Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista colombiana de Gastroenterología
versão impressa ISSN 0120-9957
Rev Col Gastroenterol vol.28 no.3 Bogotá jul./set. 2013
The utility of endoscopic ultrasound for diagnosing chronic pancreatitis and the importance of early diagnosis
Raúl Cañadas, MD. (1), Diego M. Aponte, MD. (2), Rosario Albis, MD. (3), Luis C. Sabbagh, MD. (4)
(1) Internist and gastroenterologist, Assistant Professor at the Pontificia Universidad Javeriana - Hospital Universitario San Ignacio. Chief of the Clinical Gastroenterology Unit at Clínica de Marly. Bogotá, Colombia.
(2) Internist and gastroenterologist at Clínica Reina Sofía, Graduate Academic Coordinator of Gastroenterology at Fundación Universitaria Sanitas. Bogotá, Colombia.
(3) Epidemiologist and gastroenterologist at Clínica Reina Sofía. Bogotá, Colombia.
(4) Gastroenterologist and internist, Chief of the Clinical Gastroenterology Unit at Clínica Reina Sofía. Bogotá, Colombia.
Received: 11-11-12 Accepted: 26-06-13
Abstract
Today endoscopic ultrasound (EUS) is the most sensitive test for early diagnosis of chronic pancreatitis. Early diagnosis of this disease is of fundamental importance for timely intervention to limit the inflammatory responses to possible etiological factors and to slow or prevent progression to advanced and debilitating chronic pancreatitis. The diagnostic accuracy of EUS for chronic pancreatitis (CP) has been enhanced with the use of strict criteria and the creation of scoring systems which have evolved into the Rosemont classification. This is the most restrictive classification system of all: it aims at standardizing criteria and assigning values to endoscopic ultrasound findings of chronic pancreatitis found in early or late phases.
Since this disease is difficult to diagnose using conventional methods, especially in its early stages, EUS has emerged as an excellent diagnostic and therapeutic alternative for the study of this group of patients.
Keywords
Chronic pancreatitis, endoscopic ultrasound, Rosemont criteria.
INTRODUCTION
Chronic pancreatitis (CP) is a progressive inflammatory condition which can lead to permanent structural organ damage. Histopathological evaluation of the pancreas is the gold standard for confirmation of CP. Although histopathology is the most accurate method for diagnosis, we cannot forget a fact of fundamental significance: chronic pancreatitis does not necessarily compromise the entire pancreatic parenchyma. Instead, it usually compromises patches or segments of the pancreas. This suggests that a biopsy can yield false negatives if the area where the biopsy was taken is disease free. This has led to the conclusion that biopsies are really impractical for diagnosis because of this segmental condition, difficult approaches and the risks involved in histological sampling.
Given the difficulty of diagnosing CP even with histology, attempts have been made in recent years to achieve better diagnostic methods. Moderate to severe forms of chronic pancreatitis are relatively easy to identify by using standard imaging techniques such as ultrasound, computed tomography, and cholangiopancreatography, but mild forms are difficult to detect early. This is where endoscopic ultrasound becomes important since it is able to detect these morphological changes earlier. Nevertheless, there is a tendency to over-diagnose these mild forms when minimal alterations such as hyperechoic bands and hyperechoic (slightly dilated) Wirsung's duct are present. These findings may appear in patients without CP who have normal pancreases.
These issues plus the lack of standardization and weighting for endosonographic findings inspired various proposals that made great contributions to developing diagnostic criteria for CP. Then, in 2008, the Rosemont classification appeared with the hope of giving new light and overcoming the weaknesses in the hitherto existing classification systems. This classification especially addresses attempts to establish early diagnoses that allow quick recognition of this disease and enable physicians to offer prompt treatment, monitoring and definition of a prognosis for this latent and chronic disease. Currently, endoscopic ultrasound (EUS) is a diagnostic tool of fundamental importance in the study of pancreatic disease. It is considered the method of choice for evaluation of chronic pancreatitis and pancreatic cancer even though, despite great progress achieved, some issues currently remain unresolved for diagnosis. The most important is differentiation between an inflammatory mass in the context of CP and pancreatic cancer. Today this is a real challenge for which fine-needle aspiration has a clearly defined role.
Although EUS is considered to be the most sensitive technique for CP diagnosis because it has identifying criteria not available in other imaging techniques, there are still difficulties. These are especially important when inconclusive minor criteria are found, and this will be a source of continuous study in the development of different classifications.
