Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista colombiana de Gastroenterología
versão impressa ISSN 0120-9957
Rev Col Gastroenterol vol.29 no.2 Bogotá abr./jun. 2014
The Use of NSAIDs Combined with Prophylactic Use of PPIs for Internal Medicine Patients
Álvaro Osorio, MD. (1), William Otero Regino, MD. (2), Martín Gómez Zuleta, MD. (3)
(1) Internal Medicine Resident at the Universidad Nacional de Colombia in Bogotá, Colombia.
(2) Professor of Medicine in the Gastroenterology Unit at the Universidad Nacional de Colombia and Gastroenterologist at the at the Universidad Nacional de Colombia in Bogotá, Colombia.
(3) Professor of Medicine in the Gastroenterology Unit at the Universidad Nacional de Colombia and Gastroenterologist at the Hospital EL Tunal and Hospital de Kennedy in Bogota, Colombia.
Received: 08-01-14 Accepted: 08-05-14
Abstract
Introduction: Adverse gastrointestinal events related to non-steroid anti-inflammatory drugs (NSAIDs) which are frequently prescribed in medical practice increase morbidity and mortality. These can be reduced through prophylactic use of proton pump inhibitors proton pump (PPIs) or misoprostol anti H2.
Objective: The objective of this study was to estimate the prevalence of NSAIDs use in a population of internal medicine outpatients and to determine the frequency of prophylactic prescriptions of PPIs for patients at risk of gastrointestinal bleeding.
Methods: This was a prospective and analytical study of prevalence among patients over 18 years of age in the Internal Medicine outpatient service at the Hospital San Carlos in Bogota. Patients who consume NSAIDs were classified into three risk groups based on traditionally described risk factors for gastrointestinal bleeding.
Results: Thirty percent of the 140 enrolled patients were taking NSAIDs. 47.6 % (n = 20) were classified in the low risk group, 28.5 % (n = 12) in the intermediate-risk group and 23.8 % (n = 10) in the high risk group. 47% (20 patients) of those taking NSAIDs were simultaneously taking PPIs. Eighty percent of the high risk group, 50% of the intermediate-risk group, and 30% of the low risk group were taking PPIs.
Conclusion: PPIs were prescribed more frequently for high risk patients in this study population (80%) than has been that reported in international publications (less than 50%). The prescription of PPIs for 30% of the low risk patients is unnecessary.
Keywords
NSAIDs, PPIs, prophylaxis, gastrointestinal protection.
INTRODUCTION
Non-steroid anti-inflammatory drugs (NSAIDs) are commonly used to treat various inflammatory conditions, as well as for the symptomatic relief of fever and pain without inflammation (1). They are among the most frequently prescribed medications throughout the world. It is estimated that in the United States NSAIDs are prescribed more than 100 million times every year with an estimated cost of $4.8 billion (2, 3). Alongside their proven analgesic and anti-inflammatory efficacy, NSAIDs have cardiovascular and gastrointestinal side effects that can have fatal complications (4-6). Today it is considered that NSAIDs and aspirin cause most bleeding peptic ulcers (7). Twenty-five percent of chronic NSAID users develop peptic ulcers while 2% to 4% develop bleeding or perforations. In the USA this causes more than 100,000 hospitalizations and 10,000 to 17,000 deaths every year (8). Established risk factors for development of bleeding peptic ulcers in users of NSAIDs include being older than age 65 year, a history of ulcers with or without bleeding, concomitant use of anticoagulants or steroids, severe comorbidities and use of high doses of NSAIDs (7, 8). Co-prescription of anti-secretory agents decreases the risk of bleeding ulcers in high risk patients (9). Specifically, sufficient evidence exists that 800 mcg/day of misoprostol (RR 0.17), double doses of Anti H2 (RR 0.44) or proton pump inhibitors (IBP) (RR: 0.40) decreases the risk of bleeding (9). In addition, there various guidelines for preventing ulcers as a complication of NSAID use have been published (8, 10, 11). Nevertheless, despite the wide dissemination of these guidelines, several international studies have shown that 60% to 80% of the patients at risk for complications from NSAIDs stop using protective drugs while patients with low risks of complications use them excessively (12-14). No research has been done here in Colombia on the use of prescription drugs to protect patients who chronically take NSAIDs nor has any research been done here on the population which chronically uses these medications. A recent study by Otero et al (15). found that 41% patients with duodenal ulcers use NSAIDs or aspirin. Given the importance of prescribing antisecretory agents for patients who chronically use NSAIDs, and the lack of information on this subject in our country, we decided to perform this study.
