Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista colombiana de Gastroenterología
versão impressa ISSN 0120-9957
Rev Col Gastroenterol vol.30 no.4 Bogotá out./dez. 2015
Acute Pancreatitis and Elevated Aminotransferases: What to Think? A Case Report and Literature Review
Diana Carolina Díaz T. MD (1), William Otero Regino MD (2), Martin Gómez Zuleta MD (3)
(1) Internal Medicine Resident at the National University of Colombia in Bogotá, Colombia.
(2) Professor of Medicine in the Gastroenterology Unit at the National University of Colombia and Gastroenterologist at Clínica Fundadores in Bogotá, Colombia.
(3) Professor of Medicine in the Gastroenterology Unit at the National University of Colombia and Gastroenterologist at the Hospital El Tunal in Bogotá, Colombia.
Received: 28-04-15 Accepted: 20-10-15
Abstract
Gallstones are associated with development of acute pancreatitis in 40% of cases, however the diagnostic sensitivities of abdominal ultrasound and CT scans for finding this etiology are limited. The case presented here exemplifies the predictive use of aminotransferase liver enzymes in patients with biliary cholecystectomy but abdominal ultrasound that is negative for cholelithiasis and choledocholithiasis. The case is a 55 year old patient whose clinical picture was consistent with acute biliary pancreatitis. The diagnosis was made and endoscopic treatment was recommended.
Keywords
Biliary pancreatitis, ALT, endoscopic ultrasonography, ultrasound.
INTRODUCTION
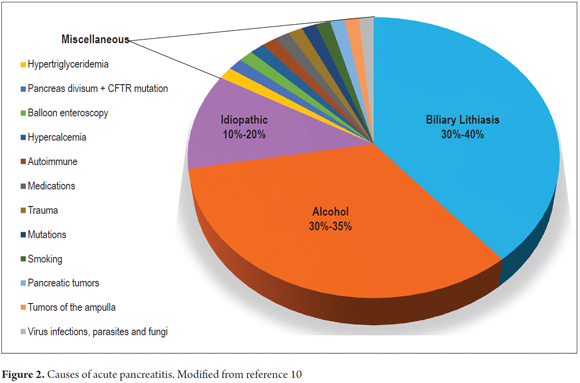
Acute pancreatitis (AP) is one of the disorders that is seen most frequently in hospital gastroenterology services (1). It is the result of inflammation and necrosis of the pancreatic tissue which generate a generalized inflammatory response that can lead to systemic involvement with multi-organ dysfunction. Death occurs in 36% to 50% of severe cases (1, 2). The most common causes are gallstones and alcohol consumption which account for 80% of all cases (3, 4). The identification of biliary AP is very important for the possibility of endoscopic treatment to control the condition and to reduce the risk of recurrence (5). Unfortunately during the initial phase of AP, the initial diagnostic imaging methods used to identify lithiasis in the bile ducts have little sensitivity and often report false negative results. This makes diagnosing biliary AP a real challenge (3, 5), but there are easily measurable biochemical alterations that predict biliary AP (1, 3, 6, 8, 9). The most important of these parameters is alanine aminotransferase (ALT) levels above 150 U/L (1, 6).
CASE REPORT
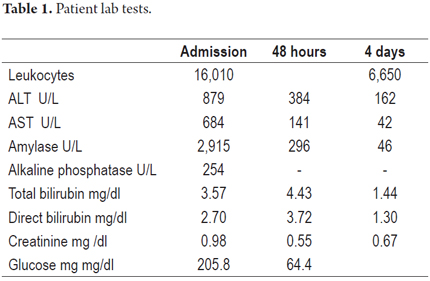
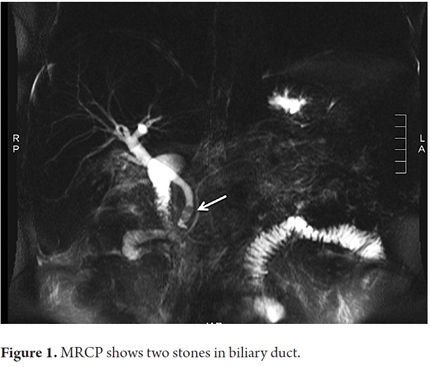
The patient was a 55 year old man who was admitted to the emergency room with abdominal pain that had suddenly developed three hours earlier. The pain was intense but did not radiate. It was accompanied by occasional vomiting. The patient had had a cholecystectomy. He did not use drugs or have any other relevant medical history. A physical examination upon admittance showed normal vital signs, tenderness in the epigastric area but no signs of peritoneal irritation. Laboratory tests showed leukocytosis of 16,010 cells/L with 87% neutrophils, ALT 879 U/L, aspartate aminotransferase (AST) 684 U/L, total bilirubin 3.57 mg/dl, direct bilirubin 2.70mg/dl, albumin 4.61 gr/dl, alkaline phosphatase 254U/L, amylase 2,915 U/L, and calcium 9.69 mg/dl. Sodium, potassium, chloride and creatinine were all within normal limits. Measures of arterial blood gases showed PaO2 73.8%, FiO2 0.21 and pH 7.41. Hepatobiliary ultrasound indicated fatty liver disease, showed a previous cholecystectomy, and indicated normal caliber intrahepatic and extrahepatic bile ducts. A diagnosis of mild acute pancreatitis without systemic complications was made. The patient-s condition was scored 1 with the modified Marshal scoring system for organ dysfunction because PaO2/FiO2 was only 351 and there was neither renal nor cardiovascular impairment (2). Supportive of treatment intravenous fluids, intravenous meperidine for pain control and a liquid diet was begun. Because of the indications of altered liver function, lithiasis and biliary AP were suspected. Magnetic resonance cholangiopancreatography (MRCP) was ordered to evaluate the bile ducts. Liver profiles at 48 and 72 hours after admission showed marked reduction in aminotransferase levels and bilirubin (Table 1). The MRCP showed an 11 mm bile duct with 5 mm stones at its distal end. The patient-s pancreas was normal (Figure 1). Endoscopic retrograde cholangiopancreatography (ERCP) with sphincterotomy was requested. After the procedure there was significant improvement of the abdominal pain.