Historically, CP is diagnosed with x-ray images that can show the presence of pancreatic calcifications, twisting or dilatation of the main pancreatic duct. Nevertheless, these images cannot identify early stages of morphologic alterations of the pancreas. Unfortunately, ERCP is an invasive procedure which only shows changes in late stages of the disease (1). In the absence of histology or pancreatic calcifications ERCP was historically accepted as the gold standard for minimally invasive CP diagnosis. The Cambridge classification which has been validated in several studies was used to evaluate severe anatomical changes. Setting ERCP as the gold standard, the sensitivity of EUS for CP diagnosis varies from 68% to 100% and its specificity varies from 78% to 97%, but its concordance in mild forms is only 83%. In a study of 38 patients with suspected or known diagnoses of CP but who were diagnosed with normal pancreases using ERCP, USE showed CP changes in 84.2% of patients. During 18 month follow-up checkups ERCPs showed classic cholangiography findings of CP. In fact, this study confirms that EUS has greater sensitivity than ERCP and reconfirms that EUS was able to identify early parenchymal changes even when the initial ERCP was normal (2).
Similarly, the other method for CP diagnosis, the pancreatic function test, has its weaknesses. The secretin stimulus test is considered a sensitive measure of the exocrine function but has the same drawback. Studies have shown sensitivity up to 40% in cases of early stage chronic pancreatitis. Duodenal bicarbonate use has recently been described for diagnosis of pancreatic exocrine insufficiency. After secretin is administered, duodenal fluid is endoscopically collected and the bicarbonate concentration is analyzed. Bicarbonate levels are markedly reduced in patients with chronic pancreatitis. This test could be useful for patients who have histories of abdominal pain suggestive of pancreatic disease but who have normal image studies (3).
ENDOSCOPIC ULTRASOUND FINDINGS IN CHRONIC PANCREATITIS
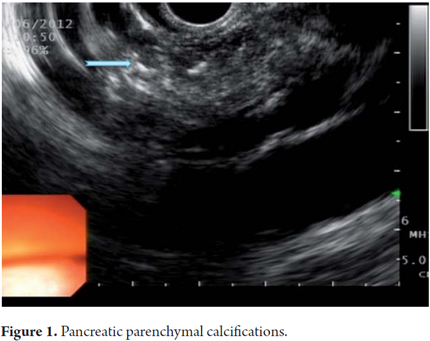
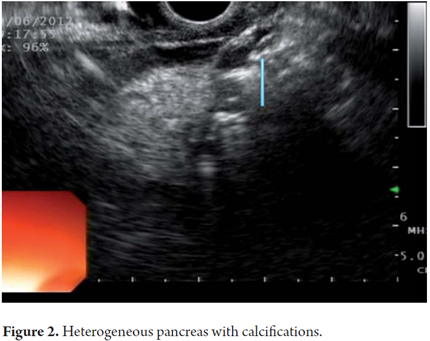
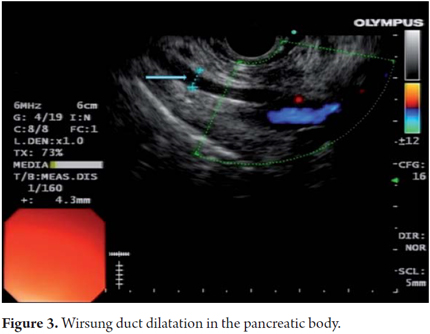
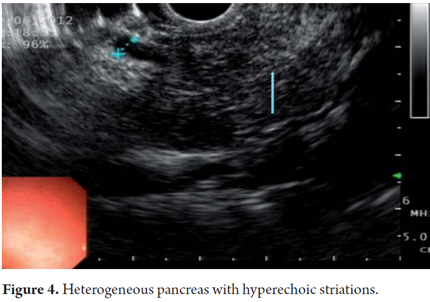
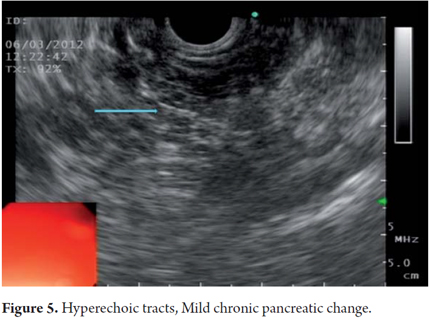
EUS is an imaging method capable of detecting both early and late changes. Diagnosis is based on parenchymal and ductal changes. Parenchymal changes include hyperechoic foci with or without acoustic shadowing, hyperechoic striations or tracts, honeycomb lobularity in the parenchyma, calcifications and cysts. Ductal changes include main duct dilatation, irregular main duct dilatation in secondary branches, hyperechoic duct wall and calculi (Figures 1, 2, 3, 4 and 5).
