Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista colombiana de Gastroenterología
versão impressa ISSN 0120-9957
Rev Col Gastroenterol vol.30 no.3 Bogotá jul./set. 2015
Utility of Probe-based (Cellvizio) Confocal Laser Endomicroscopy in Gastroenterology
Elías Alfonso Forero Piñeros MD. (1), Héctor José Cardona MD. (2), Kunal Karia MD. (3), Amrita Sethi MD. (4), Michel Kahaleh MD. (5)
(1) Gastroenterologist and Chief of Gastroenterology and Endoscopy at the Central Police Hospital in Bogota, Colombia.
(2) Gastroenterologist and Chief of Gastroenterology and Hospital Simon Bolivar and GastroinvestSAS in Bogota, Colombia.
(3) Internist and Fellow in Endoscopy at Presbyterian Hospital and Weill Cornell Medical College at Cornell University in Ithaca, New York USA.
(4) Gastroenterologist and interventional endoscopist at Columbia University in New York, New York USA.
(5) Gastroenterologist, interventional endoscopist, Chief of Endoscopy and Professor of Clinical Medicine at Weill Cornell Medical College in Ithaca, New York USA.
Received: 08-09-14 Accepted: 21-07-15
Abstract
Probe based confocal laser endomicroscopy (Cellvizio Mauna Kea, Paris) is a new technology that allows performance of histological analysis (optical biopsy) during any endoscopic procedure. This improves diagnosis and helps define the treatment needed for multiple digestive diseases. Its utility for diseases that are difficult to diagnose such as indeterminate biliary strictures and pancreatic cystic neoplasms is noteworthy. Early and accurate diagnoses can be very difficult with currently available techniques, but they are exactly what are needed to determine whether or not expensive surgical treatments with great potential morbidity, for example the Whipple procedure, need to be performed. This review looks at the contribution that this technology can make in our country where it is now available for the diagnosis and study of digestive diseases.
Keywords
Confocal laser endomicroscopy, gastrointestinal cancer, early detection.
Gastrointestinal cancer contributes significantly to overall cancer morbidity and mortality rates. Early detection improves patient outcomes, but the current clinical practice still fails to achieve this in most cases even though early detection allows for less invasive, less expensive and more successful treatments than the current practice (1).
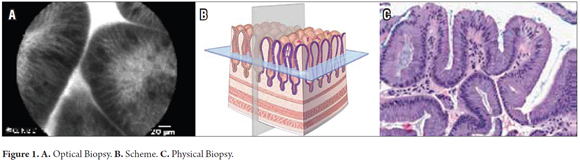
Confocal laser endomicroscopy, also called optical biopsy, is a new technology that provides very high resolution images through fiber optic probes which are passed through channels of endoscopy equipment. They produce images with resolutions of 1 to 2 microns in a field of view of about 500 to 700 microns. An image of fluorescent light reflected within a cell or on the cell surface is captured by exogenous fluorescein-type fluorescent contrast agents such as acriflavine or proflavine. Through this method, in vivo imaging of the microscopic architecture of tissue is achieved. This allows for immediate histopathological evaluation and diagnosis within the endoscopic procedure (Figure 1).
Neoplastic changes are usually accompanied by an increase in the density of microvessels, progressive loss of regularity, and disorganization of cell structure but also by capacity of cells to absorb fluorescein. As a result, cells turn dark in this examination and, in general, these findings this technology can be used for early diagnosis.
The probes used in these studies consist of more than 10,000 optical fibers. Each probe has different specifications to allow evaluations at different levels of the digestive tract and biliopancreatic system. Diameters range from 0.9 mm to 2.5 mm and have different resolutions and depths of examination. Each has a half-life of 20 procedures (2). Available probes include:
- Esophageal and gastric (Gastroflex - Mauna Kea): diameter of 2.6 mm; lateral resolution of 1 micron. Magnifies 1000 times.
- Biliary (CholangioFlex miniprobe - Mauna Kea): less than 1 mm diameter; lateral resolution of 3.5 micron. Magnifies 400 times (3, 4).
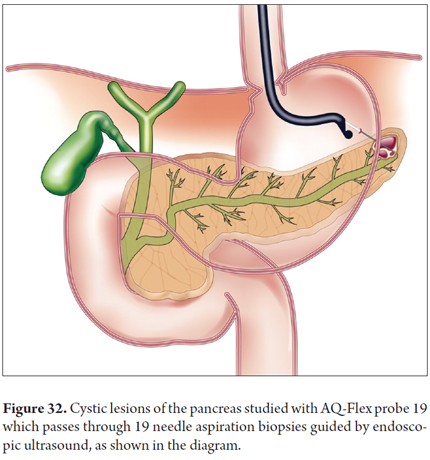
- Intra-needle (AQ-Flex 19 - Mauna Kea). For passing through aspiration needles guided by 19 G ultrasound. Useful for study of cystic pancreatic lesions. Diameter of 0.85 mm; Field of view of 320 microns. Lateral resolution of 3.5 micron. Length of 4 m, depth of penetration of 30 to 70 microns (5).
- Colonic (Coloflex - Mauna Kea): diameter of 2.6 mm; 1 micron resolution. Field of view of 240 microns. Penetration of 65 microns (Figure 2) (6).
An injection of 2.5 ml of intravenous fluorescein should be injected 10 seconds before starting the examination. The fluorescein will remain in the cells for up to 45 minutes, but eight minutes after administration image quality begins to deteriorate significantly. Consequently, it is recommended that evaluation be done during this eight minute initial time period. Adverse reactions to administration occur in between 1% and 6% of patients. Most are mild. They include nausea, transient skin discoloration, transient hypotension, injection site erythema, fuzzy self-limiting rash, and mild epigastric pain. The overall rate of adverse events is 1.4%. Anaphylaxis has not been reported. Fluorescein is not used in pregnant or lactating women (7-10).
Occasionally, acriflavine is used as a topical contrast agent in the colon, but it has been found to have carcinogenic potential in animals so its use has been limited.
This review shows the potential of confocal microscopy to improve early diagnosis of digestive tumors that are potentially malignant.
BARRETT'S ESOPHAGUS AND ESOPHAGEAL CANCER
The incidence of esophageal adenocarcinoma is increasing. Early detection increases five-year survival rates by 2.8% for advanced cases up to 49.3% for local tumors. However, if diagnosis is made at stage 0, the five-year survival rate increases to 95%. The precursor, Barrett's esophagus, evolves into low-grade dysplasia, then to high-grade dysplasia (intraepithelial or tumor), then to early cancer and finally to invasive cancer.
Current high definition white light endoscopy (HDWLE) with narrow-band imaging (NBI) and chromoendoscopy cannot clearly discriminate among simple columnar epithelium, Barrett's esophagus and areas of dysplasia, especially low-grade dysplasia. Malignancy in these patients is not clearly evident even with careful endoscopic inspection. The currently accepted Seattle protocol for follow-up calls for random biopsies from the four quadrants: every 2 centimeters when there is no suspicion of dysplasia, and every centimeter if there is suspicion. Nevertheless, even when this protocol is followed, 48% of early neoplasms are not diagnosed. In any case, the average endoscopist does not usually follow this protocol which makes it essential to improve diagnostic techniques through better imaging techniques during endoscopy with or without endoscopically guided biopsies (11).
After esophagectomy to treat high-grade dysplasia unsuspected foci of adenocarcinoma are found in 40% of the patients, but the Seattle protocol calls for study of only 3.5% of the mucosal surface at risk.
The addition of HDWLE and optical and electronic chromoendoscopy magnification with NBI have reduced the number of biopsies needed from each patient, but dysplasia is still seriously underdiagnosed. Other emerging techniques such as autofluorescence (Olympus), have failed to show any benefits because, even though they have higher detection sensitivity, they produce up to 40% false positives. The addition of chemical chromoendoscopy with 5% iodine detects additional lesions, but has little routine clinical application. The mixture of these techniques increases accuracy, but still does not detect every case.
