Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista colombiana de Gastroenterología
versão impressa ISSN 0120-9957
Rev Col Gastroenterol vol.32 no.1 Bogotá jan./mar. 2017
https://doi.org/https://doi.org/10.22516/25007440.123
Prevention of Hepatitis B Recurrence in Liver Transplant Recipients Using Low Doses of Anti-Hepatitis B Immunoglobulin and Nucleoside Analogue
Diana Moncada MD (1), Octavio Muñoz MD (2), Salomón Daguer MD (1), Óscar Santos MD (2), Juan Ignacio Marín MD (2), Sergio Hoyos MD MSc (2), Carlos Guzmán MD (3), Álvaro Mena MD (3), Juan Carlos Restrepo MD MSc PhD (2)
(1) Internal Medicine at the Universidad Pontificia Bolivariana in Medellin, Colombia
(2) Gastrohepatology Group in the Faculty of Medicine of the University of Antioquia and Pablo Tobón Uribe Hospital in Medellin, Colombia
(3) Faculty of Medicine of the University of Antioquia and Pablo Tobón Uribe Hospital in Medellin, Colombia
Received:Â Â Â 18-04-16Â Accepted:Â Â Â 16-12-16
Abstract
Introduction: Hepatitis B results in one million deaths every year and it is an important reason for liver transplantation. The use of anti-hepatitis B immunoglobulin at high doses and nucleoside analogues have reduced reinfection of the graft by 90%. Objective: This study evaluated the efficacy of low doses of immunoglobulin to prevent reinfection of grafts after transplantation. Methodology: This is a retrospective study of a series of patients who had been transplanted and who received immunoglobulin after transplantation at the Hospital Pablo Tobón Uribe between January 2004 and September 2014. Hepatitis B viral load, transaminase and serological markers were used to document relapses. Other variables studied included mortality, complications, graft dysfunction, adverse reactions and costs. Results: There were 18 patients with hepatitis B who had transplants: 50% had hepatocarcinoma, 22% had cirrhosis, and 22% had acute liver failure. The median follow-up time was 43.27 months with a range of 14.7 to 65.2 months. Two patients tested positive for surface antigen in the post-transplant period and one relapsed and had a positive viral load at 41 months. The graft reinfection rate was 5.5%. There were no deaths. It was estimated that the cost of using low doses of immunoglobulin was lower than that of using high doses at 6 months of therapy, but no cost-effectiveness study was done. Graft dysfunction was 10% to 33 months. Conclusion: Low doses of immunoglobulin prevented reinfection of grafts in a way that is similar to that reported in other series. While immunoglobulin free schemes have proven to be useful for the long term, low doses of immunoglobulin remain useful.
Key words
Liver transplantation, hepatitis B, immunoglobulin, antiviral.
INTRODUCTION
Worldwide, hepatitis B is a common infection, and about 2 billion people have had contact with the virus while 400,000 have chronic hepatitis B. (1) Colombia has intermediate endemicity with an overall incidence of 4.36 cases per 100,000 inhabitants as of November 2014. (2) Regions with higher than average incidence include Amazonas, Norte de Santander, Guaviare and Chocó with 23.8, 12.5, 9.13 and 8.89 cases per 100,000 inhabitants, respectively. (3)
Cirrhosis or hepatocellular carcinoma due to chronic infections and acute liver failure due to recent infections or to reactivation in chronic carriers result in 600,000 deaths worldwide every year. Chronic hepatitis B infections account for 10% of hepatic transplants, (1) but reinfection of the graft is a possible outcome.