Some of these criteria have also been found in normal pancreases of elderly men (OR = 1.8, 95% CI: 1.3 to 2 .55) people with a history of frequent alcohol consumption, smokers and those with a history of acute pancreatitis. This should be taken into account to avoid over diagnosing when using EUS. However, for many experts the most important diagnostic criterion for CP is the presence of pancreatic calcifications. Next in importance are lobularity and dilatation of secondary branches. Main duct dilatation which is often given a lot of value in clinical practice is less significant.
Within the various classifications we perceived the concept that the longer that patient has CP, the more likely that abnormalities will be found. This assumption is unclear, and efforts to understand the successive events of inflammatory pancreatic phenomenon continue. In this sequence the most common classification used for endoscopic ultrasound diagnosis of CP was described by Jones and developed by Wiersema et al. in 1993. This classification which describes the standard terminology for pancreatic inflammation has been extensively evaluated by experts at EUS who have agreement on the final diagnosis (k = 0.45). Wiersema described 9 pancreatic criteria incouding hyperechoic foci, fiber tracts, lobularity, cysts, calcifications, dilated Wirsung's duct, dilated side branches, main pancreatic duct irregularities and hyperechoic duct walls (4). All these criteria have the same value for a final CP diagnosis which is made when more than 4 criteria are present. An important point in the development of these classifications is that they discriminate between parenchymal and ductal findings as can be seen in the classifications of Lees and Wiersema as well as in the Milwaukee classification (Table 1).
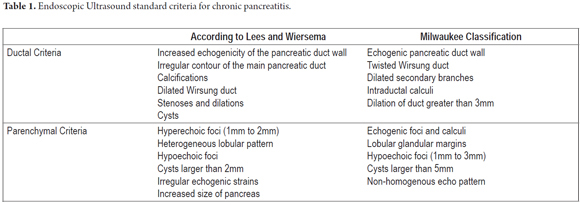
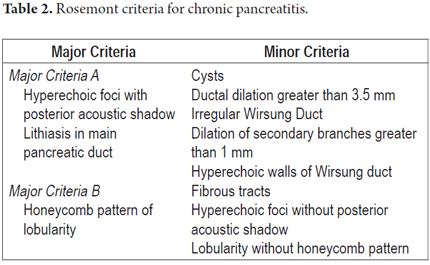
In April 2007 an international group of experts met in an effort at overcoming the limitations of standard scoring systems. They proposed a new classification in the Rosemont Hotel in Chicago, Illinois USA. The primary objective of this meeting was to generate a scoring system based on a consensus for standardizing CP diagnosis. At this meeting the proposal provided a different value for each criterion thereby establishing major and minor criteria. Six parenchymal criteria and five ductal criteria were finally included. In addition, each criterion was precisely defined to improve the objectivity of the score. For example the honeycomb lobularity (major criteria B) is defined by echogenic appearance with peripheral enhancement and echo poor centers with minimum size greater than 5mm, spatially contiguous relation and an intra-pancreatic location within the body and tail. Each criterion has a similar checklist for adequate detection with the idea of improving interobserver variability and alleviating over diagnosis. Certainly the Rosemont classification adds complexity to the standard scales and is part of the natural evolution of diagnostic tests for pancreatitis. Nevertheless further validation, especially with histological correlation studies, is required to assess its level of accuracy.