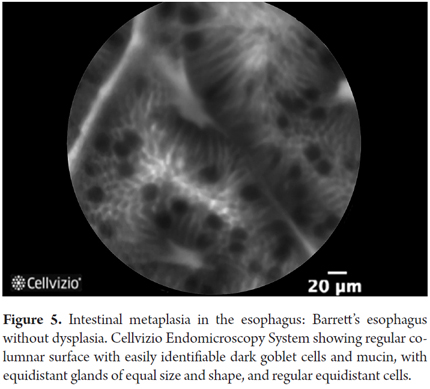
There are also many studies of the usefulness of confocal laser microscopy (Cellvizio, Mauna Kea) for the study of this disease. Generally, these studies describe views of columnar epithelium with goblet cells which are dark because the mucin contained in them does not capture fluorescein well. In addition, the progressive disruption of the vascular structure produces thicker, irregular vessels and disruption of the epithelial structure which results in fewer cells to absorb the fluorescein which also results in darker and disordered images. Gradually, confocal laser endoscopy (CLE) diagnostic criteria have been developed for this condition:
In 2008, the first five CLE diagnostic criteria suggestive of neoplasia in cases of Barrett's esophagus were developed. They are considered to be highly suggestive of malignancy when two or more are present.
- Irregular epithelial lining
- Variable thickness of epithelial lining
- Irregular vascular pattern
- Merger of glands
- Dark areas suggestive of decreased uptake of fluorescein
For both high-grade dysplasia and early cancer, these diagnostic criteria have a sensitivity of 75%, a specificity from 89% to 91%, a positive predictive value (PPV) of 30% to 35%, a negative predictive value (NPV ) of 98%, accuracy (EXAC) of 88% to 90%, and good interobserver agreement (IOA) with a kappa of 0.6. Adding HDWLE and NBI has resulted in a sensitivity of 100%, specificity of 83%, NPV of 67%, and PPV of 100%. Because the negative predictive value is so low, the Miami Classification has been developed. It consists of the following criteria:
- Irregular Vessels
- Merger of crypts and villi
- One of three of the following: irregular epithelial thickness, absence of epithelial homogeneity, dark epithelial edges.
With this classification, experts have achieved 91% sensitivity, 100% specificity, 95% accuracy, and near-perfect interobserver agreement (kappa = 0.83). Non-experts have achieved 50% to 75% sensitivity, 93% to 97% specificity, and a Kappa of 0.64.
According to the Miami classification, the characteristics of each stage of disease are as follows:
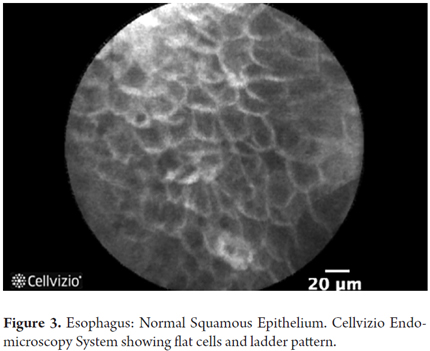
- Normal squamous epithelium: squamous cells without crypts or villi and with bright vessels located within papillae.
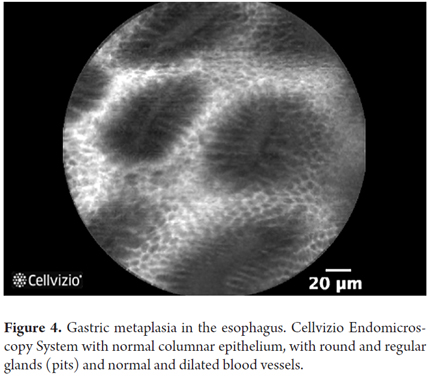
- Barrett's esophagus without dysplasia: villiform architecture with uniform columnar cells regularly interspersed with dark goblet cells.
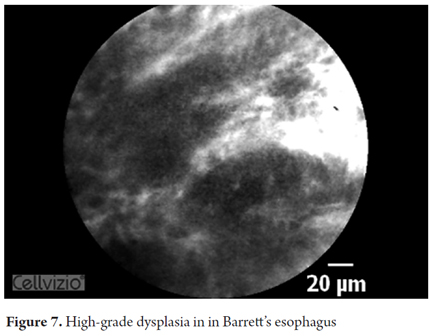
- Barrett's esophagus with high-grade dysplasia: irregular villiform architecture with dark edges and irregular, thickened, dilated vessels.
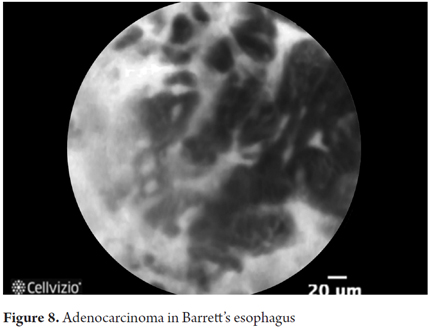
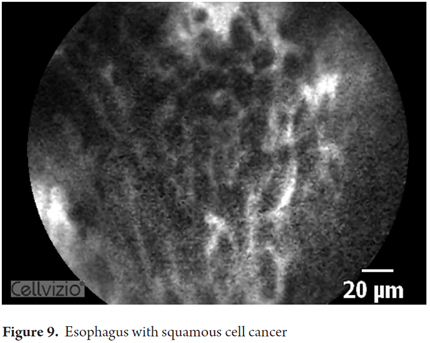
- Adenocarcinoma: irregular, disorganized non-villiform with multiple dark columnar cells and irregular dilated vessels.
After a short formal training session, use of this classification has shown good accuracy and interobserver agreement among both experts and non-experts.
The best images are obtained with the probe perpendicular to the mucosa. Some endoscopists use a 4mm cap at the tip of the endoscope use suction towards the cap. Images must be taken before the biopsy because bleeding may obscure the image as the result of escaping fluorescein.
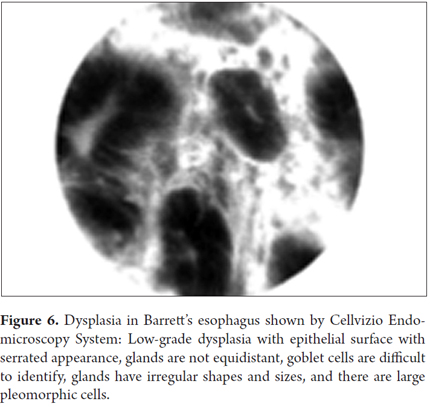
Another specific classification for the diagnosis of dysplasia was recently created. It has the following criteria:
- Glands of unequal size and shape
- Glands not equidistant
- Difficult to identify goblet cells
- Pleomorphic cells
- Enlarged cells
- Sawtooth epithelial surface
When two or more of these criteria are present, this classification for diagnosis of dysplasia has been shown to have 75% sensitivity, 90% specificity, 82% PPV, 85% NPV, 84% accuracy and good interobserver agreement among experts (Kappa = 0.66).
The use of this classification system in combination with the Miami classification system facilitates identification of neoplasia in patients with Barrett's esophagus. The use of CLE diminishes the need for biopsies to diagnose neoplastic lesions. In current practice, suspicious sites are marked with argon plasma to facilitate taking of biopsy samples and treatment. It is believed that this is most useful in cases in which there is doubt about using HDWLE and NBI. Confocal microscopy seems like the perfect complement in these cases. In general, CLE has the following uses in cases of Barrett's esophagus:
- Additional detection of neoplasia. Probe-based confocal laser endomicroscopy (pCLE) is certainly superior to HDWLE (p = 0.002), especially in apparently normal flat areas. pCLE has twice the sensitivity of HDWLE for diagnosing dysplasia and cancer (positive predictive value of 68.3% vs. 34.2% with HDWLE alone). Besides its high negative predictive value with the sum of criteria mentioned, it provides the best test for excluding neoplasia in Barrett's esophagus. The use of pCLE could improve the effectiveness of monitoring programs for these patients (12, 13).
