 100 g; I 1 g.
3Vitamin premix (per kg of premix): vit. A, 24,000,000 IU; vit. D3 8,000,000 IU; pyridoxine, 8 g; thiamine, 6 g; riboflavin, 16 g; niacin ,100 g; cyanocobalamin, 60 mg; menadione, 10 g; calcium pantothenate, 28 g; folic acid, 3 g.
¥Coccidiostat, Olistimax®, PiSA Agropecuaria, Guadalajara, Jalisco, Mexico.
Experiment 2. The starter phase lasted 21 d (from 6 to 27 d of age) during which the 2 experimental diets with different concentration of CP and ME were offered. Similar to Exp.1, the chickens were offered a common basal finisher diet containing 19% CP and 3,050 kcal ME kg -1 from 28 to 46 d of age (Table 2).
Table 2 Composition of experimental diets (Experiment 2)1.
| Starter (6-27 d of age) | Finisher (28-46 d of age) | ||
|---|---|---|---|
| Ingredients (%) | 21/3,000; Control | 16/3,000 | Basal |
| Yellow Corn | 58.03 | 73.04 | 63.93 |
| Dehulled Soybean meal | 35.82 | 19.97 | 30.29 |
| Soybean oil | 1.92 | 0.00 | 1.74 |
| Calcium carbonate | 1.35 | 3.16 | 1.42 |
| Dicalcium phosphate | 1.92 | 2.02 | 1.36 |
| L-Lysine-HCI | 0.17 | 0.68 | 0.16 |
| DL-Methionine | 0.18 | 0.24 | 0.12 |
| L-Threonine | 0.02 | 0.25 | 0.00 |
| L-Tryptophan | 0.00 | 0.06 | 0.00 |
| Salt (NaCl) | 0.35 | 0.35 | 0.35 |
| 2Vitamin premix | 0.03 | 0.03 | 0.03 |
| 3Mineral premix | 0.06 | 0.06 | 0.06 |
| Xanthophylls | 0.00 | 0.00 | 0.40 |
| Choline chloride (75%) | 0.10 | 0.10 | 0.10 |
| Coccidiostat¥ | 0.05 | 0.05 | 0.05 |
1In the starting period, the calculated diet composition was as follows: CP content: 13, 17 and 21%. Energy: 2,900, 3,000 and 3,025 kcal of ME kg-1, respectively. The finishing diet contained: 19% CP and 3,100 kcal of ME kg-1. Synthetic amino acids added to meet or exceed NRC requirements (1994).
2Mineral premix (per kg of premix): Zn 100 g; Fe 50 g; Cu 10 g; Mn 100 g; I 1 g.
3Vitamin premix (per kg of premix): vit. A, 24,000,000 IU; vit. D3 8,000,000 IU; pyridoxine, 8 g; thiamine, 6 g; riboflavin, 16 g; niacin ,100 g; cyanocobalamin, 60 mg; menadione, 10 g; calcium pantothenate, 28 g; folic acid, 3 g.
¥Coccidiostat, Olistimax®, PiSA Agropecuaria, Guadalajara, Jalisco, Mexico.
Leg abnormality assessment
Gastrocnemius tendon (GTeBS) and tibia breaking strength (TiBS). A total of 15 (5 birds per replicate pen) and 16 (2 birds per replicate pen) chickens per treatment were euthanized by cervical dislocation at 51 and 46 d of age in Experiments 1 and 2, respectively. In Experiment 1, the tibia and gastrocnemius tendons were carefully dissected from both legs. In Experiment 2, only the right tibia and right gastrocnemius tendon were collected. Tibia and tendons were subjected to breaking strength using a Vernier Force Plate (Vernier Software & Technology, Beaverton, OR, USA). The tibia was placed on an adjustable three-point loading system with a distance bone support of 60 mm, and a vertical force was applied at midpoint by a 2.54 cm fulcrum. The proximal and distal portion of each tendon were fastened with sandpaper and attached to the mounting brackets of the Vernier Force Plate. Breaking strength was recorded in newtons (N).
