Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista colombiana de Gastroenterología
versão impressa ISSN 0120-9957
Rev Col Gastroenterol vol.30 no.3 Bogotá jul./set. 2015
Malignant Hepatic Neoplasms. Part 2. Morphology and important ancillary studies useful for histopathological diagnosis
Rocío del Pilar López Panqueva MD. (1)
(1) Medical Pathologist in the Anatomical Pathology Section at the Hospital Universitario Fundación Santa Fe de Bogotáand Professor in the Faculty of Medicine at Universidad de los Andes in Bogotá, Colombia.
Received: 15-06-15 Accepted: 06-08-15
Abstract
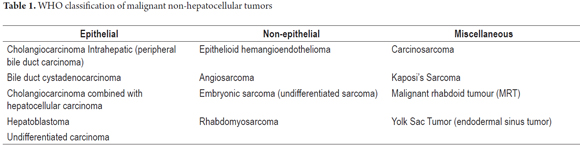
We continue with the review of primary malignant liver tumors. This article covers the most important aspects of primary tumors that are not hepatocellular. Those that originate in the epithelium of the bile duct such as cholangiocarcinoma, cystadenocarcinoma and mesenchymal tumors occur most frequently. Epithelioid hemangioendothelioma and angiosarcoma occur less frequently. The main difficulty lies in making a definitive diagnosis which must be based on the exclusion of extrahepatic primary neoplasms and benign liver lesions. Additional immunohistochemistry and molecular studies as well as diagnostic cloning of cells can be very useful.
Keywords
Malignant liver tumors, cholangiocarcinoma, cystadenocarcinoma, epithelioid hemangioendothelioma, angiosarcoma, immunohistochemistry.
For evaluation of a liver tumor, it is very important to remember the most common malignant tumors are metastases and that these are precisely the main problems for differential diagnosis of the tumors discussed in this article. As mentioned, neoplasms are classified by their cells of origin. Table 1 summarizes non-hepatocellular malignant tumors.
INTRAHEPATIC CHOLANGIOCARCINOMA
Cholangiocarcinoma (CCA) is an adenocarcinoma originating in the epithelium of the bile ducts of any portion of the intrahepatic biliary tree. It accounts for 10% to 20% of primary liver tumors and is the second most common malignant tumor. It accounts for 3% of all gastrointestinal cancers (1, 2).
Epidemiological data
CCA clearly has a geographical distribution that reflects variations in environmental factors and regional risk. The highest incidence has been described in Southeast Asia in Laos, northern Thailand, Japan and Korea where there are endemic infections by fasciola hepatica (common liver fluke), clonorchis sinensis and opisthorchis viverrini (Southeast Asian liver fluke) (1, 2).
The data show that the incidence of and deaths due to intrahepatic CCA is increasing at an alarming rate, by at least three times in recent decades, in several regions including the USA and UK even though incidence of extrahepatic CCA has been decreasing (2).
It is not clear why this is occurring. It might be an actual increase in the incidence of CCA, but more probably it is the result of because more diagnoses of CCA because immunohistochemistry, molecular methods and imaging techniques have all improved.
Most cases of CCA occur sporadically without a known etiological factor. Chronic inflammation and bile duct cell damage induced by obstruction of bile flow are two of the main conditions responsible for the development of CCA (3, 4).
The prevalence off CCA shows wide geographical variations with higher incidence in Asia, especially Thailand, Laos and southern China and Korea where the disease region is considered endemic (rates above 90%) and where infectious agents play an important role in its etiology. This is because cytokines released in the biliary microenvironment during inflammation are responsible for malignant transformations. This is associated with regional dietary factors that produce a synergistic effect on infections by opisthorchis viverrini (5, 6).
Etiology and pathogenesis
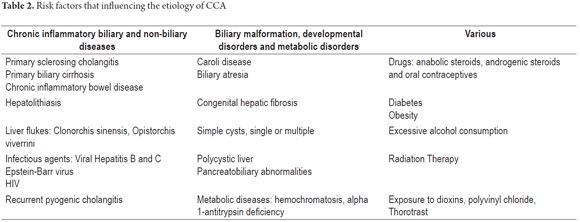
The etiology of most cases remains unknown. Among the many factors have been described are some clearly established risk associated with previous biliary and liver diseases. Those associated with biliary diseases include choledochal cysts, biliary-pancreatic malformations, chronic inflammation of the biliary tract due to primary sclerosing cholangitis (9% to 23% risk) and chronic inflammation due to hepatolithiasis while those associated with chronic liver disease include cirrhosis, biliary atresia, hepatitis B and C, liver fluke infections, clonorchis sinensis and opisthorchis viverrini. The last two are both classified as carcinogenic to humans by the International Agency for Research on CCA. Table 2 summarizes some of the risk factors (5-7).
Carcinogenesis is probably related to the duration and severity of exposure to etiologic trigger factors when known. For example, chronic inflammation of the bile duct induces an immune response in the host that damages DNA, increases endogenous production of mutagens in the bile, triggers production of free radicals, causes biliary stasis and ultimately results in abnormal bile duct epithelial cell proliferation. This occurs in steps from epithelial hyperplasia to dysplasia to noninvasive adenocarcinoma and finally to invasive adenocarcinoma (8, 9).