- pCLE immediately changes the diagnosis of patients 34% of the time resulting in changes in treatment.
- pCLE reduces the need for biopsies, and therefore the costs of biopsies, by 80%. Nevertheless, for now, pCLE does not completely eliminate the need for biopsies (14).
- pCLE is easy to interpret for Barrett's esophagus and the learning curve for this condition is short.
- pCLE is a guide for therapy. When areas of high-grade dysplasia are found, the attending physicians can proceed immediately to mucosectomy (EMR) or endoscopic submucosal dissection (ESD) in the same procedure depending on the protocol of each institution. There is no need to wait a week or more for pathology results that require a second stage of the procedure. This could also affect both costs and morbidity.
- The use of pCLE together with NBI and high-definition endoscopy achieves detection of most dysplasia with a negative predictive value of 96% and a sensitivity of almost 100%.
- pCLE identifies sites of residual Barrett's esophagus in patients undergoing radiofrequency ablation. It is also useful for reviewing margins of other types of treatment, such as endoscopic submucosal dissection (ESD) and endoscopic mucosal resection (EMR). The use of pCLE reduces both overtreatment and undertreatment although studies are required to define the criteria in these cases and the cost-effectiveness of pCLE. A recent study of these types of cases found no evidence that the use of pCLE improves diagnosis of residual neoplasia compared with the use of HDWLE alone (15).
The use of pCLE to study Barrett's esophagus has also been criticized. Some studies have shown rates of false positives for low-grade dysplasia of up to 64% for which reason it must be insisted that diagnoses be confirmed with biopsies of suspicious areas. Other studies only speak of real usefulness for flat lesions, and therefore of the need for these studies at referral centers only rather than in all endoscopy services. They justify this on the basis of the very low prevalence of early neoplastic lesions among these patients. When the lesion is suspected after HDWLE, the pretest probability of malignancy is so high that the addition of another diagnostic technique would be questionable especially since a negative result does not necessarily rule out malignancy, and thus does not change treatment (16). Additional research may finally clarify the subgroup of patients among whom this technology is most cost effective and useful.
Images showing the usefulness of Cellvizio for Esophageal Pathology: See Figures 3, 4, 5, 6, 7, 8 y 9.
THE STOMACH
There are limitations on the use HDWLE and NBI for diagnosis in the stomach similar to, but less severe than, those in the esophagus. Limitations of endoscopic diagnosis of incomplete intestinal metaplasia, of gastric dysplasia and of early gastric cancer with only HDWLE, NBI, and the use of magnification are not as great in the stomach as in the esophagus primarily thanks to development of the Japanese criteria (Yao et al.). Although excellent results with only the combination of NBI and magnification have been achieved in Japan and elsewhere in the world, still only 10% of early gastric cancer cases are diagnosed.
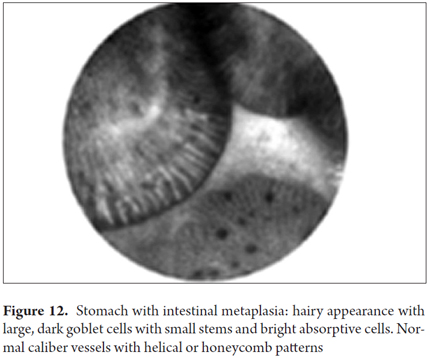
In these cases, pCLE provides diagnostic benefits, but less than in the esophagus. Consequently, there are fewer studies so definition of the role of pCLE in clinical practice will take longer and will require cost-effectiveness studies. The training required is short, as is interobserver agreement (17). pCLE can identify the goblet cells that typically define intestinal metaplasia and can clearly show microvilli which allows identification of columnar goblet cells with brush borders. Studies have shown that, for early gastric cancer, pCLE has 81.8% sensitivity, 90.9% specificity, and 96.2% accuracy. Isolated studies have also reported that pCLE is useful for diagnosing gastric lymphoma and for differentiating adenomatous polyps, hyperplastic polyps and fundic gland polyps (FGPs) with an accuracy of 97%. By using acriflavine contrast, Helicobacter pylori infections can be diagnosed with 83% sensitivity and 91% specificity, gastric atrophy can be diagnosed with 93% sensitivity and 95% specificity, and metaplasia can be diagnosed with 99% sensitivity and 100% specificity. Studies are needed to define the clinical utility of pCLE in this patient group.
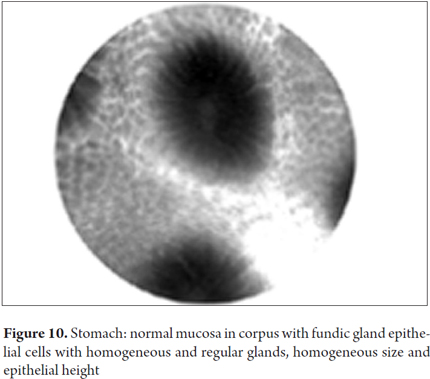
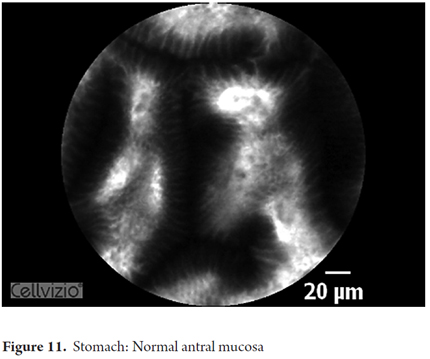
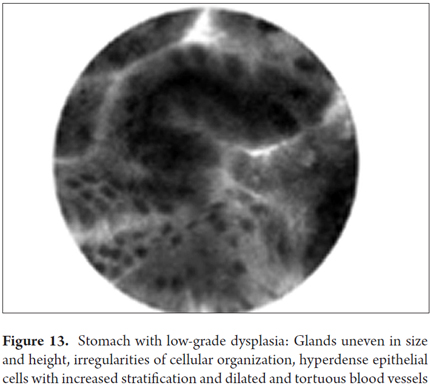
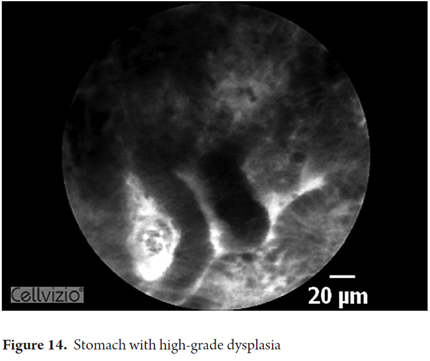
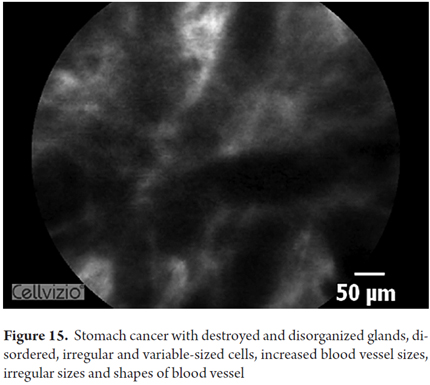
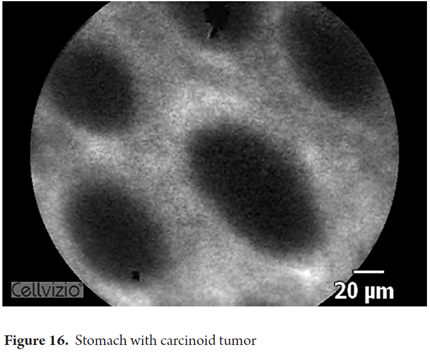
Images showing the usefulness of Cellvizio for Gastric Pathology: See Figures 10, 11, 12, 13, 14, 15 y 16.