MATERIALS AND METHODS
This is a prospective study of patients over the age of 18 who were seen in the internal medicine outpatient clinic at Fundación Hospital San Carlos from January to April 2013. The minimum sample size required to estimate the prevalence of NSAIDs in the target population was calculated to be 138 patients based on the estimated that 10% of this population consume NSAIDs with a 95% confidence interval and a precision of 5%.
During the study period the residents and medical professors who work in the internal medicine outpatient clinic enrolled outpatients who agreed to participate in the study and who signed informed consent forms. A questionnaire was designed to collect data. Information requested on the form included patient demographics, treatment indications, dosages and duration of NSAID/aspirin usage, current and/or prior diagnoses of peptic ulcers whether or not they were complicated, symptoms of gastroesophageal reflux (heartburn and/or regurgitation), dyspepsia (pain or discomfort in the upper abdomen with or without nausea, bloating, or the feeling of bloating), intolerance to NSAIDs, and concomitant use of PPIs. Usage of misoprostol or anti H2 was not recorded because the former is restricted because of its abortive effects, and anti H2 is rarely or never prescribed to be taken twice a day which is the protective dose. Serious concomitant diseases such as congestive heart failure, hypertension, stroke, diabetes and hypercholesterolemia were also recorded. The simultaneous use of anticoagulants such as clopidogrel, steroids, and consumption of two or more NSAIDs was also recorded. To reduce and/or prevent recall bias, photographs of the packages of the most frequently used NSAIDs were kept on hand at the clinic to show patients who had any doubts about whether or not they had been using these drugs.
Significant NSAID use was defined as one month in the previous year of using at least the dosage defined in the EPISER dosage study (16). Aspirin dosages of less than 300 mg/day and NSAID dosages of more than 300 mg/day were considered to be antithrombotic (17). Patients were stratified into three groups. High risk of bleeding was defined as the presence of two or more risk factors, moderate risk as the presence of one risk factor, and low risk as the absence of all risk factors (8). Medical residents who participated in information collection in the outpatient department were given a talk about the importance of NSAID prescriptions, the gastrotoxicity of NSAIDs, risk factors for gastrointestinal bleeding in patients taking NSAIDs, gastroprotection for patients taking NSAIDs, protocol definitions and use of the data collection instrument. The research protocol and informed consent form were approved by the ethics committee of the institution.
Study End Points
The principal objectives of the study were to determine the prevalence of NSAID use (whether spontaneous or prescribed by a doctor) among patients seen in the General Internal Medicine clinic, to determine the use of gastro-protective drugs among these patients, and to categorize these patients according to the level of risk (low, moderate or high) of presenting gastrointestinal complications.