Mutations in oncogenes and tumor suppressor genes have been one of the mechanisms contributing to the development of CCA that have been studied most. Various groups have shown p53 mutations with overexpression of the protein in 25% to 75% of cases. Mutations of p62 c-myc, c-ras, p21, p10 and c-erb have also been described. Overexpression of bcl-2 which produces dysregulation and inhibition of apoptosis has also been described (5, 8, 9).
Important Clinical Data
CCA is more frequent over the sixth decade of life, and - although there appears to be no difference between men and women - most series refer to a predominance among men. It is an aggressive tumor with a high mortality rate due to early invasion, regional and distant metastases and an unfortunate lack of effective treatments.
The clinical picture depends on the anatomical location of the tumor, its growth pattern and the stage of disease (4).
Integration of 100% of the clinical information including imaging, tumor markers and histology is required for the diagnosis of cholangiocarcinoma. The main differential diagnosis is metastatic adenocarcinoma.
Classification
CCA is classified according to its anatomical location. Approximately 40% to 50% are intrahepatic (developing in small ducts, also called peripheral CEC). The rest are extrahepatic (40% develop between the hepatic hilum and the ampulla of Vater) and hilar (10% originate at the confluence of the right and left hepatic ducts) which are known as Klatskin tumors (1, 3).
Morphological, phenotypical and molecular patterns
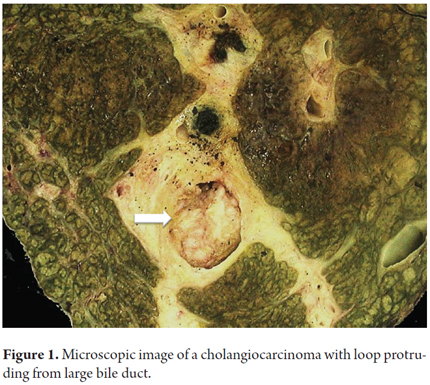
Macroscopically CCA may be diffuse of massive and nodular. Diffuse CCA is usually found in non-cirrhotic livers. They are firmer and whiter than HCC due to the greater amount of fibrous stroma. They are often multicentric. Three macroscopic types of cholangiocarcinoma have been recognized depending on the type growth: those that form masses (40-42%), intraductal (8-14%) (Figure 1), and periductal infiltrating cholangiocarcinoma (8-20%). Mixed types account for 26% to 42 % of CCA. This classification has better results and correlates well with images which allows for appropriate planning for surgical resection (1, 8, 10, 11).
Intraductal growth type has the best prognosis with a 10-year survival rate of 58%. Mass CCA has a three year survival rate of 40% and a five year survival rate of 21%. Periductal-invasive CCA has the worst prognosis with a 7% 5-year survival rate (8, 9).
The main problem for differential diagnosis is identification of whether the tumor is a primary such as hepatocellular carcinoma, is a metastatic adenocarcinoma, or is a benign lesion formed by a gland. This is especially true for differential diagnosis in a non-cirrhotic liver.
Histologically, CCA may resemble extrahepatic adenocarcinoma of any origin, so a reliable diagnosis depends on exclusion of potential primary sites.
Histological and cytological confirmation is essential for diagnosis of CCA, but they are not easy. This is especially due to issues of location, size and the marked desmoplasia often associated with this tumor. Similarly, metastatic adenocarcinomas are mainly derived from locations in the foregut such as the lungs, esophagus, stomach, and pancreas and are indistinguishable from CCA when you consider their morphological characteristics alone (12).
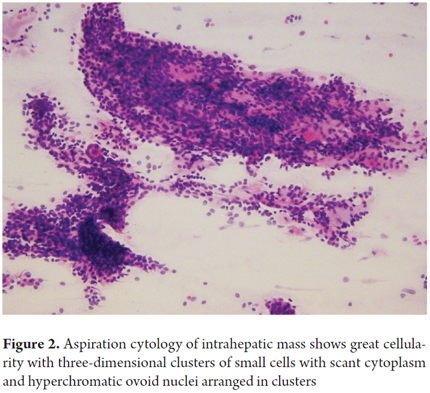
Samples for biliary cytology and brush cytology can be obtained by fine needle aspiration directed by ultrasound or CT scan, during endoscopic retrograde cholangiopancreatography, (ERCP), by endoscopic transpapillary biopsy or by percutaneous transhepatic cholangiography (Figure 2) (8, 9).
Brushing is widely used for evaluation of biliary strictures. Cytology has low sensitivity and is only positive in approximately 30% of cases of cholangiocarcinoma. Combining it with brush cytology biopsy allows a correct diagnosis in 40% to 70% of cases.
The World Health Organization classifies the histological types of CCA as adenocarcinoma, adenosquamous carcinoma, cholangiocellular carcinoma, lymphoepithelioma-like carcinoma (LELC) which has clear cells, and mucoepidermoid carcinoma. Adenocarcinoma is the most common and accounts for 90% of cases at least 80% of which may be classified into either cholangiolar carcinomas and conventional biliary carcinomas (13, 14).