THE INTESTINE AND INFLAMMATORY BOWEL DISEASE
HDWLE only has 83% sensitivity and specificity between 6% and 94% for diagnosis of celiac disease. This means that it is necessary to improve endoscopic diagnosis of celiac disease. It is also necessary to improve the study of specific antibodies which are not 100% final, are expensive, and are not available everywhere. Moreover, underdiagnosis is common in mild cases. Utilizing the water immersion technique and adding optical magnification can increase the sensitivity to 94% and specificity to 88% (18). The utility of pCLE has been demonstrated in diagnosis of villous atrophy in patients who have celiac disease, even when atrophy is mild to moderate. pCLE can identify increased amounts of intraepithelial lymphocytes with 94% sensitivity and 92% specificity. The clinical utility of pCLE for celiac disease still needs to be defined.
pCLE has also proven useful for early detection of changes associated microcirculatory deterioration due to sepsis in the intestinal mucosa (19), and early diagnosis of changes in the intestinal mucosa in graft-versus-host disease (GvHD). This is important because it is not always possible to take intestinal biopsies since most of these patients have had hematological transplants and have had bleeding disorders. pCLE can observe GvHD at an early stage in these patients by identifying the characteristic apoptotic bodies, alterations in the density of gap junctions and epithelial changes characteristic of crypts consisting of focal destruction of crypts and apoptosis. Early diagnosis of GvHD allows faster adjustment of immunosuppressive treatments and avoids biopsies (20).
Studies have been done of the entire small intestine. Double-balloon enteroscopy is used to pass the gastric or colonic probe through the intestine where it can detect all kinds of changes including loss of villi, atrophy, crypt hyperplasia, increased blood flow in inflammatory states and where it can be used to diagnose tumors. In the future, this will help with the management of many other intestinal diseases (21).
pCLE is also useful for the evaluating and monitoring inflammatory bowel disease (IBD). The probability of cancer increases in patients with ulcerative colitis 8 to 10 years after diagnosis. From that point on, annual colonoscopies are currently recommended after this time. The protocol calls for the taking of biopsy samples from suspicious lesions plus random biopsies at regular intervals of 10 cm. This normally requires taking up to 50 biopsy samples in each follow-up procedure. Nevertheless, this really only examines less than 1% of the colon's surface whereas dysplasia can occur in both inflamed areas and normal areas with no apparent lesions. One study has shown that a combination of chromoendoscopy and pCLE detected intraepithelial dysplasia 4.75 times more often than do conventional protocol biopsies (19 vs. 4, p = 0.005) and reduced the need for biopsies in half (21.2 vs. 42.2, p = 008) without increasing the average time of procedure. In these patients, pCLE also helps differentiate the mass associated with dysplasia from the simple adenomatous mass of patients with chronic ulcerative colitis. This helps define the required treatment but cannot be done with HD endoscopy alone. pCLE is also superior for defining subclinical inflammation in apparently normal mucosa by defining crypt architecture, microvascular alterations, and fluorescein leakage. It can also see intramucosal bacteria whose numbers increase in patients with active disease above the levels seen in normal patients. pCLE has also been used to detect subtle changes in ileal pouches in patients who have undergone restorative proctocolectomies. It can detect dysplasia and pouchitis at the subclinical level with sensitivity of 88.9% compared to 38.9% using HD endoscopy alone.
Similarly, for patients with Crohn's disease, pCLE seeks to define disease activity at an early stage but can also be used for early diagnosis of neoplasms during long-term monitoring. In this disease, the risk of cancer associated with colitis is 8% at 20 years, and 18% at 30 years when there is no associated liver involvement (primary sclerosing cholangitis). When primary sclerosing cholangitis is present, the risk increases to 33% at 20 years and to 40% at 30 years. The overall mortality rate from colon cancer among patients with inflammatory bowel disease is 15%. The combination of pCLE to HD colonoscopy can detect dysplasia in the colon with 93% sensitivity, 100% specificity, and 99% accuracy. In a follow-up study, low-grade intraepithelial neoplasia was found in 20% of patients. Sixty percent of the lesions were extremely small ones located in the right colon.
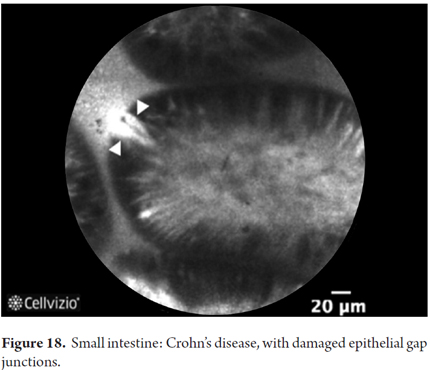
In active Crohn's disease, pCLE can be used to observe increases in the tortuosity and volume of colonic crypts, microscopic erosion, increased inflammation and inflammatory infiltrate in the lamina propria. In quiescent Crohn's disease, the number of crypts and goblet cells increases (22). For these patients, pCLE seeks to predict relapse of the disease for which the best predictors are extrusion of epithelial cells and associated epithelial barrier dysfunction. Quantitative analysis can be done to see increased changes in these barriers. The terminal ileum can also be viewed if there is colitis. Quantitative analysis can also be done of the structure of pits in the colon. This allows microscopic detection of residual inflammation with greater accuracy than endoscopy (23). Studies are still needed to define the role of pCLE in inflammatory bowel disease (24, 25).
The usefulness of pCLE has also been tested for other inflammatory diseases of the colon. High definition endoscopy is normally used in cases of microscopic colitis, but pCLE detects the shell formed by increased subepithelial collagen around the crypts which indicates the diagnosis (26). pCLE can identify early histological changes in Clostridium difficile infections which are associated with pseudomembranous colitis with 88.9% sensitivity and 96.3% accuracy. In addition, it can see the bacteria if acriflavine is used as a dye for FISH (fluorescence in situ hybridization). The rapid and accurate diagnosis of this infection is of crucial importance for patient treatment and for preventing nosocomial transmission (27).
Images showing the usefulness of Cellvizio for Small Intestine Pathology: See Figures 17 and 18.
POLYPS AND COLON CANCER
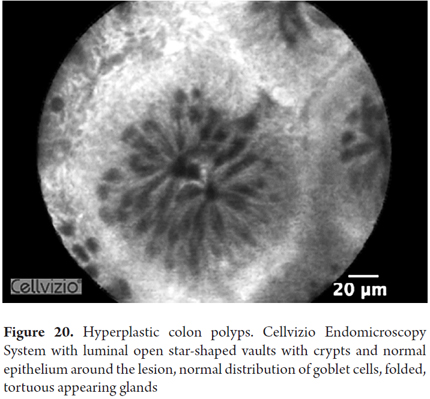
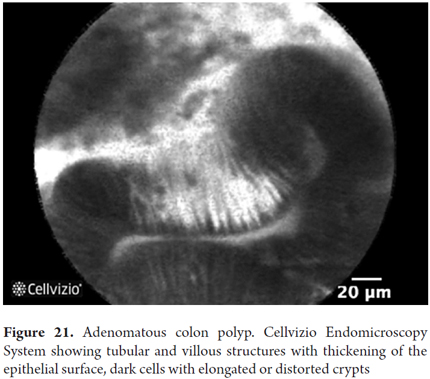
Colorectal cancer is the third among the different types of cancer and produces 9% of all deaths from this cause. Early detection increases five-year survival rates from 64% to 90%. Only two-thirds of colorectal cancer originates from adenomas, and up to one third originate from normal mucosa. Aberrant crypt foci have been proposed as very early stages of malignant transformation, but they are impossible to detect with colonoscopy alone. Colonoscopy does not detect 25% of polyps, particularly those which are very small. Mass screening programs to prevent colon cancer only prevent 65% of cases and cannot determine the possibility of development from some polyps. Consequently, additional improvements in imaging techniques is still needed. pCLE has shown that it can identify hyperplastic adenomatous polyps with 97% sensitivity and 96% specificity, and that it can identify tubular and villous adenomas with 100% sensitivity and 92% specificity. By adding NBI, specificity increases to 99%. In general, for definition of changes related to colonic neoplasia, pCLE has 97% sensitivity, 99% specificity and 99% accuracy. However, its use increases the duration of procedures and does not completely avoid biopsies. Moreover, interobserver agreement is not perfect (76%). For these reasons, the use of pCLE has not yet been included in routine screening programs. It has also been proven useful for detecting residual tumor after mucosal resection (28-30).