Statistical Analysis
The database was designed in Excel® (Microsoft Office 2010) and IBM® SPSS® Statistics Version 18 was used for statistical analysis. Demographic and clinical variables of patients who used NSAIDs and proton pump inhibitors were descriptively analyzed. Categorical variables were expressed as absolute and relative frequencies. Continuous variables were described using measures of central tendency. Normality tests were performed for continuous quantitative variables. ANOVA analysis with a significance level of 95% and a maximum error of 0.05 was used to estimate the difference between age and consumption of NSAIDs. The Chi square test was used for dichotomous variables
RESULTS
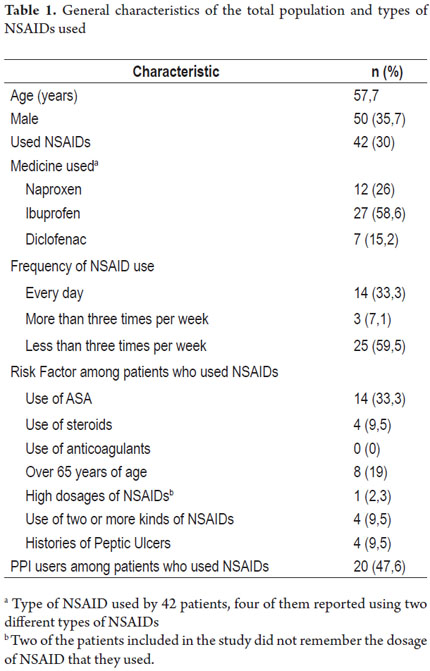
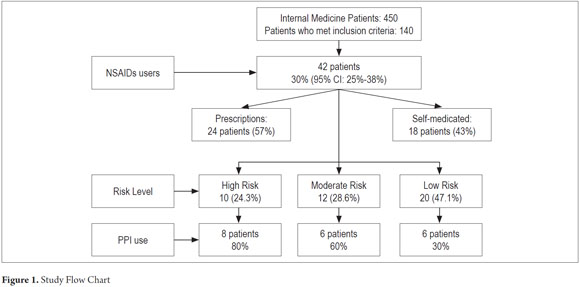
Of a total of 450 patients seen in the internal medicine clinic during the study period, 140 met the inclusion criteria and chose to participate in the study. 35.7% of these were women. The average age was 57.7 years with a range of 19 to 90 years. Of the 140 patients included, 42 patients (30%, 95% CI 16% -30%) used NSAIDs, and 29 of these (69%) were women. Their average age 53.8 years with a range of 19 to 90 years. The general characteristics of the population and the percentages who used different types of NSAIDs are shown in Table 1.
Fourteen patients (33.3%) used NSAIDs daily, three patients (7.1%) used them three or more times per week, and 25 patients (59.5%) used them less than three times a week. Twenty-seven patients (64.2%) used significant amounts of NSAIDs over the previous year (NSAID use for more than one month during the year prior to the study). Patients' risk factors included concomitant use of doses of acetylsalicylic acid for primary or secondary prevention of cardiovascular disease (14 patients, 33.3%), ages over 65 years (8 patients, 19%), use of steroids (four patients, 9.5%), histories of peptic ulcers diagnosed by endoscopy (four patients, 9.5%), simultaneous use of two or more NSAIDs (four patients, 9.5%), and high intake of NSAIDs (one patient, 2.4%). Two patients did not remember the daily dose of the drug they consumed. No patients consumed anticoagulants.
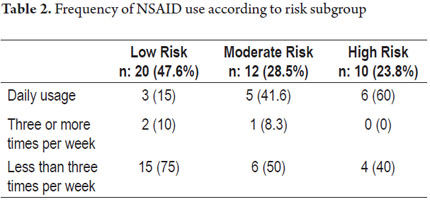
Twenty patients had no risk factors for gastrointestinal bleeding and were classified in the low risk category), twelve patients (28.5%) had one risk factor (intermediate risk), seven patients (16.6%) had two factors risk, and three patients (7.1%) had more than two risk factors. These last ten qualified as high-risk patients. Table 2 shows the frequency of NSAID usage according to risk groups.
Of the 42 patients taking NSAIDs, twenty (47.6%) were also taking PPIs. Eight of these patients (80%) were among the ten patients in the high risk group. Six patients, half of the moderate risk group, took PPIs while six of the twenty patients in the low risk used PPIs. Twenty-four patients used NSAIDs that had been prescribed (57%) the rest self-medicated. The study flowchart is shown in Figure 1.