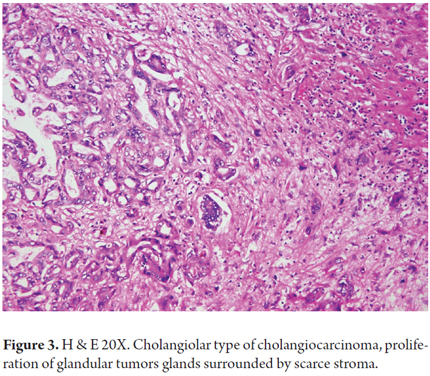
Cholangiolar carcinomas are formed by proliferation of glandular structures lined by low columnar tumor cells containing little eosinophilic cytoplasm. They can be arranged in anastomosing trabeculae, cribriform patterns, micropapillary patterns or solid patterns. They generally show high cellularity with little surrounding stroma (Figure 3). The cores are generally low-grade or intermediate grade. Lymphoepithelioma-like carcinoma is a variant with high-grade tumors with abundant lymphocytes infiltrating the tumor and surrounding glands. It has the best prognosis.
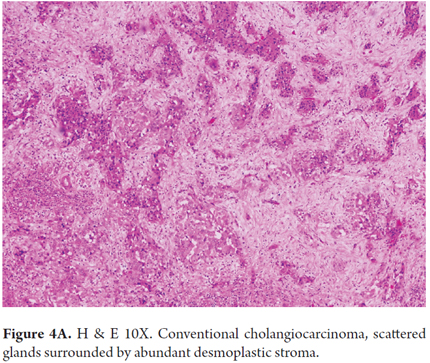
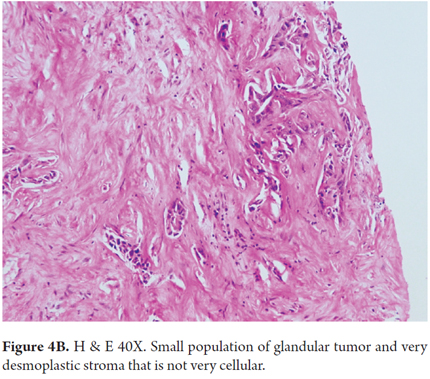
Conventional biliary carcinomas the most frequently observed. They are characterized by the proliferation of high columnar cells arranged in a large glandular pattern. They have lower cellularity and abundant desmoplastic stroma (Figures 4A and 4B). They can be identified by intraductal bile, tumor cells that line the ducts and have altered nuclei and cytoplasm and abundant eosinophilic or mucinous cytoplasm. They are more frequently associated with lymph node metastases and poor prognoses.
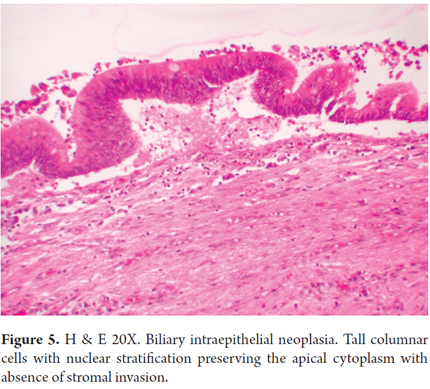
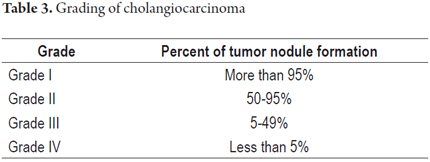
Precursor lesions such as biliary intraepithelial neoplasia and intraductal papillary neoplasms may be seen in both types (Figure 5). CCA is graded on the basis of the percentage of glandular formation (Table 3) and to the degree of differentiation (well differentiated, moderately differentiated and poorly differentiated).
The principal diagnostic difficulty is that metastatic adenocarcinomas and all variants of CCA are very similar morphologically. For this reason, immunophenotyping can be very useful, especially when there is no history of extrahepatic adenocarcinoma. However, because there no specific tumor markers for CCA, identifying a combination of two or more antibodies may assist in finding the source of an intrahepatic tumor (12).
Immunophenotyping can also be useful for differentiating the type of intrahepatic CCA. Bile duct CCA is typically negative for N-cadherin and positive for TFF1, S100P and AGR2, while the cholangiolar CCA is typically positive for N-cadherin and negative for TFF1, S100P and AGR2 (15),
Key points for diagnosis
- Medical history and images to discard extrahepatic adenocarcinoma, particularly elsewhere in the gastrointestinal tract and gall bladder, stomach, bile duct and pancreas
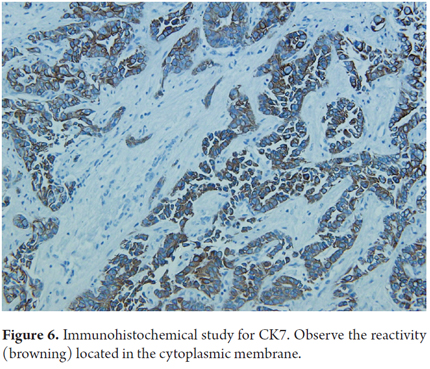
- Reactivity (90% of cases) for cytokeratin 7 and 19 (Figure 6)
- Reactivity (80-85% of cases) for polyclonal and monoclonal carcinoembryonic antigen (CEA) in non-canalicular membrane pattern. CA19-9, CD15 and epithelial membrane antigen (EMA) are expressed on the luminal surface, MOC31.