Without post-transplant antiviral prophylaxis, graft infection occurs in 75% to 80% of cases, with a 50% two year mortality rate. (4) With the use of antiviral prophylaxis, recurrence is reduced to 10%. (1)
High dosages of hepatitis B immunoglobulin (HBIG) (10,000 IU/IV) were one of the first prophylaxis strategies used to reduce graft infection rates by 20% to 35%. (5) Nevertheless, reinfection occurred in more than half of these cases due to the emergence of viruses with surface antigen (HBsAg) escape mutations. (6) Next, lamivudine showed better efficacy than did short-term administration of HBIG, (7) but, lamivudine monotherapy produced high rates of resistance which led to relapses in 40% to 50% of patients at three years. (8) Subsequently, the combination of large dosages of HBIG and lamivudine has reduced recurrences to less than 10% with a lower probability of resistance. (6, 9) Nevertheless, this scheme's high cost is a problem, especially in developing countries. To overcome the cost problem, protocols that call for low-dosage monthly administration of HBIG (400 IU/intramuscular) have been developed and have demonstrated similar effectiveness to that evidenced by high dosages. (9, 10). The aim of this study, conducted at the Hospital Pablo Tobón Uribe in Medellín , Colombia, is to describe the efficacy of the combination of low intramuscular doses of HBIG and a nucleoside analogue to prevent reinfection of the hepatic graft by hepatitis B virus after transplantation
METHODS
Population
This is a retrospective case series based on review of medical records of patients at the Hospital Pablo Tobón Uribe (HPTU) between January 2004 and September 2014. Records reviewed were those of patients with hepatitis B virus who underwent liver transplantation due to acute liver failure, cirrhosis with complications or one or more focal points of hepatocellular carcinoma and who received prophylaxis with HBIG and an antiviral agent (entecavir, lamivudine or tenofovir) during the post-transplant period. Patients who received less than 7 days of HBIG, who were followed up for less than 6 months, or whose records had insufficient data to evaluate the primary outcome were excluded. All patients had hepatitis B diagnosed positive tests for HBsAg positive and anti-core IgM antibodies (HbcIgM) or IgG type (HbcIgG) before transplantation. Co-infections with hepatitis C virus (HCV), hepatitis D virus (HDV) and human immunodeficiency (HIV) were evaluated.
Clinical and demographic variables collected and recorded included follow-up times, months of immunoglobulin treatment, graft dysfunction defined as transaminase elevation twice normal values, mortality, HBsAg, pretransplant HBeAg, post-transplant anti-e antigen, hepatitis B viral load, transaminases, prophylaxis and long-term immunosuppression.
Prophylaxis for Reinfection
A low dose HBIG regimen of daily intramuscular injections of 400 IU doses were administered for seven consecutive days starting in the anhepatic phase and thereafter with 400 IU intramuscular injections once a month. The first patients received indefinite prophylaxis with HBIG, then a duration of 6 months was established. All patients also received an oral nucleoside analog indefinitely. Those receiving lamivudine prior to transplantation continued with the same treatment if the viral load at the time of transplantation was negative. Those who had received no treatment or had viral replicative activity received 1 mg of entecavir daily in the post-transplant period. Patients with positive viral loads at the time of transplantation received HBIG for a longer period of time.
Immunosuppression Scheme
The Gastrohepatology Group's immunosuppression protocol, used for these patients, calls for descending IV administration methylprednisolone beginning at of 1 gram the first day and continuing for six days. This is followed by daily doses of 20 mg of prednisolone for 3 months. Patients with renal impairment or risk of renal failure receive 1,000 mg of mycophenolate every 12 hours and those with normal renal function or low risk of renal failure receive 1-2 mg/kg of azathioprine. A calcineurin inhibitor, either cyclosporine or tacrolimus, is started within 6 to 18 hours, up to 72 hours post-transplant depending on renal function. All patients have are strictly followed up on an outpatient basis.
Virological Follow-up
HBsAg was measured in the post-transplant period. For those who tested positive or had elevated transaminases, HBV viral loads were measured in real time. Reinfection of the graft was defined as positive HBV viral load in the post-transplant period.
Statistical Analysis
Data collection and analysis was done in SPSS® version 20 (SPSS Inc., Chicago, Illinois, USA). Quantitative variables are expressed as means or medians, with their respective dispersion measures according to the distribution of the variable using the Shapiro-Wilk test. Kaplan-Meier survival curves were used to assess long-term graft dysfunction.
Ethical Issues
The study was approved by the Ethics Committee of the Hospital Pablo Tobón Uribe and adhered to Resolution 008430 of 1993 of the Ministry of Health of the Republic of Colombia regarding ethical issues in research on human beings.