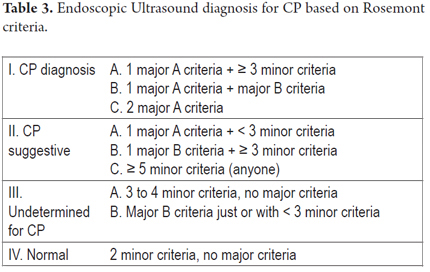
One of the most important recent works which tries to compare standard criteria with the Rosemont classification for EUS diagnosis of chronic pancreatitis was published by Cristina Jimeno-Ayllon et al. They examined differences in final CP diagnoses using standard Wiersema criteria and the Rosemont classification Rosemont for 47 patients with chronic pancreatitis. They divided these patients into 2 groups according to the Wiersema criteria. Patients with fewer than 4 criteria were diagnosed as not having CP diagnosis while those with more than 4 criteria were diagnosed with CP. Parenchymal and ductal criteria were studied. These patients were tested again using the Rosemont classification and diagnosed as having normal pancreases, indeterminate diagnoses, suggestive of CP and consistent with CP (Table 2 and Table 3). They found that patients with CP most frequently presented lobularity (66% of cases) followed by Wirsung duct dilatation and the presence of calcifications. There was a statistically significant association between the results of both classifications (p <0.05). The greatest degree of association was for patients with more than 4 standard criteria and a definitive CP diagnosis by Rosemont ranking. However, the Rosemont classification diagnosed the conditions of patients who had less than 4 standard criteria as suggestive of CP which could indicate that some cases can be detected earlier. Although the results showed no statistically significant differences for patients with four or more standard diagnostic criteria, a diagnosis of CP is virtually ruled out in the standard classification when there are less than four criteria present. In the Rosemont classification, 27.6% of these patients would be diagnosed as suggestive of CP. Consequently, this new classification could be useful when there is a high degree of suspicion that a patient who presents less than four standard criteria has chronic pancreatitis but presents major criteria such intra-parenchymal calcifications, lobularity or intraductal stones (5).
A study of 69 patients published by D. Del Pozo, E Poves et al. assessed interobserver variability between the classical form of diagnosis (Standard Minimum criteria) and the Rosemont classification for CP diagnosis. It found that the degree of concordance between both diagnostic scales was moderate: k = 0.53 for conventional criteria and 0.46 for the Rosemont criteria. The Rosemont classification did not improve interobserver variability compared to the conventional form (6) which should be kept in mind in the continuing evolution and development of classifications. In fact, interobserver variability is another important limitation of EUS for CP diagnosis (7).
EUS vs. MAGNETIC RESONANCE CHOLANGIOPANCREATOGRAPHY
Magnetic resonance cholangiopancreatography (MRCP) is being used more and more frequently because of its great virtues in diagnosing mild to severe CP. It can be used to evaluate not only from a structural point of view but also for measuring functional parameters which makes it a noninvasive alternative to ERCP (8). We know that MRCP allows us to study the main pancreatic duct and the secondary branches. Parenchymal changes are detected using gadolinium highlighted images in T1 or using secretin in T2. Consequently, post secretin duodenal filling allows us to study the exocrine function. A comparison of the accuracy of MRCP and EUS showed that EUS had greater sensitivity (93% vs. 63%) while the specificities of the two methods were similar (93% vs. 90%). Nevertheless, double-blind studies are lacking and needed for establishment of real differences including differences in cost-effectiveness.
CHRONIC ABDOMINAL PAIN AND CHRONIC PANCREATITIS
Because EUS can show parenchymal changes at initial stages of the disease process, it should have increased value for patients with a history of recurrent, sometimes debilitating, chronic abdominal pain suggestive of pancreatic etiology who have endured multiple examinations, hospitalizations and imaging studies (CT, MRCP, ERCP) without any diagnosis. In fact, chronic pancreatitis with minimal changes is currently defined as pancreatic abdominal pain syndrome without structural or minimal changes detected in images but with histological inflammation and fibrosis indicative of CP (9). Another relevant clinical fact is that many CP cases are related to chronic alcohol consumption. If changes are detected early it may be possible for abstinence to halt the progression of the inflammatory process towardn advanced CP associated with endocrine and exocrine failure.
A management algorithm published in the Cleveland Clinic Journal of Medicine in March 2012 suggests that when we have a patient with chronic abdominal pain suggestive of pancreatic etiology, the initial examination of choice is abdominal ultrasound or CT scan. If either is positive that would confirm the CP diagnosis. When not positive, the immediate next step should be EUS. If its findings are compatible with CP that would confirm the diagnosis, but if its findings are negative it excludes the diagnosis. In cases of suggestive or indeterminate CP findings, the option is to perform pancreatic function tests (10).
RADIAL EUS vs. LINEAR EUS
According to studies, both radial EUS and linear EUS can be used to diagnose CP. A prospective comparative study conducted with 100 patients using secretin stimulation recorded tests which were interpreted independently by three expert endosonographers. The sharpness of the radial EUS was not inferior to that of the linear EUS (74% vs. 84%). In another study using both radial and linear technique, interobserver variability was moderate (K = 0.50 and 0.61 respectively). The best concordance between the two methods was for detecting cysts, calcifications and dilated side branches (11).