- Absence of or occasional expression of cytokeratin 20, CA125, and estrogen receptors (less than 20% of cases)
Intrahepatic CCA is a very heterogeneous tumor, so classifications have been developed from both morphological and molecular point of views for clinical consideration. Nevertheless, it is likely that each will respond differently to treatment (3).
Activation of KRAS mutations and loss of function of mutations of p53 are often found. DNA sequencing identifies mutations in KRAS in 22% of tumors and in BRAF in 45% of tumors. Mutations in EGFR, PIK3CA, NRAS and APC are found less frequently. Cholangiolar CCA is most often associated with viral hepatitis and mutations in oIDH2 and IDH1. In 23% of cases of CCA associated with biliary stones there are mutations of the K-ras oncogene. Overexpression of erbB-2 and COX-2 may announce an early carcinogenic event in the biliary tract. This is thought to be important for defining adjuvant chemoprevention or chemotherapy for CCA. It has also been shown that overexpression of c-MET and CD151 in metastatic and invasive CCA may have an effect on targeted therapy. Expression of transcription factors such as HIF-1α serves as an independent predictor of overall survival and of disease-free survival factor and correlates with advanced stage tumors, large tumors greater than 4 cm and with vascular invasion and metastasis.
These findings indicate that genetic alterations may vary significantly according to the etiological association. Most likely, future molecular and genomic studies will help refine this classification (3).
Prognosis
CCA's natural course of development is very aggressive. The average 24-month survival rate is low, even after potentially curative surgery in early stages. Currently, there are no curative medical therapies for CCA. If there is no surgical option, systemic chemotherapy is the standard practice for patients with advanced or metastatic disease, but survival rates are still very low. The desmoplastic stroma may prevent access of drugs to the tumor and may promote tumor survival signals while genetic heterogeneity makes curative treatment options difficult.
Differential diagnosis
As mentioned, metastatic adenocarcinoma is the main differential diagnosis to consider. But some other benign entities which are very similar morphologically must be considered in the differential diagnosis. These include epithelioid hemangioendothelioma, adenoma of the bile ducts, biliary adenofibroma, biliary microhamartomas (Von Meyenburg complexes), peribiliary cysts and multifocal hyperplasia of the peribiliary glands.
HEPATOBILIARY CYSTADENOCARCINOMA
Hepatobiliary cystadenocarcinoma is a rare cystic epithelial mucinous neoplasia which accounts for less than 3% of malignant tumors. There is usually no communication with the bile ducts. Preoperative diagnosis is difficult. Hepatobiliary cystadenocarcinoma also is an adenocarcinoma with a papillary pattern. It originates in multilocular cystadenoma less often than in unilocular ductal cysts. It grows slowly over a period of several years.
Epidemiological data
Although patients' ages range from 24 to 90 years, this is a tumor of adults with an average age of 60 years. It can occur in either men or women unlike its benign counterpart that occurs almost exclusively in women.
Etiology and Pathogenesis
There are several hypotheses about its etiology and pathogenesis. It may arise from ectopic remnants of embryonic bile ducts or from intrahepatic peribiliary glands. It might originate de novo from bile ducts and ischemia induced by carcinogens or secondary malignant transformation of a preexisting cystadenoma. This latter theory is supported by the finding of benign epithelium within resected specimens of cystadenocarcinoma.
Clinical Data
Its clinical presentation varies significantly. Some patients are asymptomatic patients, others have nonspecific symptoms including abdominal pain and bloating (55% to 90%). Up to 20% of patients have abnormal liver function tests, but obstructive jaundice and cholangitis are rare.
Because of the rarity of these lesions, little is known about their prognoses, but recurrence rates are low right after surgery (5% to 10%). Extrahepatic disease is uncommon, occurring in less than 20%) of patients (16-18).
Morphological and phenotypic issues
Hepatobiliary cystadenocarcinoma appears as large multilocular cystic masses which may have solid or polypoid areas protruding toward the light and whose contents appears to be mucinous.
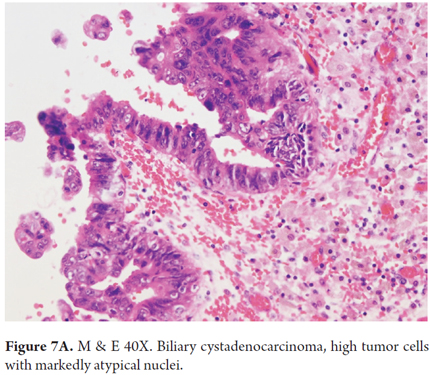
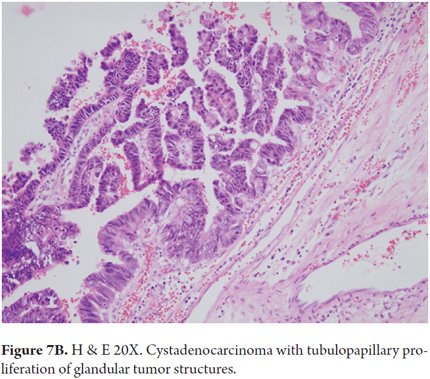
Microscopically, these tumors are characterized by glandular structures arranged in tubulopapillary, solid or tubular patterns of cuboidal or columnar mucin-producing cells arranged in several layers. There is a loss of polarity, mitosis and subepithelial stroma. In women, the subepithelial stroma is densely cellular and ovarian with smooth muscle fibers and very little collagen. In men, the subepithelial stroma is more collagenous. Those tumors that are connected to the bile ducts and which have no ovarian type subepithelial stromal may be associated with intraductal papillary neoplasia (IPN) (Figures 7A and B).