The average age of patients who used NSAIDs was 53.8 years. In contrast, patients who did not use NSAIDs had an average age of 59.48 years (p <0.05). There was no statistical difference between the proportions of women and men using NSAIDs.
DISCUSSION
In this study, 30% of the patients seen in the internal medicine outpatient clinic at Hospital San Carlos use NSAIDs. A population-based study in Spain with a sample of more than 2,900 subjects showed that 20.6% (95% CI 15.8% to 25.4%) of the sample population used NSAIDs (18). Of the NSAIDs users in our study population, 23.8% were considered to be at high risk. This percentage is similar to the 22.3% found in the Spanish LOGIC study conducted among patients with osteoarthritis (17). Our results showed that the majority of high-risk patients (80%) took PPIs and that 50% of the moderate risk patients took PPIs. These figures are higher than those found in international studies in which the gastroprotection is used by only 20% to 45% of patients (12-14, 19-21). Despite the high frequency of gastroprotection use among high-risk patients in this study, the ideal is that 100% patients at high and moderate risks should receive prophylaxis (20, 21). Our study, like other studies, found that a significant proportion of patients without risk (30%) use PPIs. This usage is unnecessary for prophylaxis in this group and is not cost-effective (22). It is possible that the high rates of prescriptions of PPIs in groups at high or moderate risk are due to the indiscriminate use of PPIs in our environment, but in this case this widespread practice favors these groups of patients. Nevertheless, the ideal would be for PPIs to be used in a rational way in which only those who need them would take them. This means that those with little likelihood of bleeding, such as the low risk group in this study, should not take PPIs. Thirty percent of this patients have unnecessary prescriptions to these drugs. This is a higher rate than the 12% over-prescription rate reported in the literature (14). Nevertheless, we do not know what proportion of patients use PPIs as a consequence of conscious over-prescription by doctors to avoid damage from NSAIDs or conditions like acid reflux or dyspepsia. As in other studies, the high rate of NSAID use demonstrates self-medication by patients which reflects the ease of use without a prescription (5). It is likely that this situation explains why this study, unlike other studies, found that NSAID consumers were younger than non-users (16, 18).
We recognize that this study has limitations. Among them are the absence of a database of prescriptions and drug dispensaries which would allow a more certain idea of the true prevalence of NSAID use. Also, the information was collected from data provided by patients to doctors during consultations which can lead to recall bias in data collection. This was partially offset by having pictures of the packaging of the most frequently prescribed NSAIDs. Another limitation was that the sample calculation was performed in relation to our population, but this number of patients is likely to be insufficient for the best study of the risk factors involved in the use of PPIs.
Despite these limitations, we believe that this study provides relevant data such as the high rate of NSAIDs use among the population of internal medicine patients (30%) almost half of which is non-prescription. Similarly, prophylactic prescription of PPIs does not cover all patients with moderate or high risk of bleeding while unnecessary prescriptions of PPIs are given to a third of the patients at low risk. Based on the information we have provided in this article, we believe that dissemination of prevention guidelines regarding bleeding peptic ulcers among NSAID users could be key (8, 10, 11).
Another large area that remains to be explored is the burden and impact of H. pylori infections on these patients. The literature has shown that this infection is a risk factor for the development of adverse gastrointestinal events in patients who use NSAIDs, and there is evidence that demonstrates the usefulness of actively searching for and eradicating H. pylori in patients receiving NSAIDs/aspirin. This has recently been recommended by the IV Maastricht consensus (23) and the latest consensus of China on the management of H. pylori infection (24). In our country it has been found that this infection is the leading cause of duodenal ulcers (73%), followed by the consumption of NSAIDs (41%) and that both risk factors exist in a third of all patients (15).