Gait score (GS) and valgus/varus angulation (VAng). In Experiment 1, gait score (GS; indicator of walking ability) and valgus/varus angulation (VAng) were measured at 51 d of age in 15 randomly selected chickens per treatment (5 birds per replicate). In Experiment 2, 40 broilers per treatment (5 birds per replicate) were randomly selected at d 46 of age. Gait score was evaluated according to the methodology described by Kestin et al. (1992) and later modified by Garner et al. (2002). Six score categories (0 to 5) were used (0: broilers with a fluid locomotion; 5: completely lame broilers that cannot walk or stand). Two observers viewed and scored each bird individually. If the evaluators did not reach consensus, they had to evaluate another bird. Valgus/varus angulation (VAng) was evaluated according to the methodology described by Leterrier and Nys (1992). Depending on the angle size of tibia-metatarsus, four scores (0 to 3) were defined (0: broilers that show no tibia- metatarsus angulation evident to the naked eye -tibia-metatarsus angle less than 10°; 3: broilers with severe angulation -angle greater than 45°).
Performance evaluation
In both experiments, body weight (BW), body weight gain (BWG), feed intake (FI) and feed conversion ratio (FCR) were recorded weekly. Mortality weight gain was recorded and used to correct FCR.
Statistical analysis
The statistical model used to analyze the data was: Yij=µ+Ti+Eij; where: Yij is the response variable in treatment i, replicate j; µ is the general mean; Ti is the effect of treatment I; and Eij is the random error. The TiBS and GTeBS variables were analyzed using the MIXED procedure of SAS, version 9.4 (SAS Institute, Cary, N.C., USA). The GS and VAng variables were analyzed using the GLIMMIX procedure 16. For TiBS, GTeBS, GS and VAng the experimental unit was each individual chicken. The BW, BWG, FI and FCR were analyzed as repeated measures using the MIXED procedure, where the experimental unit was each pen replicate. Statistical differences were considered at p<0.05.
Results
Leg abnormality assessment
Gastrocnemius tendon (GTeBS) and tibia breaking strength (TiBS). In Experiment 1, the broilers in the 17/3,000 and control treatments showed similar (p>0.05) GTeBS and TiBS. The birds in the 13/2,900 treatment had the lowest (p<0.05) GTeBS and TiBS values with respect to the broilers in the control and 17/3,000 treatments. In Experiment 2, the chickens from the 16/3,000 treatment had lower (p<0.05) GTeBS than the broilers from the control treatment, while TiBS was not different (p>0.05) between treatments (Table 3).
Table 3 Gastrocnemius tendon (GTeBS) and tibia (TiBS) breaking strength of broilers fed different crude protein (% CP) and metabolizable energy (ME) levels (Exp. 1 and 2).
| Experiment 1 -51 d of age (N)ǂ- | |||
|---|---|---|---|
| *Treatments | GTeBS | TiBS | |
| 13/2,900 | 121b | 250b | |
| 17/3,000 | 202a | 338a | |
| 21/3,025; CON | 224a | ||
| SEM¥ | 9 | 332a | |
| p-value | 16 | ||
| <0.01 | <0.01 | ||
| Experiment 2 -46 d of age (N)- | |||
| **Treatments | GTeBS | TiBS | |
| 16/3,000 | 116b | 331 | |
| 21/3,000; CON | 158a | 347 | |
| SEM | 11 | 16 | |
| p-value | |||
| <0.01 | 0.497 |
a-bMeans within the same column without a common superscript differ (p<0.05)
ǂNewtons.
*Experiment 1, treatments: 13% CP and 2,900 kcal ME kg-1 of diet, 17% CP and 3,000 kcal ME kg-1 of diet and control 21% CP and 3,025 kcal ME kg-1 of diet.
**Experiment 2, treatments: 16% CP and 3,000 kcal ME kg-1 of diet and control; 21% CP and 3,000 kcal ME kg-1 of diet.
¥SEM; standard error of the mean.
Gait score (GS) and valgus/varus angulation (VAng). In Experiment 1, GS was affected by the diet (p<0.01). When dietary CP and ME were reduced to 13/2,900 a higher number of birds with a GS 4 (less walking ability) were observed compared to the control treatment. On the other hand, the birds in the 17/3,000 treatment had more cases with a GS 3 with respect to the birds in the control treatment. Similar number of GS 5 scores were observed among 13/2,900 and 17/3,000 treatments. The GS in Experiment 2 was not different (p>0.05) between diets (Table 4).