Immunohistochemical studies shows epithelial tumors that are reactive for cytokeratin 7, 8 and 19. There is also expression of CA125, CA19-9, epithelial membrane antigen (EMA), Carcinoembryonic antigen (CEA) and ovarian inhibin α-type stroma, and estrogen and progesterone receptors. This phenotypic pattern does not help distinguish between the benign form and the malignant form (4, 17).
Prognosis and treatment
The prognoses of patients with non-invasive cystadenocarcinoma is great if resection is possible, but the behavior of invasive cystadenocarcinoma is difficult to predict. Nevertheless, the prognosis is better than for CCA with patient survival between three and eight years after surgery. Several studies have reported 5-year survival rates after complete surgical resection varying from 65% to 70%.
Differential diagnosis
From the clinical standpoint, differential diagnosis of cystic liver lesions should consider simple cysts, parasitic cysts, mucinous cystic neoplasms, mucin producing metastatic tumors, hemangiomas, lymphangiomas, foregut liver cyst, mesenchymal hamartomas, and mucinous teratomas (18).
Keys to diagnosis
- Cystic structures glands coated with glands lined with cuboidal or columnar mucin producing cells
- Tubulopapillary, solid or tubular pattern
- Ovarian type subepithelial stromal with expression of α-type-inhibin and estrogen and progesterone receptors
- Expression in the tumor epithelium of CEA, EMA, and CA19-9 cytokeratins
EPITHELIOID HEMANGIOENDOTHELIOMA
Epithelioid hemangioendothelioma is a rare vascular tumor of varying potential for malignancy and aggressivity of tumors although it is considered to be intermediate grade for malignancy. It is composed of dendritic and epithelioid spindle-cells that grow over existing and newly-formed vascular structures which are arranged in a hyalinized myxoid stroma.
Etiology and epidemiological data
The incidence of epithelioid hemangioendothelioma has been reported to be one out of every 100,000 people in the general population. It is more common in women (60%) than men (40%), and has a wide range of ages at presentation (12 to 86 years with an average of 47 years).
The etiology of this disease is unknown, but has been associated with the use of oral contraceptives, liver trauma, exposure to vinyl chloride, and to hepatitis B and C without underlying cirrhosis or chronic liver disease (19-21). Molecular alterations described include translocation t (1; 3) (p36.3; q25) and WWTR1- CAMTA 1 gene fusion (22).
Clinical Data
The symptoms of epithelioid hemangioendothelioma are very vague and nonspecific, and the finding is incidental in over 40% of cases. Patients may have abdominal pain, jaundice and ascites and epithelioid hemangioendothelioma can be complicated with Budd-Chiari syndrome or veno-occlusive disease.
Morphological and phenotypic aspects
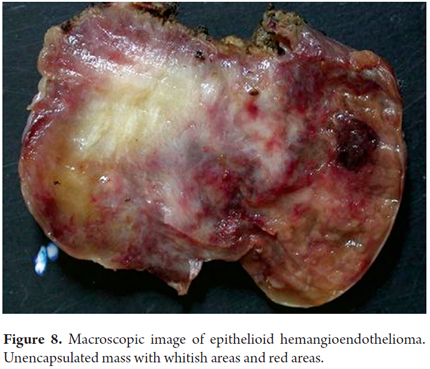
Macroscopically, epithelioid hemangioendothelioma is a poorly defined unencapsulated nodular lesion that is whitish with firm reddish nodules (Figure 8). Tumors may may be solitary or multiple lesions and can compromise one or involving both lobes of the liver. Subcapsular tumors may have umbilication (4).
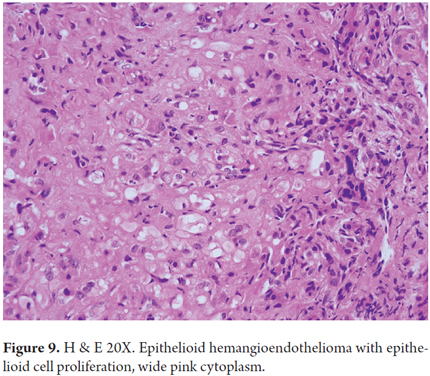
Hepatic epithelioid hemangioendothelioma has several morphological aspects related to its growth pattern, cells and stromal components. Dendritic cells, epithelioid cells, and mesenchymal cells with pleomorphic nuclei proliferate. Some areas are organized in solid blocks while others are loosely organized with vascular spaces surrounded by a very characteristic extracellular matrix which is not very cellular in hyalinized areas with eosinophilic appearance or in fibrous stromal desmoplasia. There are signet ring cells with vacuoles in the cytoplasm that appear like lights, and some of them contain erythrocytes (Figure 9). Nodules with intravascular growth are characterized by solid tops, tufted tops, or polypoid projections or which are dispersed in the fibrous stroma. Tumor cells invade the liver sinusoids causing hepatocyte atrophy. Usually, there is very little or no mitosis (21, 22).