ENDOSCOPIC ULTRASOUND GUIDED FINE NEEDLE ASPIRATION (EUS-FNA) PANCREATIC TISSUE SAMPLING FOR SUSPECTED CHRONIC PANCREATITIS
The addition of fine needle aspiration to echoendoscopic CP diagnosis is safe and increases negative predictive value although does not increase specificity. Cytology provides cellular material for microscopic examination but the correlation with histopathology remains uncertain. In fact, for many practitioners histology sampling is an impractical measure. Studies like that conducted by DeWitt show that Trucut biopsies allow us to obtain pancreatic samples in cases of non-focal chronic pancreatitis. However, undiagnosed specimens were found in 6 of 15 patients (12). Given the risks and the limited diagnostic yield, this technique is not currently recommended for routine evaluations of these patients.
Usefulness of needle aspiration changes when treating a patient with CP and masses. EUS sensitivity for detection of suspicious pancreatic masses in the parenchyma with CP changes may reach 100%. The sensitivity of EUS-FNA for malignant parenchymal masses with CP findings is only 54% to 74% compared with 89% when the surrounding parenchyma is normal (13). However, in the event of high suspicion of malignancy when EUS-FNA is negative, repeating the aspiration favors the diagnostic yield in 84% of cases. Initial diagnostic failures can be attributed to CP changes.
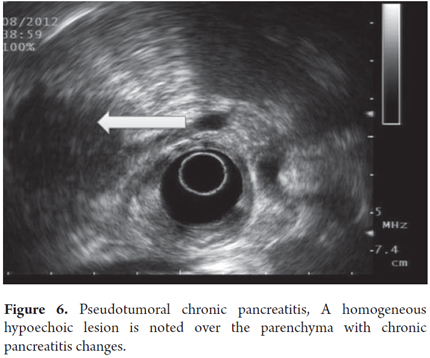
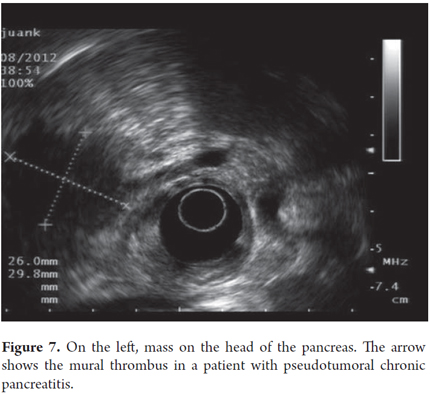
Pseudotumoral CP is suspected when lobular, homogeneous, hypoechoic or isoechoic areas with well defined borders are present and when there is a Doppler signal inside the mass (Figures 6 and 7). Pancreatic cancer is defined as a hypoechoic, heterogeneous mass with poorly defined borders and the absence of a Doppler signal within the mass. Pseudotumoral CP diagnosis can be difficult to differentiate from pancreatic carcinoma. Puncture aspiration is the only method that can distinguish between them because ultrasound findings, clinical presentations and CA 19-9 elevations for the two are all very similar. In these cases it is of fundamental importance to arrive at a diagnosis because of the implications of pseudotumoral CP surgery for patients and the morbidity that this represents. In this context the expert must evaluate topography, mass size, texture, echogenicity, borders, lobularity, septa, calcifications, pseudocysts and calculi.
Despite these findings it is not easy to distinguish pseudotumoral from pancreatic cancer. For this reason, fine needle aspiration (FNA) is recommended even though there is a low risk of metastasis. Nevertheless some studies indicate a preference for surgery so that a definitive diagnosis of the mass can be made. However, we insist that since a benign diagnosis can be found in no more than 6% of suspected cases of malignancy it is essential to avoid the complications inherent to surgical that reach the not inconsiderable rate of 21% (14).
In conclusion, even though EUS-guided FNA is not considered for routine evaluation of CP patients due to its limited diagnostic yield, its utility is still accepted in specific cases where it is essential for clarifying the etiology for therapeutic decisions specifically in cases of pancreatic pseudo-tumors whose differential diagnosis is pancreatic cancer.