RESULTS
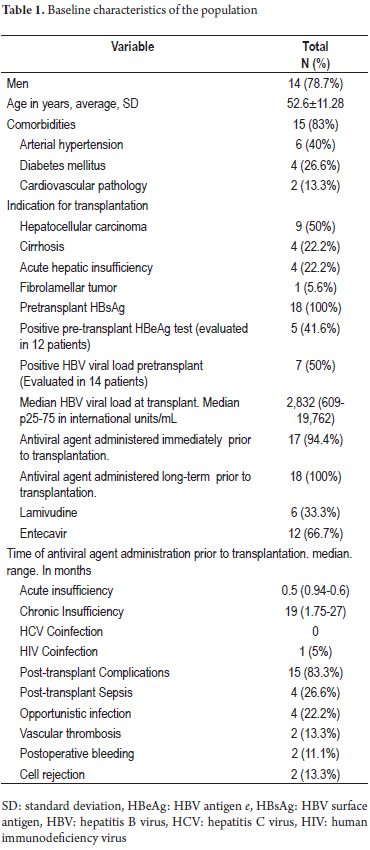
Eighteen transplant patients with hepatitis B virus infections who met the inclusion criteria were treated between January 2004 and September 2014. The baseline characteristics of the population are described in Table 1. Of these patients, 77.8% were men, the average age was 52.6 ± 11.28 years, and 83% of the patients had one or more comorbidity. The most frequent were hypertension which affected 40%, and diabetes which affected 26.6% of the cases. The median follow-up time was 43.27 months (14.7 to 65.2). Indications for transplantation were hepatocellular carcinoma (50%), cirrhosis with complications (22.2%), and acute liver failure (22.2%). Seven out of fourteen patients had positive HBV viral loads at the time of transplantation with a median of 2,832 IU/mL (609-19762 IU/mL). 94% of the patients received antiviral agents before transplantation, mean time of 17.9 ± 17.7 months in cases of chronic infection versus 0.28 ± 0.31 months in cases of acute liver failure. Entecavir was the most frequently used antiviral agent (67%).
Immunoglobulin prophylaxis
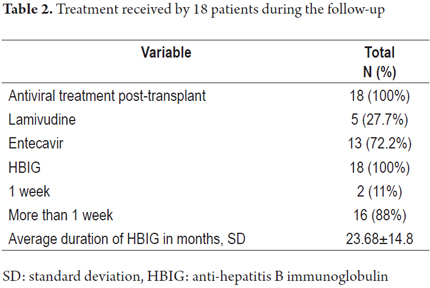
Of the 18 patients, 16 received HBIG for 23.68 ± 14.8 months accompanied by lamivudine in 27.7% of the patients and by or entecavir in 72.2% of the patients. Of the 18 patients, two received HBIG only for the first 7 days after transplantation and the continued with entecavir (Table 2).
Serological follow-up
HBeAg seroconversion was found in 11 of the 14 patients evaluated. During follow-up, two of the 18 patients (11.1%) were HBsAg positive. One relapsed at 41 months post-transplant and three months after HBIG suspension. His viral load was 464,977,202 copies/mL despite the continued use of lamivudine. This patient had received lamivudine for 19 months prior to transplantation. After the relapse was detected, entecavir therapy was modified without response. The patient was then switched to tenofovir which resulted in better control of viral load and better graft functioning. There were unexplained recurrences of hepatocellular carcinoma through viral replication in the post-transplant period in two of the 18 cases (Table 3). The overall relapse rate was 5.5% (1 out of 18 patients).
Graft dysfunction
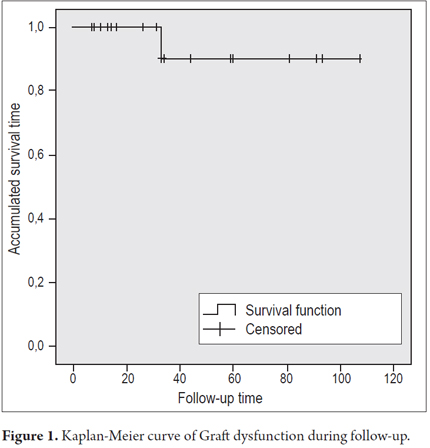
There were 8 cases of graft dysfunction during follow-up: two due to bile duct stenosis, one due to hepatotoxicity, two due to mild acute cellular rejection, one due to chronic periportal hepatitis, one due to HBV relapse, one due to relapse of a fibrolamellar tumor. All cases of dysfunction were transient, except in the patient with fibrolamellar tumor, in whom the disease progressed. Overall graft dysfunction was 10% at 33 months (Figure 1), and there were no deaths during follow-up.