In Experiment 1, VAng was affected by PC and ME levels (p<0.05). Broilers in the control treatment showed the lowest VAng values (scores 2 and 3) compared with the other treatments. Moreover, chickens in the 13/2,900 treatment showed higher VAng 2 scores (birds with obvious angulation) than birds in the control treatment. The birds in the 17/3,000 treatment had higher VAng (score 3) than chickens in the 13/2,900 group. Similar to Experiment 1, the VAng was not different (p>0.05) between treatment diets in Experiment 2 (Table 5).
Table 4 Frequency (%) of gait score (GS) at 51 d of age (Experiment 1) and at 46 d of age (Experiment 2) in broilers fed diets with different crude protein (% CP) and metabolizable energy (ME) levels.
| Experiment 1 | 1Gait score | ||||||
|---|---|---|---|---|---|---|---|
| *Treatments | 0 | 1 | 2 | 3 | 4 | 5 | |
| 13/2,900 | 0.0 | 0.0 | 20.0 | 46.7 | 26.7 | 6.7 | |
| 17/3,000 | 0.0 | 13.3 | 26.7 | 40.0 | 13.3 | 6.7 | |
| 21/3,025; CON | 66.7 | 20.0 | 13.3 | p-value | 0.0 | 0.0 | 0.0 |
| 0.0001 | |||||||
| Experiment 2 | Gait score | ||||||
| **Treatments | 0 | 1 | 2 | 3 | 4 | 5 | |
| 16/3,000 | 2.5 | 27.5 | 32.5 | 27.5 | 5.0 | 5.0 | |
| 21/3,000; CON | 15.0 | 20.0 | 50.0 | p-value | 15.0 | 0.0 | 0.0 |
| 0.1331 |
*Experiment 1, treatments: 13% CP and 2,900 kcal ME kg-1 of diet, 17% CP and 3,000 kcal ME kg-1 of diet and control 21% CP and 3,025 kcal ME kg-1 of diet.
**Experiment 2, treatments: 16% CP and 3,000 kcal ME kg-1 of diet and control; 21% CP and 3,000 kcal ME kg-1 of diet. 1Gait score: Briefly, a score of 0 denotes a bird with fluid locomotion and a furled paw when it is raised and a score of 5 denotes a bird in complete lameness that cannot walk or stand (Kestin et al. (1992) modified by Garner et al. (2002)).
Table 5 Frequency (%) of leg valgus/varus angulation (VAng) at 51 of age (Experiment 1) and at 46 d of age (Experiment 2) in broilers fed with different crude protein (% CP) and metabolizable energy (ME) levels.
| Experiment 1 | 1 valgus/varus angulation (VAng) | ||||
|---|---|---|---|---|---|
| *Treatments | 0 | 1 | 2 | 3 | |
| 13/2,900 | 13.3 | 33.3 | 46.7 | 6.7 | |
| 17/3,000 | 0.0 | 66.7 | 20.0 | 13.3 | |
| 21/3,025; CON | 13.3 | 86.7 | 0.0 | 0.0 | |
| p-value | |||||
| 0.0422 | |||||
| Experiment 2 | valgus/varus angulation | ||||
| **Treatments | 0 | 1 | 2 | 3 | |
| 16/3,000 | 15.0 | 60.0 | 22.5 | 2.5 | |
| 21/3,000; CON | 30.0 | 55.0 | 12.5 | 2.5 | |
| p-value | |||||
| Treatment | 0.0956 |
*Experiment 1, treatments: 13% CP and 2,900 kcal ME kg-1 of diet, 17% CP and 3,000 kcal ME kg-1 of diet and control 21% CP and 3,025 kcal ME kg-1 of diet.
**Experiment 2, treatments: 16% CP and 3,000 kcal ME kg-1 of diet and control; 21% CP and 3,000 kcal ME kg-1 of diet. 1Valgus/varus angulation was evaluated according to the methodology described by Leterrier and Nys (1992): 4 scores were defined: 0, normal chicken; 1, chickens with little angulation (tibia-metatarsus angle between 10 and 25°); 2, birds with obvious angulation (angle between 25 and 45°) and 3, severe angulation (angle greater than 45°).