THERAPEUTIC USEFULNESS OF EUS IN CHRONIC PANCREATITIS
The sole object of an EUS-guided celiac plexus block is to reduce pain and improve quality of life. It can be used for patients with pain and minimal CP changes who are unresponsive to analgesic therapy and for patients with severe ductal changes whose pain is not controlled after endoscopic treatment or surgical decompression. This technique is superior to percutaneous blocking and provides better results in terms of satisfaction. There is still controversy about whether central injection or bilateral plexus injection is better. Several studies show that they are substantially equal in terms of safety, but their results in relation to pain management are contradictory which highlights the need for further controlled studies. In some studies with short follow-up periods, pain control was achieved in from 55% to 70% of patients. This confirms the need for studies with longer follow-up periods. The few complications (1.8%) reported include hypotension, retroperitoneal abscesses and post-procedural pain.
The results of a meta-analysis published by Puli et al. showed that 60% of CP pain patients achieved at least partial and temporal responses (15). Questions remain to be resolved, especially because of poor results among patients younger than 45 years old and among patients who had previously undergone pancreatic surgery.
FUTURE DEVELOPMENT OF ENDOSCOPIC ULTRASONOGRAPHY WITH PANCREAS CONTRAST AND ENDOSCOPIC SONOELASTOGRAPHY
Endoscopic ultrasonography with contrast media allows enhancement of arteries, venous portals, and collateral vessels. This could help differentiate CP from ductal adenocarcinoma because of the perfusion characteristics of micro-vessels. The criteria for identifying malignant lesions are absence of detectable vascularization in a conventional power Doppler ultrasound, irregular appearance of arterial vessels and absence of detectable veins within the lesion. The criteria for chronic pancreatitis in the absence of neoplasia are absence of detectable vascularization prior to injection, normal looking vessels and visible presence of veins. These criteria increase the sensitivity of tumor diagnosis and differentiation from benign diseases (16).
On the other hand, endoscopic sonoelastography is a technique that quantifies tissue hardness. Tissue tension is evaluated according to the tissue's response to compression by the endoscope. The tension in each region is converted into a color image which is then interpreted. Different patterns of elasticity are marked on a 5 color scale. Pattern 1, marked in soft green, has low elasticity indicating a homogeneous area corresponding to normal pancreatic tissue. Pattern 2, marked in green, yellow and red, indicates a heterogeneous area with a range of soft tissues corresponding to fibrosis. Pattern 3, marked in blue, consists of hard tissue with minimal heterogeneity which corresponds to small pancreatic adenocarcinomas (less than 25 mm). Pattern 4, marked in green, has a central hypoechoic region surrounded by hard blue which corresponds to vascular lesions such as a neuroendocrine tumor or a small pancreatic metastasis. Pattern 5 is almost totally blue with heterogeneous patches of green and red for softer tissues. This corresponds to necrosis (16). In additional, a honeycomb pattern associated with hard patches differentiates CP from a normal pancreas, however the same pattern can also be seen in pancreatic cancer (17, 18).
It should be recognized that studies based on these principles have focused on differentiating malignancy from inflammation rather than on detecting early changes in chronic pancreatitis. Using the scoring system based on different color patterns in images, differentiation between benign and malignant pancreatic masses has a sensitivity of 92% and a specificity of 80%.
With the progressive development of second generation endoscopic elastography based on identifying tissue stiffness this technique will become useful for differential diagnosis of solid pancreatic masses which is key for differentiation between malignant masses and benign masses in the context of CP. There may also be progress in the development of new and better techniques, for example directing a biopsy to improve diagnostic accuracy. Despite its benefits, the interobserver variability of this technique is its main drawback. Because of the difficulty of obtaining and interpreting images, more studies will be needed to systematically apply this technique in the future.
CONCLUSIONS
EUS is a commonly used technique with good levels of sensitivity for CP diagnosis. This is especially true when imaging techniques routinely used show no early pathological changes that can be documented and which will impact the natural evolution of the disease. The Rosemont classification certainly gives us our best tool for more accurately diagnosing CP: it promotes early detection of subtle changes in the pancreatic inflammation-fibrosis sequence. This contributes to early intervention for patients with this disease and also promotes a common language for the development of future research in this area. Despite the advantages of EUS for pancreatic evaluation, it is impossible to know its true diagnostic accuracy due to the heterogeneity of designs and the inherent limitations of this technique which is particularly observer-dependent. For these reasons a judicious and appropriate learning curve will be needed to reproduce the scope shown in the literature since the interobserver variability is far from perfect.