Other experimental applications for colon cancer evaluate microvascular density of tumors to define their development, monitor response to anti-oncogene and biological tumor progression (31).
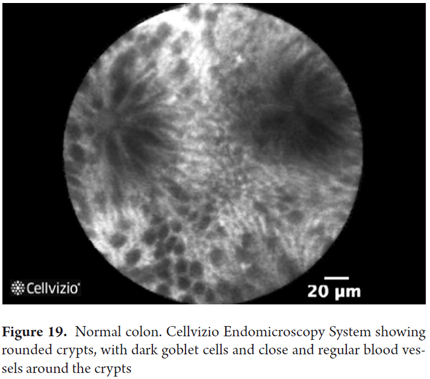
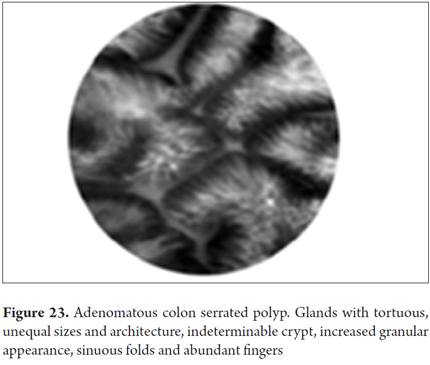
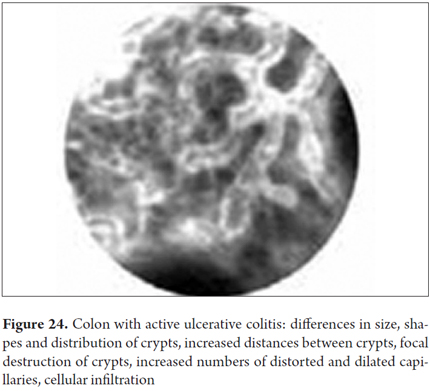
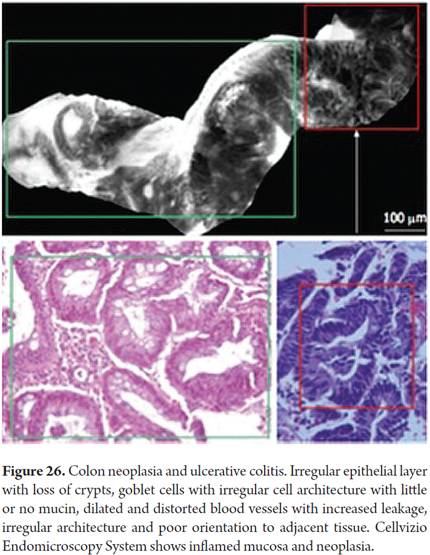
Images showing the usefulness of Cellvizio for Colonic Pathology: See Figures 19, 20, 21, 22, 23, 24, 25, 26 y 27.
BILIOPANCREATIC INDETERMINATE STRICTURES
Indeterminate biliary strictures are defined as those having at least one previous image mode, those with negative cytopathological evaluations in previous attempts at sampling (endo-biliary brushing or biopsy), during ERCP, in the absence of an alternative cause (32).
Indeterminate biliopancreatic strictures continue to be difficult diagnostic problems in medicine. Ninety-five percent of cholangiocarcinoma are adenocarcinomas some of which could be treated through surgical resection or liver transplantation if diagnosed early. In addition, inaccurate diagnoses still lead to many unnecessary operations. In 15% of the surgery performed to treat hilar cancer, the tumors turn out to be benign. Nevertheless, patients with stage T1 cholangiocarcinoma have excellent 5-year survival rates with surgical treatment. Inaccurate diagnostic methods for this condition usually slow and complicate treatment of patients who end up requiring more and more complex interventions (33).
The mere appearance through the endoscope in endoscopic retrograde cholangiopancreatography (ERCP) is not to sufficient to determine a diagnosis. Morphological criteria visible with radiology that suggest malignancy include long, irregular, asymmetric stenoses with abrupt transitions and nodular curvatures. Nevertheless, with the use of these criteria, ERCP can achieve diagnoses with 74% to 85% sensitivity, 70% to 75 specificity, positive predictive values of 74% to 78%, negative predictive values of 70% to 82%, and accuracy of 72% to 80%. Still, more than 30% of patients cannot be diagnosed with ERCP in clinical practice (34). Brush biopsies have only 30% to 60% sensitivity while forceps biopsies have 43% to 81% sensitivity. By adding brush biopsies of these stenosis the sensitivity becomes 30% to 57%, the specificity becomes 90% to 100%, PPV becomes 94% to 100%, and NPV goes to 8% to 62%. In patients with primary sclerosing cholangitis specificity decreases further. Adding brush biopsies increases sensitivity slightly. The best results are obtained when at least two methods for sampling tissue are combined. Brush biopsies combined with forceps biopsies have achieved 73.5% sensitivity. The combination of genetic analysis and FISH slightly improves sensitivity but is rarely available and is very expensive.
Endoscopic ultrasound cannot easily differentiate benign from malignant strictures of the common bile duct or the hilar area. It has a sensitivity of 78% and a specificity of 84%. Ultrasound-guided endoscopic cytological needle aspiration has sensitivities from 43% to 89% with specificity of 100% and PPV of 100%, but with NPV from 29% to 67% and accuracy from 70% to 91%. It also has the potential of producing peritoneal tumor seeding.
Intraductal endoscopic ultrasound (IDEU) has sensitivities from 83% to 91% with specificities from 50% to 92%, PPV from 92% to 96%, NPV from 67% to 100% and accuracy from 76% to 90%.
Cholangioscopy can detect irregular vessels is good for guiding biopsies. It has achieved sensitivities from 78% to 96%, specificities from 75% to 100%, PPV from 60% to 100%, NPV from 58% to 91% % and accuracy of 93.4%. When biopsies are done with the Spyglass system, the sensitivity is 82%, the specificity is 82%, PPV and NPV are 100%, and accuracy is 82%.
Evidence of the utility of pCLE evaluation of stenoses and intraductal tumors of the pancreas and biliary tree is emerging. pCLE provides real-time information about tissue and enables the physician to view both the epithelial and subepithelial tissue while incorporating dynamic information about blood flow, contrast uptake and contrast leaks. It can penetrate 40 to 70 microns below the surface epithelium and can be used to direct the taking of biopsies guided by cholangioscopy or fluoroscopy. The CholangioFlex miniprobe (Mauna Kea Technologies, Paris, France) is passed through a biliary catheter, Spyglass or cholangioscope channel. Bile does not affect the quality of the image. Initial descriptions of benign strictures have shown normal reticular patterns with gray, but not dark, scales. They do not show loss of mucosal structures irregularities in the epithelium. They show thin vessels (white elongated structures rather than thick structures). Neoplastic stenoses are shown on a dark gray background with poor mucosal structure and large irregular vessels. Initial pCLE studies of these strictures have achieved 83% sensitivity, 88% specificity and 86% accuracy. A more recent study of 102 patients who had indeterminate pancreatic and/or biliary stenosis showed that the diagnostic accuracy of ERCP plus tissue acquisition was 73%. By adding pCLE, sensitivity increased from 45% to 98%, negative predictive value increased from 69% to 97% and accuracy increased to 90% (p = 001).