Performance evaluation
In Experiment 1, broilers in the 17/3,000 treatment had similar (p>0.05) BW, BWG, FI and FCR (from 2 to 7 weeks age) to the chickens fed the control diet. Broilers in the 13/2,900 treatment showed lower (p<0.05) BW and FI than those in the control treatment (wk 3-7) (Figure 1).
In Experiment 2, broilers fed the 16/3,000 diet exhibited reduced (p<0.05) BW at weeks 3-6 compared to the control. At week 7 no difference in BW (p>0.05) was detected between treatments. BWG of broilers fed the 16/3,000 diet was higher (p<0.05) than the control treatment after week 5 of the trial. FI was lower (p<0.05) for broilers fed the 16/3,000 diet than the control treatment at weeks 4 and 5. Feed conversion ratio (FCR) was higher (p<0.05) for the broilers fed the 16/3,000 diet at weeks 2, 3 and 4 than the broilers in the control treatment; conversely, in weeks 5, 6 and 7, the birds fed the control treatment had higher FCR (p<0.05) than those in the 16/3,000 group (Figure 2).
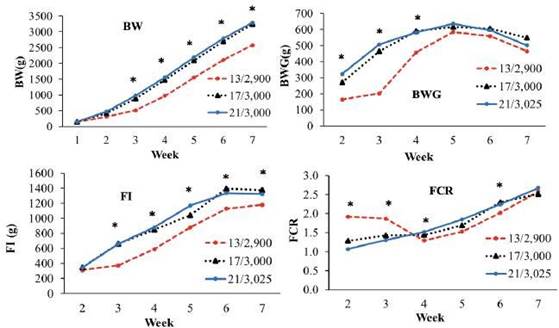
Figure 1 Performance Experiment 1. BW, body weight (g); BWG, body weight gain (g); FI, feed intake (g) and FCR, feed conversion ratio. Broilers fed diets with different CP and ME levels during the starter period (Treatments: 13% CP and 2,900 kcal ME kg-1 of diet, 17% CP and 3,000 kcal ME kg-1 of diet, and control, 21% CP and 3,025 kcal ME kg-1 of diet). *p<0.05.
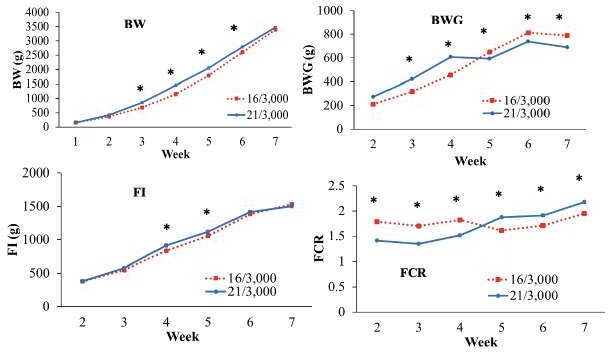
Figure 2 Performance Experiment 2, BW, Body weight (g); BWG, body weight gain (g); FI, feed intake (g) and FCR; Feed conversion ratio. Broilers fed diets with different CP and ME levels during the starter period (treatments: 16% CP and 3,000 kcal ME kg-1 of diet and control, 21% CP and 3,000 kcal ME kg-1 of diet). *p<0.05.