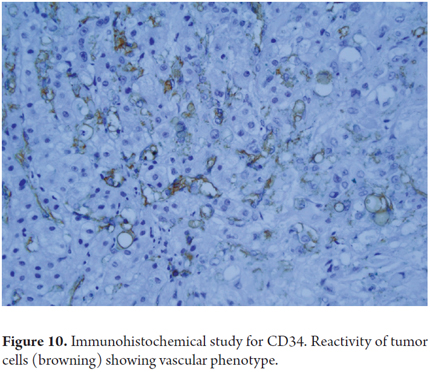
Even when immunohistochemical studies do not help differentiate whether a mucinous cystic lesion is benign or malignant tumor, they can identify expression of immunophenotype markers such as CD10, CD34 (Figure 10), CD31, ERG, and Factor VIII. In addition, some tumor cells are positive for cytokeratin 7, 18 and Podoplanin (D2-40) and have low rates of Ki-67 proliferation (4, 23).
Prognosis and treatment
Epithelioid hemangioendotheliomas are slow-growing tumors with unpredictable potential for malignancy. The prognosis depends on the extent of disease at presentation. Disease limited to the liver occurs in less than 10% of patients, but these patients are candidates for surgical resection or liver transplantation. In 90% of the patients, the disease is multifocal and affects both lobes. The tumor metastasizes in 27% to 45% of patients. The most common locations of metastases are the bones and lungs (4, 22).
Differential diagnosis
The main imitators of this tumor are cholangiocarcinoma, angiosarcoma, hepatocellular carcinoma and metastatic adenocarcinoma.
Keys to diagnosis
- Identify the 3 cell types: mesenchymal, epithelioid and dendritic (signet ring cells
- Extracellular eosinophilic matrix, hyalinized, or marked desmoplasia
- Very little or no mitosis
- Vascular reactivity markers: Factor VIII (99%), CD34 (94%), CD31 (86%), ERG (97%)
- Focal reactivity for cytokeratin 7 and 18
- Podoplanin Reactivity (D2-40) and CD10.
ANGIOSARCOMA
Angiosarcomas are the most common hepatic sarcomas. They originate in very aggressive endothelial cells, even though they occur infrequently and account for less than 2% of all primary tumors.
Epidemiological data and Etiology
The incidence of angiosarcomas in the western world is estimated at 0.5 to 1 per million people while the prevalence is estimated to be 0.14 to 0.25 per million people. Angiosarcoma usually develops during the sixth and seventh decades of life, and is more common in men than women> the male: female ratio is 3: 1 (24, 25). Angiosarcomas are unusual in young children (26).
In most cases the etiology is unknown, and less than half of the cases are associated with specific etiologies. These include exposure to vinyl chloride, inorganic arsenic, copper sulfate, phosphorus pesticides, Thorotrast, anabolic steroids and androgenic steroids. Some cases may be associated with alcoholic cirrhosis or hemochromatosis (26-27). TP53 gene mutations have been associated with exposure to vinyl chloride while KRAS mutations are common to sporadic angiosarcomas and are associated with exposure to thorotrast and vinyl chloride (4, 25-29).
Clinical manifestations
Angiosarcomas are difficult to diagnose because of their nonspecific clinical presentation. Symptoms and signs of liver disease such as hepatomegaly, ascites, abdominal pain, and abnormal liver function occur in most cases. Acute abdomen with hemoperitoneum due to tumor rupture occurs in up to 15% of cases. Less frequently, this tumor presents as acute fulminant liver failure. Most cases are identified in advanced stages or after they have metastasized (25, 26).
Morphological and phenotypic aspects
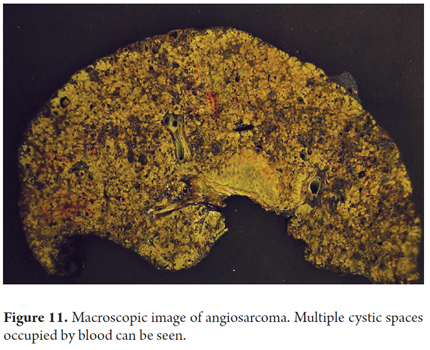
Macroscopically, compromise due to angiosarcoma appears as a poorly defined gray-white mass with hemorrhagic areas, and cystic cavities filled blood which are usually multifocal. Both lobes are usually affected, and sometimes the entire parenchyma is compromised (Figure 11).
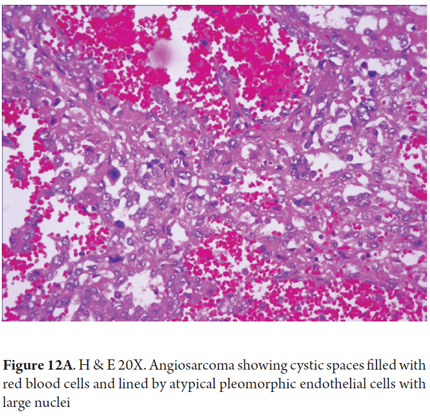
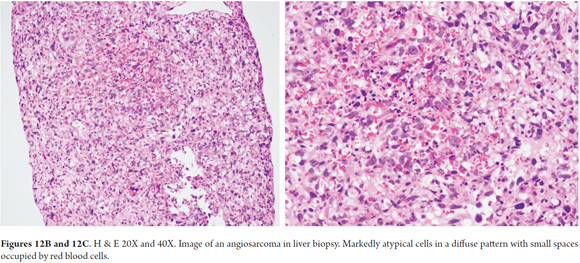
Endothelial tumor cells, spindle cells or rounded which occasionally are pleomorphic, hyperchromatic and multinucleated, are arranged along the vascular channels. Mitosis occurs frequently (Figure 12A). The growth pattern is sinusoidal. The tumor also compromises hepatic portals and progressively produces atrophy and disruption of liver cells. Large vascular channels filled with tumor cells form. Sometimes there are polypoid or papillary projections surrounded by small amounts of fibrous stroma. Sometimes the pattern is diffuse with small spaces occupied by red blood cells (Figures 12B-C) (24, 27).