The commonly accepted criteria for determining malignancy with pCLE in these indeterminate biliary strictures criteria are as follows:
- Loss of less than 20 microns of reticular pattern
- Irregular epithelial lining
- Villi or gland-like structures
- Loss of mucosal structures suggestive of fibrosis
- Sac-like vessels which are dilated and tortuous with inconsistent bifurcations
- Black areas of decreased fluorescein uptake
Of these, the presence of irregular vessels and loss of mucosal structures identified using the CholangioFlex miniprobe from Mauna Kea have the highest predictive power for malignancy (35, 36).
Subsequently the criteria have been improved by the development of the Miami criteria:
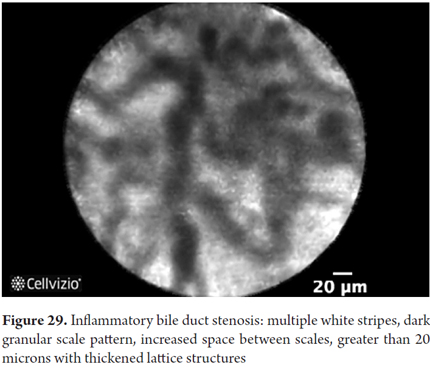
- Benign stricture: thin branching bands less than 20 microns in diameter, reticular network of dark bands and vessels less than 20 microns thick.
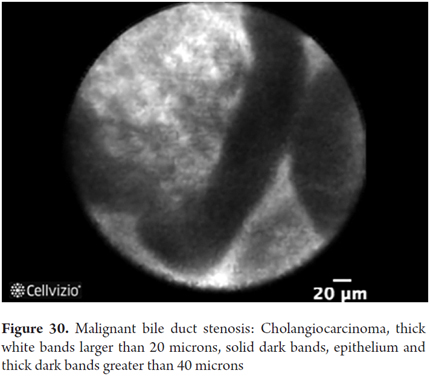
- Malignant stenosis: dark glandular clusters, bands of thick branches more than 20 microns thick and bright, thick, tortuos vessels of more than 20 microns thickness
After the development of criteria Miami, sensitivity increased to 98%, but specificity fell to 67%, and accuracy fell to 81% with many false positives for inflammation. In addition, interobserver agreement with the Miami criteria is poor for this condition although it improves with training (37, 38). For these reasons, particularly to reduce false positives, the classification of Paris was developed (39).
The criteria added for defining benign strictures include:
- Vascular congestion
- Thickened reticular structure
- Increased space between glands
- Small dark granular patterns with scales
With the modifications of the Paris classification to the Miami criteria, specificity of 75% to 83.3% and accuracy of 86% has been achieved. These criteria still need to be refined, and doctors need to be better trained in their use for this pathology.
For patients with dominant stenosis due to primary sclerosing cholangitis, diagnostic evaluation is much more difficult and there are fewer criteria. Among these patients, the incidence of cholangiocarcinoma varies between 7% and 14%. Despite using multiple modalities of tissue sampling, detection is still very difficult. pCLE permits a sufficiently good view of dominant stenosis in 95% of cases (40). A study that added pCLE to the usual histological sampling techniques to exclude neoplasia found 100% sensitivity, specificity increased from 61% to 94.4%. The study also found a positive predictive value of 22.2% and a negative predictive value of 89.5% to 100%. The combination is a good test for ruling out malignancy in these patients, but it also has poor interobserver agreement. pCLE could help determine the appropriate intervals for monitoring these patients in order to reduce repetition of ERCP and its associated risks and costs. It could also be used to determine when early surgery should be performed due to high levels of suspicion of malignancy. Although there are no specific criteria for this, the criteria for evaluating indeterminate strictures can be used.
Attempts to improve the diagnostic evaluation of ampullary lesions using pCLE have had poor results because of the lack of specific criteria and poor interobserver agreement. For this condition further studies are needed (41, 42).
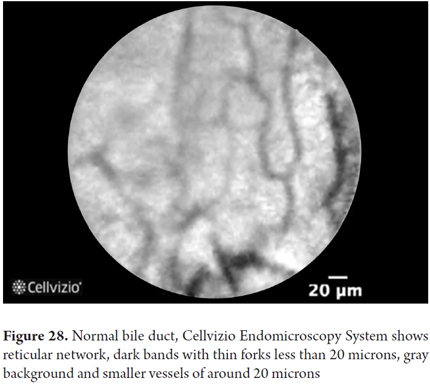
Images showing the usefulness of Cellvizio for biliary duct and pancreatic pathlogies: See Figures 28, 29, 30 y 31.
PANCREATIC CYSTIC NEOPLASMS
Pancreatic cysts are most often detected incidentally in abdominal imaging. Of these cysts, 60% are neoplasms, 30% are pseudocysts and 10% are congenital cysts or miscellaneous cysts. All cysts smaller than 2 cm are difficult to differentiate by imaging techniques alone (43). Pancreatic cystic neoplasms continue to be a diagnostic problem even with the development of endoscopic ultrasound and endoscopic ultrasound guided fine needle aspiration (EUS-FNA) for cytological evaluation of tumors and genetic markers which has improved diagnostic accuracy. Considerable difficulties persist and many unnecessary operations are still performed with high rates of morbidity and mortality (e.g. Whipple procedures) because the risk of malignancy cannot be completely ruled for many of these lesions. Endoscopic ultrasound provides diagnostic accuracy of only 51% to 73% for determining morphology. In addition, it over-diagnoses nodular wall cysts. This can be slightly improved by adding cytology and fluid studies, especially if carcinoembryonic antigen is measured. This increases accuracy to 79%. To date, genetic studies of liquid with K-ras and other RNA extraction analyses are experimental, expensive and unproven for diagnostic efficacy.
During pancreatoscopy, pCLE using the CholangioFlex miniprobe can help diagnose intraductal papillary mucinous neoplasms (IPMN) with the criteria for biliopancreatic stenosis. However, needle-based Confocal Laser-induced Endomicroscopy (nCLE, Cellvizio AQ-Flex prototype, Mauna Kea Technologies) has now been developed. It uses a special probe (AQ-Flex 19) which is passed through a 19G EUS-needle to study pancreatic cystic lesions in real time and define whether they have malignant potential and require surgery. Those with malignant potential include IPMN, mucinous cystadenoma, cystadenocarcinoma, cystic endocrine neoplasia while those which do not include serous cystadenomas and pancreatic pseudocysts. Prior to aspiration of fluid and tissue for analysis, puncture of the lesion is followed immediately by study of the image from confocal laser microscopy.
In the initial studies, nCLE was able to detect epithelial villous structures which were associated with neoplastic cysts. The sensitivity was 59% with 100% specificity, 100% PPV, and 50% NPV. Detection of these villous structures alone defines the need for surgical management. There were no serious complications, and the total complication rate was 9%: mild pancreatitis accounted for 3%, and the other 6% were cases of transient abdominal pain and bleeding that did not require additional intervention. These results showed low sensitivity but high specificity. nCLE still requires more evaluation of its safety.