An association of H. pylori infection with NSAIDs increases the risk of peptic ulcers by 64 times and increases the risk of bleeding ulcers over six times (25). Given the design of our study, it was impossible to get data regarding H. pylori infections, but our misgivings about this connection are supported by data from other Colombian patient populations showing that the prevalence of this infection ranges from 77% to 82% (26-28), although recently it was found that the prevalence in Bogotá is only 60% (29). In conclusion, prescription of PPIs for patients at high risk is higher than that reported in international studies (80% vs. less than 50%), but, as in many of those studies, our study shows that there is also unnecessary prescription of PPIs for patients without risk of bleeding ulcers. We consider that a multicenter study including more patients is need to better estimate the rate of NSAID use among these patients.
REFERENCES
1. Tenenbaum J. The epidemiology of nonsteroidal anti-inflammatory drugs. Can J Gastroenterol J Can Gastroenterol. 1999;13(2):119-22. [ Links ]
2. Shaheen NJ, Hansen RA, Morgan DR, Gangarosa LM, Ringel Y, Thiny MT, et al. The burden of gastrointestinal and liver diseases, 2006. Am J Gastroenterol. 2006;101(9):2128-38. [ Links ]
3. Laine L. Approaches to nonsteroidal anti-inflammatory drug use in the high-risk patient. Gastroenterology. 2001;120(3):594-606. [ Links ]
4. Risser A, Donovan D, Heintzman J, Page T. NSAID prescribing precautions. Am Fam Physician. 2009;80(12):1371-8. [ Links ]
5. Lee I, Cryer B. Epidemiology and role of nonsteroidal antiinflammatory drugs in causing gastrointestinal bleeding. Gastrointest Endosc Clin N Am. 2011;21(4):597-612. [ Links ]
6. Lanas A, Hunt R. Prevention of anti-inflammatory drug-induced gastrointestinal damage: benefits and risks of therapeutic strategies. Ann Med. 2006;38(6):415-28. [ Links ]
7. Malfertheiner P, Chan FKL, McColl KEL. Peptic ulcer disease. Lancet. 2009;374(9699):1449-61. [ Links ]
8. Lanza FL, Chan FKL, Quigley EMM, Practice Parameters Committee of the American College of Gastroenterology. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104(3):728-38. [ Links ]
9. Rostom A, Dube C, Wells G, Tugwell P, Welch V, Jolicoeur E, et al. Prevention of NSAID-induced gastroduodenal ulcers. Cochrane Database Syst Rev. 2002;(4):CD002296. [ Links ]
10. Abraham NS, Hlatky MA, Antman EM, Bhatt DL, Bjorkman DJ, Clark CB, et al. ACCF/ACG/AHA 2010 expert consensus document on the concomitant use of proton pump inhibitors and thienopyridines: a focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. Am J Gastroenterol. 2010;105(12):2533-49. [ Links ]
11. Lanza FL. A guideline for the treatment and prevention of NSAID-induced ulcers. Members of the Ad Hoc Committee on Practice Parameters of the American College of Gastroenterology. Am J Gastroenterol. 1998;93(11):2037-46. [ Links ]
12. Clinard F, Bardou M, Sgro C, Lefevre N, Raphael F, Paille F, et al. Non-steroidal anti-inflammatory and cytoprotective drug co-prescription in general practice. A general practitioner-based survey in France. Eur J Clin Pharmacol. 2001;57(10):737-43. [ Links ]
13. Abraham NS, El-Serag HB, Johnson ML, Hartman C, Richardson P, Ray WA, et al. National adherence to evidence-based guidelines for the prescription of nonsteroidal anti-inflammatory drugs. Gastroenterology. 2005;129(4):1171-8. [ Links ]