REFERENCES
1. Bolondi L, y cols. Sonography of chronic pancreatitis. Radio Clin North Am 1989; 27: 815-833. [ Links ]
2. Kahl S, et al. EUS in the diagnosis of early chronic pancreatitis: a prospective follow-up study. Gastrointestinal endoscopy 2002; 55: 507-11. [ Links ]
3. Conwell DL, y cols. An endoscopic pancreatic function test with synthetic porcine secretin for the evaluation of chronic abdominal pain and suspected chronic pancreatitis. Gastrointestinal endosc 2003; 57: 37-40. [ Links ]
4. Wallace MB. Chronic pancreatitis. Gastrointest Endosc 2009; 69(Supl.2): S117-120. [ Links ]
5. Cristina Jimeno-Ayllón, José Ignacio Pérez-García. Criterios estándar versus clasificación de Rosemont para el diagnóstico ecoendoscópico de pancreatitis crónica. Rev Esp Enferm Dig (Madrid) 2011; 103: 626-631. [ Links ]
6. D. Del Pozo, E Poves, S Tabernero, I. Beceiro, I. Moral, M. Villafruela, C. Sanz, G. Borrego. Conventional versus Rosemont endoscopic ultrasound criteria for chronic pancreatitis: Interobserver agreement in same day back-to-back procedures. Pancreatology 2012; 12: 284-287. [ Links ]
7. Stevens T, López, et al. Multicenter comparison of the interobserver agreement of standard EUS scoring and Rosemont classification scoring of chronic pancreatitis. Gastrointestinal Endosc 2010; 71: 519-526. [ Links ]
8. Gareth Morris-Stiff, Phillip Webster, Ben Frost, Wyn G Lewis, Malcom CA Puntis, S Ashley Roberts. Endoscopic Ultrasound reliably identifies chronic pancreatitis when other imaging modalities have been non-diagnostic. JOP. J Pancreas 2009; 10(3): 280-283. [ Links ]
9. Walsh TN. Minimal change chronic pancreatitis. Gut 1992; 33: 1566-1571. [ Links ]
10. Siwar Albashir MD, y cols. Endoscopic ultrasonography to evaluate pancreatitis. Cleveland Clinic Journal of Medicine 2012; 79: 202-206. [ Links ]
11. Stevens T, et al. Prospective comparison of radial and lineal endoscopic ultrasound for diagnosis of chronic pancreatitis. Endoscopy 2009; 41: 836-41. [ Links ]
12. DeWitt J, et al. EUS-guided Trucut biopsy of suspected nonfocal chronic pancreatitis. Gastrointestinal endoscopy 2005; 62: 76-84. [ Links ]
13. Krishna NB, et al. EUS/EUS-FNA for suspected pancreatic cancer: influence of chronic pancreatitis and clinical presentation with o without obstructive jaundice on performance characteristics. Gastrointestinal endoscopic 2009; 70: 70-79. [ Links ]
14. José Celso Ardengh, Cesar Vivian López, Antonio Dorival Campos, Luiz Felipe Pereira de Lima, Filadelfio Venco, José Luiz Pimenta. Endoscopic Ultrasound and fine needle aspiration in chronic pancreatitis. Differential diagnosis between pseudotumoral masses and pancreatic cancer. JOP. J Pancreas 2007; 8(4): 413-421. [ Links ]
15. Puli, SR, Reddy, et al. EUS-guided celiac plexus neurolysis for pain due to chronic pancreatitis or pancreatic cancer pain a meta-analysis and systematic review. Dig Dis Sci 2009; 54: 2330-7. [ Links ]
16. Andrada Seicean. Endoscopic ultrasound in chronic pancreatitis: Where are we now? World Journal of Gastroenterology 2010; 16(34): 4253-4263. [ Links ]
17. MJ Varas Lorenzo. Ultrasonografía endoscópica. Aplicaciones diagnósticas y terapéuticas. Sonoelastografía por ultrasonografía endoscópica. Editorial Panamericana; 2008. p. 291. [ Links ]
18. Julio Iglesias-García, Jose Lariño-Noia, J. Enrique Domínguez-Muñoz. Elastografía en la evaluación de la pancreatitis crónica. Gastroenterol Hepatol 2011; 34(9): 629-34. [ Links ]











 texto em
texto em