DISCUSSION
AP is the most common acute pancreatic disease (1). In the USA, the incidence is 13 to 45 cases per 100,000 people (1). Although multiple etiologic factors have been identified, as previously mentioned, gallstones and alcohol consumption produce 75% of all cases (3, 9,10). The ratio between biliary origins and alcoholic origins may vary in different geographical areas. In the populations of the United States and Europe, 60% of cases have biliary etiologies while in countries like South Africa the prevalence of alcoholic origins reaches as high as 83% (3, 7, 8). Biliary AP is the first manifestation of gallstones in 40% of those patients who have no previous histories of stones in the gallbladder or biliary colic (5). Other risk factors include diagnostic or therapeutic ERCP, drugs, hypertriglyceridemia and pancreas divisum (PD) (9, 10). The prevalence of PD in the population is 7.5%. Although the hypothesis that it can cause AP is still controversial, it is currently believed that AP can develop when a patient with PD also has associated genetic mutations such as the gene for cystic fibrosis (CFTR gene) and the serine protease inhibitor Kazal-type 1 (SPINK1) gene (Figure 2) (1, 5, 9, 10).
While the pathophysiological mechanisms of AP have not yet been fully elucidated, it is now considered that many of the etiological factors can cause obstruction at the ampulla of Vater. This increases pressure in pancreatic and biliary ducts resulting in retrograde flow of pancreatic and biliary secretions producing trypsin activation and self-digestion of the pancreatic parenchyma (8-10). In the initial pathophysiological phases, zymogen granules within acinar cells are not exported and join the rich lysosomes in cathepsin B which converts trypsinogen to trypsin (1, 10).
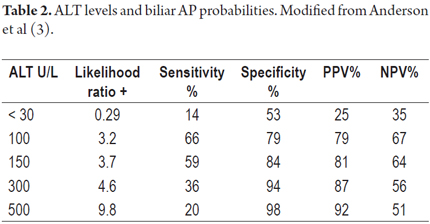
This mechanism is considered to be the "classical trypsin theory". Recently it has become controversial because many cases have demonstrated that this series of events does not always occur but rather nuclear factor kappa B (NF-kB) is activated within acinar cells independently of the presence of trypsin (1, 10-12). Therefore, at present it is considered that both mechanisms are active causes of AP. The activation of NF-kB, involved in both the initial outbreak of the disease and its perpetuation by inducing the production of pro-inflammatory substances (7-10). In cases of cholelithiasis, there are several risk factors for biliary obstruction including the presence of 20 or more small stones of less than 5 mm each, cystic ducts with diameters greater than 5 mm, and anatomical variations of the pancreaticobiliary ducts. These can include longer than usual common bile ducts, increased angles of convergence between the biliary and pancreatic ducts, and papillary diverticula (5). As in the case of any medical disorder, identification of the etiological agent is fundamental. Hence, determination that AP is biliary allows interventions such as cholecystectomy and ERCP to decrease recurrence of the entity (5, 13, 14, 15). If the biliary etiology of an initial AP episode is not identified, the entity will recur within six months in about 60% of patients (5, 7, 11, 16). The first line examination is hepatobiliary ultrasound. In expert hands its sensitivity is 92% to 98% for uncomplicated cholelithiasis. However, its sensitivity drops to 76% to 87% in cases of biliary AP and drops even further in cases of choledocholithiasis to 20% to 50% (3, 17, 18). Because of this limitation, more sensitive and specific tests such as MRCP or biliopancreatic endoscopic ultrasonography (EUS) need to be used when choledocholithiasis is suspected as the cause of AP associated with elevated ALT (3, 17-19). The association between biliary AP and elevated ALT was first described by McMahon and Pickford in 1979 (5, 20). A meta-analysis conducted in 1994 by Teener et al. found that more than 150 U/L or 3 times the normal upper limit of ALT has a positive predictive value (PPV) of 95% (6). Subsequently, several studies have found that the PPV for biliary AP of high levels of ALT is between 75% and 100% (21-23). Variation in the degree of accuracy depends on the value of ALT established (Table 2) (3, 15).