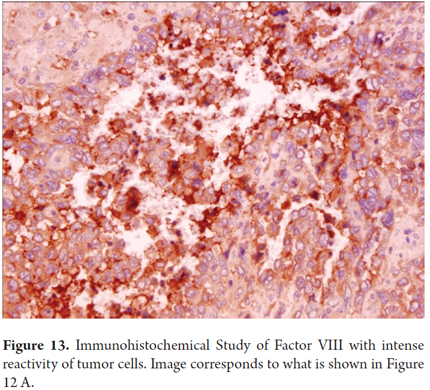
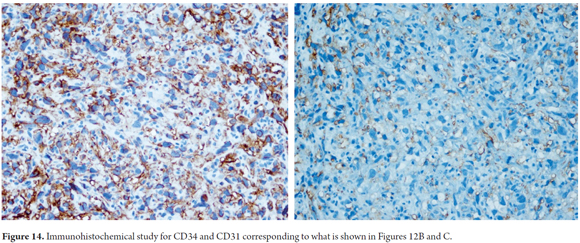
Immunohistochemical studies may help confirm the endothelial phenotype of tumor cells, by indicating reactivity to Factor VIII, CD31 and CD34 (Figures 13 and 14). Up to 40% of cases have aberrant expression of AE1/AE3 and cytokeratin CAM5.2 that can sometimes make diagnosis difficult (24-29).
Prognosis and treatment
The prognosis is poor for these patients: most deteriorate rapidly and die within 12 months of diagnosis as the result of massive abdominal bleeding or liver failure (4). When surgery is possible, resection plus radiation therapy is the best treatment choice, but recurrence is frequent. The role of chemotherapy is not well defined. There are several case reports of liver transplantation, but none have shown good results (25, 29).
Differential diagnosis
Primary hepatic angiosarcoma cannot be differentiated from metastatic angiosarcoma, only the patient's medical history and the absence of extrahepatic angiosarcoma can confirm the diagnosis. Vascular tumors such as epithelioid hemangioendothelioma and Kaposi's sarcoma cause additional problems for diagnosis. Other sarcomas that usually metastasize to the liver, including fibrosarcomas and leiomyosarcomas, may have similar images. For this reason, immunohistochemistry is a fundamental tool for demonstrating tumor phenotype (4, 24, 29).
Keys to diagnosis
- Tumor Growth with sinusoidal pattern and acinar destruction
- Markedly atypical endothelial cells with multinucleation
- Mitosis
- Presence of red blood cells in neoplastic vessels
- Absence of extracellular matrix
- Reactivity factor VIII (80-90%), CD34 (75%) and CD31 (30%)
CONCLUSION
Malignant hepatic tumors are aggressive and are frequently preceded by underlying chronic liver disease that create microenvironments that favor the oncogenic process. Their morphological, phenotypic and molecular heterogeneity explain their development and also hinder diagnosis and management of these patients. Many efforts are being made to improve our knowledge about these tumors. Next-generation sequencing has greatly advanced our understanding of the development of liver cancer, has created a detailed map of genetic alterations and has identified somatic changes that may contribute to future development of new therapeutic approaches to neoplasias such as malignant liver tumors which currently have poor prognosis.