14. Valkhoff VE, van Soest EM, Sturkenboom MCJM, Kuipers EJ. Time-trends in gastroprotection with nonsteroidal anti-inflammatory drugs (NSAIDs). Aliment Pharmacol Ther. junio de 2010;31(11):1218-28. [ Links ]
15. Otero W, Gómez M, Ruiz X. Etiología de las úlceras duodenales en una población colombiana. Rev Col Gastroenterol 2009; 24: 266-271. [ Links ]
16. Ballina J, Carmona L, Laffon A, Grupo de Estudio EPISER. Impacto del consumo de AINES en la población general española. Resultados del estudio EPISER. Rev Esp Reumatol. 2002;29:337-42. [ Links ]
17. Lanas A, Tornero J, Zamorano JL. Assessment of gastrointestinal and cardiovascular risk in patients with osteoarthritis who require NSAIDs: the LOGICA study. Ann Rheum Dis. 2010;69(8):1453-8. [ Links ]
18. Talley NJ, Evans JM, Fleming KC, Harmsen WS, Zinsmeister AR, Melton LJ 3rd. Nonsteroidal antiinflammatory drugs and dyspepsia in the elderly. Dig Dis Sci. 1995;40(6):1345-50. [ Links ]
19. Ho CW, Tse YK, Wu B, Mulder CJJ, Chan FKL. The use of prophylactic gastroprotective therapy in patients with nonsteroidal anti-inflammatory drug- and aspirin-associated ulcer bleeding: a cross-sectional study. Aliment Pharmacol Ther. 2013;37(8):819-24. [ Links ]
20. Smalley W, Stein CM, Arbogast PG, Eisen G, Ray WA, Griffin M. Underutilization of gastroprotective measures in patients receiving nonsteroidal antiinflammatory drugs. Arthritis Rheum. 2002;46(8):2195-200. [ Links ]
21. Abraham NS, El-Serag HB, Johnson ML, Hartman C, Richardson P, Ray WA, et al. National adherence to evidence-based guidelines for the prescription of nonsteroidal anti-inflammatory drugs. Gastroenterology. 2005;129(4):1171-8. [ Links ]
22. Lanas A. Economic analysis of strategies in the prevention of non-steroidal anti-inflammatory drug-induced complications in the gastrointestinal tract. Aliment Pharmacol Ther. de 2004;20(3):321-31. [ Links ]
23. Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon ATR, Bazzoli F, et al. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61(5):646-64. [ Links ]
24. Chinese Society of Gastroenterology, Chinese Study Group on Helicobacter pylori, Liu WZ, Xie Y, Cheng H, Lu NH, Hu FL, et al. Fourth Chinese National Consensus Report on the management of Helicobacter pylori infection. J Dig Dis. 2013;14(5):211-21. [ Links ]
25. Huang JQ, Sridhar S, Hunt RH. Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic-ulcer disease: a meta-analysis. Lancet. 2002;359(9300):14-22. [ Links ]
26. Campuzano-Maya G, Hoyos-Castaño D, Calvo-Betancur VD, Suárez-Ramírez OA, Lizcano-Cardona D, Rojas-Arbeláez CA. [Prevalence of Helicobacter pylori infection in physicians in Medellín, Colombia]. Acta Gastroenterol Latinoam. junio de 2007;37(2):99-103. [ Links ]
27. Bravo LE, Cortés A, Carrascal E, Jaramillo R, García LS, Bravo PE, et al. Helicobacter pylori: patología y prevalencia en biopsias gástricas en Colombia. Col Med 2003; 34:124-31. [ Links ]
28. Porras C, Nodora J, Sexton R, Ferreccio C, Jimenez S, Dominguez RL, et al. Epidemiology of Helicobacter pylori infection in six Latin American countries (SWOG Trial S0701). Cancer Causes Control CCC. 2013;24(2):209-15. [ Links ]
29. Trespalacios AA, Rimbara E, Otero W, Mercado M, Caminos JE, Reddy R, Graham D. PCR Alelo-Específica para detección de resistencia de Helicobacter pylori a claritromicina y fluoroquinolonas en biopsias gástricas de Colombia incluyendo la identificación de una nueva mutación en gyrA; manuscrito en preparación. [ Links ]











 texto em
texto em