In populations with high levels of alcohol consumption and high prevalences of AP due to alcohol, ALT measurements over 150 U/L has a sensitivity of 51%, a specificity of 97%, a PPV of 80% and an NPV of 90% regardless of gender (7). In contrast, amylase values have no correlation with biliary AP (7). Because AST is present in various tissues other than hepatobiliary tissues, it cannot discriminate for biliary AP. When its level is 10 times higher than the limit, it is primarily associated with hepatic ischemia and biliary obstruction (24). To date, the mechanism by which transaminases increase in biliary AP remains unknown although it may be due to choledocholithiasis in which elevated ALT levels have been described (25). Mossberg and Ross have proposed three mechanisms to explain the liver enzyme elevation after an obstructive process: 1) regurgitation of transaminases from clogged biliary canaliculi to the liver sinusoids, 2) increased production of the enzymes, and 3) secretion of transaminases by hepatocytes in response to increased intrabiliary pressure (26).
During follow-up of acute pancreatitis, it is necessary to repeat the measurement of transaminases at least 48 hours after admission because a peak in the levels of transaminases followed by a rapid decline is additional b evidence of gallstones (15, 19, 20, 27). It is important to keep in mind the facts that 10% to 15% of all cases of biliary AP have normal transaminase levels and that 16.7% have high levels that are less than 3 times the upper limit of normal (3, 5). In 10% to 20% of all acute pancreatitis patients there are no associated risk factors or etiological agents found, and they are often diagnosed with idiopathic pancreatitis (1, 29), yet in 78% of the patients whose abdominal ultrasound during the initial phase of pancreatitis shows no AP, a biliary cause is detected in studies with better diagnostic performances such as EUS, MRCP and diagnostic ERCP (7, 30, 31).
Because of the high resolution of its images, EUS has much better diagnostic accuracy than does conventional abdominal ultrasound. It finds stones in the biliary tract in 59% to 79% of patients with negative ultrasound results and finds microlithiasis in 40% to 80% of AP patients who are initially classified as idiopathic (15, 32, 33). Since the diagnostic yields of EUS and MRCP are comparable, the choice between them depends on availability and expertise of the person performing the EUS. In a meta-analysis, Giljaca and colleagues found that the operational characteristics of these two studies is similar with sensitivities of 95% and 93% and specificities of 97% and 96% for EUS and MRCP respectively. (34) One advantage that endoscopic ultrasound has over resonance is that EUS can detect stones smaller than 5 mm (33, 35, 36).
Other studies that compare MRCP and ERCP have produced similar data. Both have sensitivities of 90% to 100%, and both have specificities of 92%. ERCP has the advantage of the providing an opportunity for therapeutic sphincterotomy (3, 15, 19, 34). ERCP is recommended only for patients with biliary AP for whom endoscopic sphincterotomy with therapeutic intent is indicated, for example patients with associated acute cholangitis and persistent obstruction of the bile duct (33, 35). The procedure must be performed early, within the first 72 hours, and even earlier if the patient's condition is not developing well (15, 33). Early ERCP is also indicated in patients with acute pancreatitis and high probabilities of choledocholithiasis such as bilirubin over 4 mg/dl or dilatation of the bile duct with bilirubin between 1.8 and 4 mg/dl (33). Similarly, this procedure can be performed upon hospital admission for patients with indications for cholecystectomy but who, because of other medical conditions, cannot undergo laparoscopic cholecystectomy (33).