Discussion
Results of the present study suggest that levels below 17% CP and 3,000 kcal kg-1 ME reduce GTeBS, and concentrations below 16% CP and 3,000 kcal kg-1 impair the walking ability of broilers. A high number of birds in the 13/2,900 treatment showed mild to severe leg abnormalities. Research done in rats has shown that low dietary protein decreases collagen synthesis (Deyl et al., 1981) and bone mineralization (Mardon et al., 2009). Similarly, human diets that contain high levels of protein were correlated with high mineral (Ca and Mg) absorption and consecutively, high excretion. (McCance et al., 1942; Kerstetter et al., 2003; Dawson-Hughes et al., 2007). Thus, a relationship between amino acid type and concentration has been established with calcium homeostasis ultimately influencing bone turnover and structural protein metabolism. In the case of poultry, limited research was found regarding low dietary protein concentrations and intestinal calcium absorption. Bruno et al., 2007 concluded that when broilers are fed varying concentrations of CP and ME in the diet, it is possible to modulate the deposition of bone collagenous proteins and structure of organic components. Additionally, Bruno et al., 2007 observed that broilers fed 18.5% CP and 3,200 kcal kg-1 of diet had lower femur width and humerus weight than broilers from control group (22% CP and 2,950 kcal kg-1 of diet). The high incidence of leg abnormalities (GTeBS, TiBS, GS, VAng) and poor performance observed in the 13/2900 treatment could be explained by the low dietary protein levels used in this study. Less mineral deposition in the tibia could be the cause of deformity and reduction of breaking strength. In this study, no mineral analysis of the tibia was done; total mineral content or tibia bone ash analysis could provide more information to explain the dynamics of dietary protein and skeletal integrity in growing boiler chickens. This should be taken into consideration for further studies investigating the effect of low dietary protein in collagen synthesis and intestinal Ca absorption. The results of this study suggest that it is possible to reduce dietary CP concentration up to 17% without affecting TiBS. In agreement with these results, Yalcin et al. (1998) found that reduction from 23 to 18% CP does not affect TiBS. Collagen is the main structural component in the extracellular matrix of connective tissues such as tendons, and amino acids are the building units of proteins. Therefore, impaired collagen synthesis due to low protein intake could result in weak structural tissues and abnormalities. Type I collagen is the most abundant component in tendons (Wang, 2006). Deyl et al. (1981) reported that low protein intake affects the synthesis of Type I collagen in rats. To further investigate the effects observed in this study, where GTeBS was reduced with lower dietary protein, collagen synthesis in broiler chicks could be analyzed and provide insights on key steps being affected by low amino acid bioavailability and their relation with other nutrients such as vitamin C (Rath et al., 2000; Wu et al., 2020). Vitamin C has a key role on collagen synthesis, especially in broiler chickens under heat stress (Abidin, and Khatoon, 2013); therefore, vitamin C supplementation to broiler diets could be a strategy to ameliorate the negative effects of low dietary CP levels.
In both experiments conducted in this study, low dietary CP treatment birds appeared to recover from the nutrient restriction. Broiler chickens tended to grow at similar or even higher rates compared to controls when the finisher diets were offered. Compensatory weight gain is usually present after phases or periods of nutrient restriction in young animals. Leeson et al. (1991) observed that broilers may tolerate a 7-d period of undernutrition during the starter period (from 4 to 11 d of age), and concluded that the compensatory response depends on age and the severity of the undernutrition period. The importance of feeding a nutritionally complete diet after a restriction period to observe compensatory weigh gain has been investigated by Malomo et al. (2013) who observed that birds fed with 16% CP for 42 d had lower weight gain and higher feed conversion than those fed with 21% CP. No nutritionally complete diet was offered in the Malomo and colleagues’ study after nutrient restriction and no compensatory weight gain was observed. On the other hand, in agreement with the results obtained in this study, compensatory weigh gain was observed in a recent study conducted by Hadaeghi et al. (2021) which involved a 14-d nutrient restriction followed by nutritionally adequate diets in broiler chickens.
Nutrient restriction is a common strategy to decrease the incidence of metabolic and skeletal disorders (Rodríguez-Ortega et al., 2014). From an animal welfare standpoint, the use of diets with low content of CP and ME constitutes a feed restriction method that reduces growth rate in chickens without having to restrict full access to feed and, thus, hunger (Sahraei, 2012).
In conclusion, feeding broiler chickens with 16% CP and 3,000 kcal ME kg-1 of diet does not affect tibia breaking strength, gait score, valgus/ varus angulation, body weight, and feed intake of broilers. However, diets below 16% CP and 3,000 kcal ME kg-1 of diet reduce gastrocnemius tendon and tibia breaking strength, body weight, and feed intake of broilers. The results of the present study suggest that starter diets for broiler chickens should not be formulated below 17% CP and 3,000 ME kcal kg-1 of diet.