Recently, Dr. Napoleon collected the diagnostic criteria for various pancreatic structures that are listed here (44):
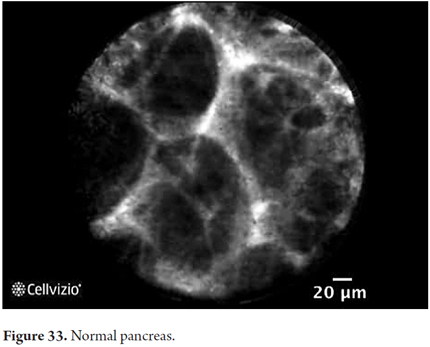
- Normal Pancreas: composed of oval gray structures (adipocytes) and dark lobular structures (acini)
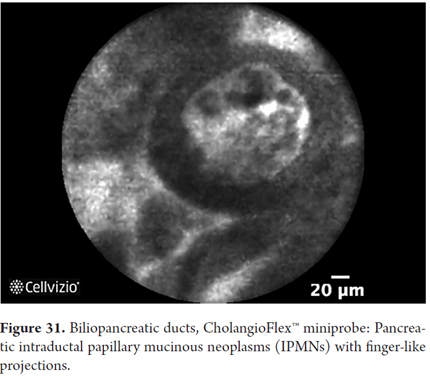
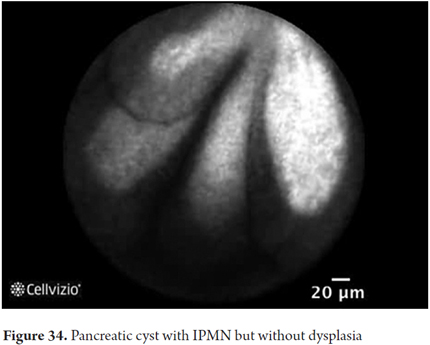
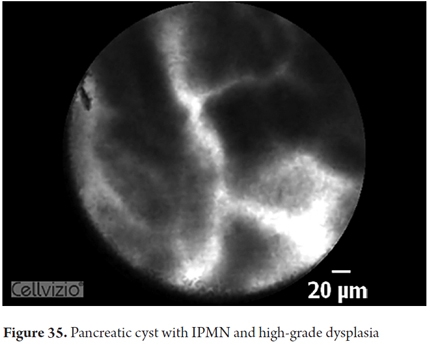
- IPMN: detection of papillas which in longitudinal view appear as fingerlike papillary projections but in transverse view appear as dark rings with light nuclei
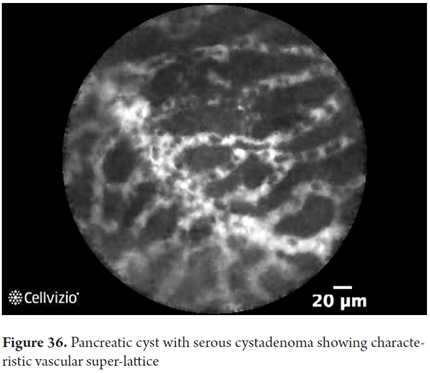
- Serous cystadenoma: thin superficial vascular network and connected vessels that are surprisingly dynamic
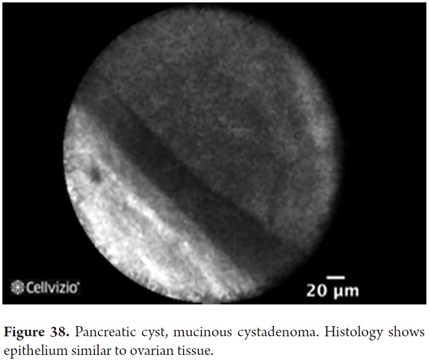
- Mucinous cystadenoma: cyst wall with epithelial border and normal vascular flow
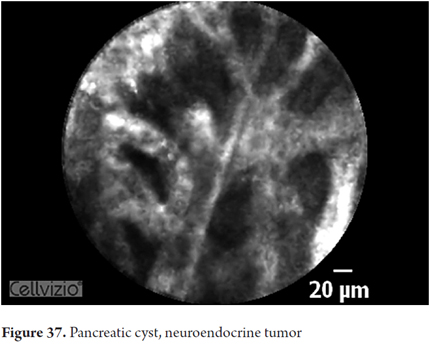
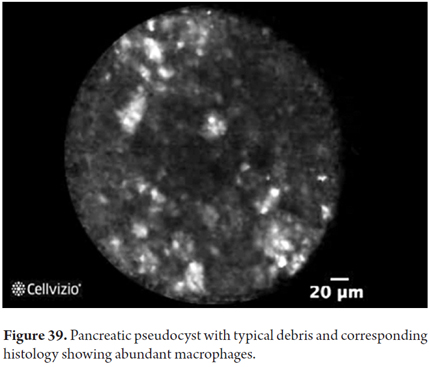
- Pseudocyst: dark field with glowing particles (macrophages).
There are isolated reports of the combination of Spyglass and nCLE increasing diagnostic sensitivity close to 100% without increasing the rate of complications. So far there are too few studies and improvement is still needed in the quality of the images from Spyglass, but this is still a promising combination.
Interest in ablation of some cysts is reviving. Publications show resolution of up to 80% of cases using ablation with ethanol and paclitaxel. Complications are acceptable and transient: abdominal pain in 7.9% of the cases and acute pancreatitis in 2% of the cases. It is not known whether these ablations also completely prevent the risk of subsequent malignancy. In addition, ablation is not applicable to IPMN since these cases have very high risks of pancreatitis and require very closer monitoring (45).
Work is currently underway to assess the usefulness of nCLE for diagnosis of pseudo-tumoral autoimmune pancreatitis and other types of pancreatitis.
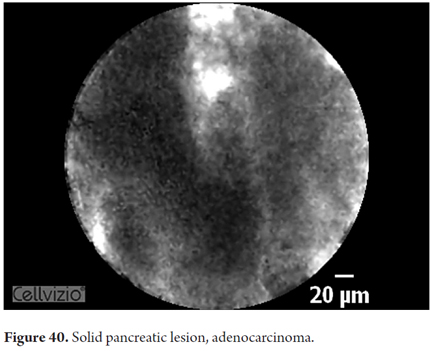
Images showing the usefulness of Cellvizio for pancreatic pathologies: See Figures 32, 33, 34, 35, 36, 37, 38, 39 y 40.
FUTURE DEVELOPMENTS
Currently, and simultaneously with advances in confocal laser microscopy, other technologies are being investigated. Many of them complement the existing diagnostic arsenal. Among them are:
- HRME (high-resolution microendoscopy) which is similar to CLE but with less expensive catheter technology. So far, there are very few studies.
- Optical coherence tomography
- Metabolomics Imaging
- Mass spectrometry and light scattering spectrometry
- Multiphoton microscopy
- Second generation harmonic imaging
- RAMAN Endoscopy
- Coherent scattering microscopy
- FLIM fluorescence microscopy imaging, etc.
In addition, new developments in CLE technology are under consideration:
Development of new nonspecific contrast media such as proflavine, lectins, agglutinin of wheat germ, agglutinin of Helix pomatia, and Aspergillus oryzae and lecithin
- Development of new specific contrast media such as fluorescent deoxyglucose which is preferentially taken up by tumor cells of Barrett's esophagus
- Simultaneous development of new classifications based on the new images
- Development of specific molecular and genetic contrast agents for each disease
- Specific antibodies of various diseases which can also be marked with fluorescein to improve visualization and diagnosis.
This remains an area in constant development whose progress will improve information on the structure and nature of tissues and allow increasingly accurate and early diagnosis of these diseases. The future is promising (46).
Acknowledgements
The authors thank Dr. Monica Gaidhane of Presbyterian Hospital of Cornel University in New York for her bibliographic assistance and Mr. Georges Tavary of the Mauna Kea company for providing some of the images included in this article.