In conclusion, the clinical case above is a clear example of how elevated levels of transaminases, especially ALT, combined with rapid decline during the course of acute pancreatitis, are a good predictor of biliary origin even when initial ultrasound is negative for gallstones. Confirmation of the presence of gallstones is essential either by EUS, MRCP or ERCP with therapeutic intent in the context of acute cholangitis and/or persistent obstruction of the bile duct.
Conflicts of Interest
The authors declare that they have no conflicts of interest and that the costs of this research were assumed by the authors. William Otero declares that he has received lecture fees from the Abbott-Lafrancol, Sanofi, Tecnofarma, Takeda, Janssen, Procaps and Biotoscana.
REFERENCES
1. Lankisch PG, Apte M, Banks PA. Acute pancreatitis. Lancet. 2015;386(9988):85-96. [ Links ]
2. Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. enero de 2013;62(1):102-11. [ Links ]
3. Anderson K, Brown LA, Daniel P, Connor SJ. Alanine transaminase rather than abdominal ultrasound alone is an important investigation to justify cholecystectomy in patients presenting with acute pancreatitis. HPB (Oxford). 2010;12(5):342-7. [ Links ]
4. Sakorafas GH, Tsiotou AG. Etiology and pathogenesis of acute pancreatitis: current concepts. J Clin Gastroenterol. 2000;30(4):343-56. [ Links ]
5. van Geenen EJM, van der Peet DL, Bhagirath P, Mulder CJJ, Bruno MJ. Etiology and diagnosis of acute biliary pancreatitis. Nat Rev Gastroenterol Hepatol. 2010;7(9):495-502. [ Links ]
6. Tenner S, Dubner H, Steinberg W. Predicting gallstone pancreatitis with laboratory parameters: a meta-analysis. Am J Gastroenterol. octubre de 1994;89(10):1863-6. [ Links ]
7. Moolla Z, Anderson F, Thomson SR. Use of amylase and alanine transaminase to predict acute gallstone pancreatitis in a population with high HIV prevalence. World J Surg. 2013;37(1):156-61. [ Links ]
8. Alexakis N, Lombard M, Raraty M, Ghaneh P, Smart HL, Gilmore I, et al. When is pancreatitis considered to be of biliary origin and what are the implications for management? Pancreatology. 2007;7(2-3):131-41. [ Links ]
9. Sugiyama M, Atomi Y. Risk factors for acute biliary pancreatitis. Gastrointest Endosc. agosto de 2004;60(2):210-2. [ Links ]
10. Otero W, Otero L, Gómez M. Fisiopatología de la pancreatitis aguda. En Aponte DM (ED). Tratado de Pancreatología. Editorial Panamericana Formas e Impresos S.A, Bogotá, 2016 pp:69-76. [ Links ]
11. Samuel I, Yorek MA, Zaheer A, Fisher RA. Bile-pancreatic juice exclusion promotes Akt/NF-kappaB activation and chemokine production in ligation-induced acute pancreatitis. J Gastrointest Surg. 2006;10(7):950-9. [ Links ]
12. Dawra R, Sah RP, Dudeja V, Rishi L, Talukdar R, Garg P, et al. Intra-acinar trypsinogen activation mediates early stages of pancreatic injury but not inflammation in mice with acute pancreatitis. Gastroenterology. 2011;141(6):2210-7.e2. [ Links ]
13. Acosta JM, Katkhouda N, Debian KA, Groshen SG, Tsao-Wei DD, Berne TV. Early ductal decompression versus conservative management for gallstone pancreatitis with ampullary obstruction: a prospective randomized clinical trial. Ann Surg. enero de 2006;243(1):33-40. [ Links ]
14. Hammarström LE, Stridbeck H, Ihse I. Effect of endoscopic sphincterotomy and interval cholecystectomy on late outcome after gallstone pancreatitis. Br J Surg. 1998;85(3):333-6. [ Links ]
15. Johnson C, Lévy P. Detection of gallstones in acute pancreatitis: when and how? Pancreatology. 2010;10(1):27-32. [ Links ]
16. Samuel I. Bile and pancreatic juice exclusion activates acinar stress kinases and exacerbates gallstone pancreatitis. Surgery. 2008;143(3):434-40. [ Links ]