REFERENCES
1. Ustundag Y, Bayraktar Y. Cholangiocarcinoma: A compact review of the literature. World J Gastroenterol. 2008;14(42):6458-66. [ Links ]
2. Mosconi S. Cholangiocarcinoma. Crit Rev Oncol Hemat. 2009;69:259-70. [ Links ]
3. Rizvi S, Borad MJ, Patel T, Gores GJ. Cholangiocarcinoma: Molecular pathways and therapeutic opportunities. Semin Liver Dis. 2014;34(4):456-64. [ Links ]
4. Bosman FT, Carneiro F, Hruban RH, Theise ND (editors). WHO Classification or tumours of the digestive system. Lyon, France: IARC; 2010. [ Links ]
5. Tushar P. Cholangiocarcinoma. Nat Clin Pract Gastroenterol Hepatol. 2006;3(1):33-42. [ Links ]
6. Shin HR. Epidemiology of cholangiocarcinoma: An update focusing on risk factors. Cancer Sci. 2010;101:579-85. [ Links ]
7. Villaveces VA, López R. Orthotopic liver transplantation for biliary atresia complicated by incidental cholangiocarcinoma: Case report and literature review. J Pediatr Gastr Nutr. 2012;55(3):336-7. [ Links ]
8. Mosconi S. Cholangiocarcinoma. Crit Rev Oncol Hemat. 2009;69:259-70. [ Links ]
9. Leong T, Wannakrairot P, Lee ES, Leong A. Pathology of cholangiocarcinoma. Curr Diag Path. 2007;13:54-64. [ Links ]
10. Shaib YH, Davila JA, McGlynn K, et al. Rising incidence of intrahepatic cholangiocarcinoma in the US: A true increase? J Hepatol. 2004;40:472-7. [ Links ]
11. Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Sem Liver Dis. 2004;24:115-24. [ Links ]
12. Goodman ZD. Neoplasms of the liver. Modern Pathol. 2007;20:S49-60. [ Links ]
13. Liau JY. Morphological subclassification of intrahepatic cholangiocarcinoma: Etiological, clinicopathological, and molecular features. Modern Pathol. 2014;27:1163-73. [ Links ]
14. Nakanuma Y, Sato Y, Harada K, Sasaki M, Xu J, Ikeda H. Pathological classification of intrahepatic cholangiocarcinoma based on a new concept. World J Hepatol. 2010;2(12):419-27. [ Links ]
15. Dabbs DJ, Geisinger KR, Ruggiero F, Raab SS, Nalesnik M, Silverman JF. Recommendations for the reporting of tissues removed as part of the surgical treatment of malignant liver tumors. The Association of Directors of Anatomic and Surgical Pathology. Am J Clin Pathol. 2005;123:494-8. [ Links ]
16. Mosnie JF. N-cadherin serves as diagnostic biomarker in intrahepatic and perihilar cholangiocarcinomas. Modern Pathol. 2009;22:182-90. [ Links ]
17. Zen Y, Pedica F, Patcha VR, Capelli P, Zamboni G, Casaril A, et al. Mucinous cystic neoplasms of the liver: A clinicopathological study and comparison with intraductal papillary neoplasms of the bile duct. Modern Pathol. 2011;24:1079-89. [ Links ]
18. Lee CW, Tsai HI, Lin YS, Wu TH, Yu MC, Chen MF. Intrahepatic biliary mucinous cystic neoplasms: Clinicoradiological characteristics and surgical results. BMC Gastroenterol. 2015;15:67-75. [ Links ]
19. Soares KC, Arnaoutakis DJ, Kamel I, Anders R, Adams RB, Bauer TW, et al. Cystic neoplasms of the liver: biliary cystadenoma and cystadenocarcinoma. J Am Coll Surg. 2014;218(1):119-28. [ Links ]
20. Baron PW, Amankonah T, Cubas RF, Kore AH, Elihu A, de Vera ME, et al. Diffuse hepatic epithelioid hemangioendothelioma developed in a patient with hepatitis C cirrhosis. Case Rep Transplant. 2014;2014:694903. [ Links ]
21. Zhao XY, Rakhda MI, Habib S, Bihi A, Muhammad A, Wang TL et al. Hepatic epithelioid hemangioendothelioma: A comparison of Western and Chinese methods with respect to diagnosis, treatment and outcome. Oncol Lett. 2014;7:977-83. [ Links ]
22. Campionea S, Cozzolino I, Mainenti P, DAlessandroa V, Vetrani A, DArmiento M. Hepatic epithelioid hemangioendothelioma: Pitfalls in the diagnosis on fine needle cytology and "small biopsy" and review of the literature. Pathol Res Pract. 2015;211:702-5. [ Links ]
23. Weinreb I, Cunningham KS, Perez-Ordoñez B, et al. CD10 is expressed in most epitheliod hemangioendotheliomas: A potential diagnostic pitfall. Arch Pathol Lab Med. 2009;133 (12):1965-8. [ Links ]
24. Bioulac-Sage P, Laumonier H, Laurent C, et al. Benign and malignant vascular tumors of the liver in adults. Semin Liver Dis. 2008;28(3):302-14. [ Links ]
25. López R, Castro-Villabón D, Álvarez J, Vera A, Andrade R. Hepatic angiosarcoma presenting as acute liver failure in young adults. Report of two cases and review of literature. Case Reports in Clin Med. 2013;2:439-44. [ Links ]
26. Srinivasa S, Lee WG, Aldameh A, Koea JB. Spontaneous hepatic hemorrhage: A review of pathogenesis, etiology and treatment. HPB (Oxford). 2015 Aug 7. doi: 10.1111/hpb.12474. [ Links ]
27. Deyrup AT, Miettinen M, North PE, Khoury JD, Tighiouart M, et al. Angiosarcomas arising in the viscera and soft tissue of children and young adults. Am J Surg Pathol. 2009;33:264-9. [ Links ]
28. Wiland HO, Pai RK, Purysko AS. Hepatic angiosarcoma mimicking sinusoidal obstruction syndrome/venoocclusive disease: A pathologic-radiologic correlation. Ann Diagnos Pathol. 2012;16: 275-9. [ Links ]
29. Zhu YP, Chen YM, Matro E, Chen RB, Jiang ZN, Mou YP, et al. Primary hepatic angiosarcoma: A report of two cases and literature review World J Gastroenterol. 2015;21(19):6088-96. [ Links ]











 texto em
texto em