REFERENCES
1. Carns J. Optical molecular imaging in the gastrointestinal tract. Gastrointest Endosc Clin N Am. 2013;23(3):707-23. [ Links ]
2. Templeton A. Confocal microscopy in the esophagus and stomach. Clin Endosc. 2013;46:445-9. [ Links ]
3. Shieh F. High-definition confocal endomicroscopy of the common bile duct. J Clin Gastroenterol. 2012;46(5):401-6. [ Links ]
4. Meining A. Classification of probe-based confocal laser endomicroscopy findings in pancreaticobiliary structures. Endoscopy. 2012;44:251-7. [ Links ]
5. Konda V. A pilot study of in vivo identification of pancreatic cystic neoplasms with needle-based confocal laser endomicroscopy under endosonographic guidance. Endoscopy. 2013;45:1006-13. [ Links ]
6. Liu J. Beyond white light endoscopy: The role of optical biopsy in inflammatory bowel disease. World J Gastroenterol. 2013;19(43):7544-51. [ Links ]
7. Humphris J. Status of confocal laser endomicroscopy in gastrointestinal disease. Trop Gastroenterology. 2012;33(1):9-20. [ Links ]
8. Coda S. State of the Art in advanced endoscopic imaging for the detection and evaluation of dysplasia and early cancer of the gastrointestinal tract. Clin Exp Gastroenterol. 2014;7:133-50. [ Links ]
9. Neumann H. Confocal laser endomicroscopy for in vivo diagnosis of clostridium difficile associated colitis- a pilot study. PLos One. 2013;8(3):e58753. [ Links ]
10. Dunbar KB. Endomicroscopy in Barretts esophagus. Gastrointest Endoscopy Clin N Am. 2013;23:565-79. [ Links ]
11. Hyun Ko K. Recent advances in molecular imaging of premalignant gastrointestinal lesions and future application for early detection of Barrett esophagus. Clin Endosc. 2014;49(1):7-14. [ Links ]
12. Sharma P. Real-time increased detection of neoplastic tissue in Barretts esophagus with probe-based confocal laser endomicroscopy: Final results of an international multicenter prospective, randomized, controlled trial. Gastrointest Endosc. 2011;74(3):465-72. [ Links ]
13. Bertani H. Improved detection of incident dysplasia by probe-based confocal laser endomicroscopy in a barretts esophagus surveillance program. Dig Dis Sci. 2013;58(1):188-93. [ Links ]
14. Canto MI. In vivo endomicroscopy improves detection of Barretts esophagus-related neoplasia: A multicenter international randomized controlled trial. Gastrointest Endosc. 2014;79:211-21. [ Links ]
15. Wallace M. Multicenter randomized controlled trial of confocal laser endomicroscopy assessment of residual metaplasia after mucosal ablation or resection of GI neoplasia in Barretts esophagus. Gastrointest Endosc. 2012;76:539-49. [ Links ]
16. Boerwinkel D. The clinical consequences of advanced imaging techniques in Barretts esophagus. Gastroenterology. 2014;146:622-9. [ Links ]
17. Pittayanon R. The learning curve of gastric intestinal metaplasia interpretation on the images obtained by probe-based confocal laser endomicroscopy. Diag Ther Endosc. 2012;2012:278045. [ Links ]
18. Ianiro G. Endoscopic tools for the diagnosis and evaluation of celiac disease. World J Gastroenterol. 2013;19(46):8562-70. [ Links ]
19. Schmidt C. Confocal laser endomicroscopy reliably detects sepsis-related and treatment-associated changes in intestinal mucosal microcirculation. Br J Anaesth. 2013;111(6):996-1003. [ Links ]
20. Hundorfean G. Highlighting crypt necrosis by using confocal laser endomicroscopy for the in vivo and real-time diagnosis of gi graft versus-host disease. J Clin Oncol. 2012;30(35):e368-9. [ Links ]
21. Miehlke S. Probe-based confocal laser endomicroscopy in double balloon enteroscopy. Z Gastroenterol. 2011;49(12):1529-34. [ Links ]
22. Neumann H. Assessment of Crohns disease activity by confocal laser endomicroscopy. Inflamm Bowel Dis. 2012;18(12):2261-9. [ Links ]
23. Musquer N. Probe-based confocal laser endomicroscopy: A new method for quantitative analysis of pit structure in healthy and Crohns disease patients. Dig Liv Dis. 2013;45:487-92. [ Links ]
24. Kraus E. Characterization of lymphoid follicles with rearing signs as first manifestation of early Crohns disease by conventional histopathology and confocal laser endomicroscopy. Int J Clin Exp Pathol. 2012;5(5):411-21. [ Links ]
25. Bessissow T. Advanced endoscopic imaging for dysplasia surveillance in ulcerative colitis. Expert Rev Gastroenterol Hepatol. 2013;7(1):57-67. [ Links ]
26. Neumann H. In vivo diagnosis of lymphocytic colitis by confocal laser endomicroscopy. Gut. 2013;62(2):333-4. [ Links ]
27. Hundorfean G. In vivo detection of mucosal healing-involved histiocytes by confocal laser endomicroscopy. World J Gastroenterol. 2012;18(32):4447-9. [ Links ]
28. Su P. Efficacy of confocal laser endomicroscopy for discriminating colorectal neoplasms from non-neoplasms: A systematic review and meta-analysis. Colon Dis. 2013;15(1):e1-12. [ Links ]
29. Goetz M. Real-time histology in colonoscopy. Gastrointest Clin North Am. 2013;42(3):567-35. [ Links ]
30. Shahid M. Diagnostic accuracy of probe-based confocal laser endomicroscopy in detecting residual colorectal neoplasia after EMR: A prospective study. Gastrointest Endosc. 2012;75:525-33. [ Links ]
31. Cartana T. Confocal laser endomicroscopy for the morphometric evaluation of microvessels in human colorectal cancer using targeted anti-CD31 antibodies. PLos One. 2012;7(12):e52815. [ Links ]
32. Wani S. Probe-based confocal laser endomicroscopy for the diagnosis of indeterminate biliary strictures. Curr Opin Gastroenterol. 2013;29:319-23. [ Links ]
33. Smith I, Kahaleh M. A review on the use of confocal laser endomicroscopy in the Bile Duct. Gastroenterol Res Pract. 2012;2012:454717. [ Links ]
34. Yoon WJ. Endoscopic evaluation of bile duct strictures. Gastrointest Endoscopy Clin N Am. 2013;23:277-93. [ Links ]
35. Loeser C. Confocal endomicroscopic examination of malignant biliary strictures and histologic correlation with lymphatics. J Clin Gastroenterol. 2011;45(3):246-52. [ Links ]
36. Yoon WJ. Endoscopic evaluation of bile duct strictures. Gastrointest Endosc Clin N Am. 2013;23(2):277-93. [ Links ]
37. Talreja J, Kahaleh M. Interpretation of probe-based confocal laser endomicroscopy of indeterminate biliary strictures: Is there any interobserver agreement? Dig Dis Sci. 2012;2338-6. [ Links ]
38. Kalaitzakis E. Establishing a diagnosis in indeterminate pancreatic-biliary strictures: Is confocal laser endomicroscopy the answer? Dig Dis Sci. 2012;57:3052-4. [ Links ]
39. Caillot F, Kahaleh M. Refined Probe-based confocal laser endomicroscopy classification for biliary strictures: The Paris Classification. Dig Dis Sci. 2013;2533-5. [ Links ]
40. Heif M. ERCP with probe-based confocal laser endomicroscopy for the evaluation of dominant biliary stenosis in primary sclerosing cholangitis patients. Dig Dis Sci. 2013;58(7):2068-74. [ Links ]
41. Ho HC. Confocal laser endomicroscopy of ampullary lesions, can we agree on black or white? J Clin Gastroenterol. 2013;47(5):377-8. [ Links ]
42. Bakhru MR, Kahaleh M. Interobserver agreement for confocal imaging of ampullary lesions: A multicenter single-blinded study. J Clin Gastroenterol. 2013;47(5):440-2. [ Links ]
43. Iftimia N. Cystic lesions of the pancreas: More reliable differentiation with in situ high-resolution optical imaging? Expert Rev Gastroenterol Hepatol. 2012;6(2):125-27. [ Links ]
44. Napoleón B. Criterios diagnósticos de las diferentes estructuras pancreáticas. En: VI Reunión Internacional de Usuarios de Cellvizio. Lisboa, Portugal; 2015. [ Links ]
45. Nakai Y. Role of endoscopic ultrasonograph in pancreatic cystic neoplasms: Where do we stand and where will be go. Dig Endosc. 2014;26:135-43. [ Links ]
46. Shahid M. Diagnostic accuracy of probe-based confocal laser endomicroscopy and narrow band imaging for small colorectal polyps: a feasibility study. Am J Gastroenterol. 2012;107:231-9. [ Links ]











 texto em
texto em