17. Lévy P, Boruchowicz A, Hastier P, Pariente A, Thévenot T, Frossard JL, et al. Diagnostic criteria in predicting a biliary origin of acute pancreatitis in the era of endoscopic ultrasound: multicentre prospective evaluation of 213 patients. Pancreatology. 2005;5(4-5):450-6. [ Links ]
18. Dholakia K, Pitchumoni CS, Agarwal N. How often are liver function tests normal in acute biliary pancreatitis? J Clin Gastroenterol. enero de 2004;38(1):81-3. [ Links ]
19. Liu CL, Fan ST, Lo CM, Tso WK, Wong Y, Poon RTP, et al. Clinico-biochemical prediction of biliary cause of acute pancreatitis in the era of endoscopic ultrasonography. Aliment Pharmacol Ther. 2005;22(5):423-31. [ Links ]
20. McMahon MJ, Pickford IR. Biochemical prediction of gallstones early in an attack of acute pancreatitis. Lancet. 1979;2(8142):541-3. [ Links ]
21. Davidson BR, Neoptolemos JP, Leese T, Carr-Locke DL. Biochemical prediction of gallstones in acute pancreatitis: a prospective study of three systems. Br J Surg. marzo de 1988;75(3):213-5. [ Links ]
22. Kazmierczak SC, Catrou PG, Van Lente F. Enzymatic markers of gallstone-induced pancreatitis identified by ROC curve analysis, discriminant analysis, logistic regression, likelihood ratios, and information theory. Clin Chem. abril de 1995;41(4):523-31. [ Links ]
23. Ammori BJ, Boreham B, Lewis P, Roberts SA. The biochemical detection of biliary etiology of acute pancreatitis on admission: a revisit in the modern era of biliary imaging. Pancreas. 2003;26(2):e32-5. [ Links ]
24. Matull WR, Pereira SP, O'Donohue JW. Biochemical markers of acute pancreatitis. J Clin Pathol. 2006;59(4):340-4. [ Links ]
25. Nathwani RA, Kumar SR, Reynolds TB, Kaplowitz N. Marked elevation in serum transaminases: an atypical presentation of choledocholithiasis. Am J Gastroenterol. 2005;100(2):295-8. [ Links ]
26. Mossberg SM, Ross G. High serum transaminase activity associated with extrahepatic biliary disease. A clinical and pathologic study of sixty patients with serum glutamic-oxalacetic transaminase levels of 300 units or greater. Gastroenterology. 1963;45:345-53. [ Links ]
27. Whitehead MW, Hawkes ND, Hainsworth I, Kingham JG. A prospective study of the causes of notably raised aspartate aminotransferase of liver origin. Gut. 1999;45(1):129-33. [ Links ]
28. Davidson BR, Neoptolemos JP, Leese T, Carr-Locke DL. Biochemical prediction of gallstones in acute pancreatitis: a prospective study of three systems. Br J Surg. 1988;75(3):213-5. [ Links ]
29. Vege SS, Whitcomb DC. Etiology of acute pancreatitis. Up to Date 2015. [ Links ]
30. Liu CL, Lo CM, Chan JK, Poon RT, Fan ST. EUS for detection of occult cholelithiasis in patients with idiopathic pancreatitis. Gastrointest Endosc. 2000;51(1):28-32. [ Links ]
31. Sajith KG, Chacko A, Dutta AK. Recurrent acute pancreatitis: clinical profile and an approach to diagnosis. Dig Dis Sci. 2010;55(12):3610-6. [ Links ]
32. Draganov P, Forsmark CE. «Idiopathic» pancreatitis. Gastroenterology. marzo de 2005;128(3):756-63. [ Links ]
33. Itoi T, Draganov PV. Endoscopic Management of Severe Gallstone Pancreatitis. En: Forsmark CE, Gardner TB, editores. Prediction and Management of Severe Acute Pancreatitis. Springer New York; 2015. p. 169-78. [ Links ]
34. Giljaca V, Gurusamy KS, Takwoingi Y, Higgie D, Poropat G, timac D, et al. Endoscopic ultrasound versus magnetic resonance cholangiopancreatography for common bile duct stones. Cochrane Database Syst Rev. 2015;2:CD011549. [ Links ]
35. Tse F, Yuan Y. Early routine endoscopic retrograde cholangiopancreatography strategy versus early conservative management strategy in acute gallstone pancreatitis. Cochrane Database Syst Rev. 2012;5:CD009779. [ Links ]
36. Aubé C, Delorme B, Yzet T, Burtin P, Lebigot J, Pessaux P, et al. MR cholangiopancreatography versus endoscopic sonography in suspected common bile duct lithiasis: a prospective, comparative study. AJR Am J Roentgenol. enero de 2005;184(1):55-62. [ Links ]











 texto em
texto em