Adverse reactions
There were no adverse events related to therapy.
Costs
Because the recurrence rates reported in this study are similar to those reported in studies using high doses of HBIG, it can be assumed that both protocols coincide in effectiveness. Nevertheless, even though we did not do a cost-effectiveness study and these are only observational data, the two protocols costs are very different.
DISCUSSION
Currently, liver transplantation is the best alternative for patients with chronic complications of HBV infections. (11, 12) In the early 1990s, high-dose HBIG prophylaxis became the best therapeutic alternative for decreasing graft reinfections and mortality. (4) Markowitz optimized prophylaxis by combining HBIG with lamivudine, (13) but the cost of high doses of immunoglobulin has been a limiting factor and has led to the emergence of protocols with lower doses. Nevertheless, ability to generate neutralizing antibodies and to avoid recurrence with low doses has been questioned.
McCaughan et al. used low doses of HBIG in 9 patients to 17 months after transplantation without relapses. (14) Angus et al. used low doses of HBIG in 32 patients without recurrences up to 18 months after transplantation. (10) Finally, in 2007, Gane et al. used low doses of HBIG with lamivudine in an Australian and New Zealand cohort and found a reinfection rate of 4% up to 5 years. The one year survival rate was 82%, and the five-year survival rate was 88% which is similar to those reported with high dose protocols. (9) We report our experience using a low-dose HBIG protocol combined with lamivudine or entecavir in transplant patients with hepatitis B at a liver transplant referral center in Colombia. The results agree with those of other series. With a recurrence rate of infection only 5.5% (One out of 18 patients). The viral load at the time of transplantation of the patient who relapsed is unknown, even though he had received 19 months of lamivudine monotherapy previously. The relapse occurred 3 months after discontinuing HBIG. We suspect resistance to the nucleoside analogue, but he also relapsed with entecavir and showed improvement only after starting tenofovir and reducing immunosuppression to control viremia.
Most patients became negative for HBsAg during follow-up, so it is considered to be a good parameter for follow-up. Only one patient was positive for HBsAg during follow-up but without correlation with a positive viral load. Similar to other cohorts in which there are very few reports of adverse events with low doses of HBIG, we found no adverse effects related to the administration of HBIG. Nevertheless, more similar studies are required to support the use of low-dose immunoglobulin as a prophylactic alteration in low-income countries. This is due to the fact that duration of prophylaxis can be long-term or indefinite (15% to one year and 8% to two years). (15) In this regard, studies have been published suggesting a limited time use of HBIG combined with a high genetic antiviral barrier (tenofovir or entecavir) (13 16 17) HBIG-free schemes have even been suggested. (18).
A study by Choudhary et al. that used HIBG for 12 months followed by tenofovir or entecavir reported a relapse rate of 4.3% at an average of 43 months of follow-up (range: 12-117). (19) Similarly, Tanaka et al. found no relapses at 29 months after using HBIG for 12 months followed by tenofovir combined with lamivudine. (20) An HBIG-free protocol using entecavir monotherapy once viral load has been controlled has also been described with a relapse rate of 1.26% up to 26 months. (21). The mean time of HBIG administration in our patients was 23 ± 14 months. The reason for this is that patients transplanted in the early years used to use HBIG indefinitely, but in recent years with the advent of new antivirals we decided to shorten the time of HBIG treatment, especially when seroconversion of HBeAg and HBsAg was achieved. (22) Use of nucleoside analogues in monotherapy or in combination therapy with another analogue is progressively increasing for prophylaxis. One result is decreasing use of HBIG. Nevertheless, long-term studies of the safety of HBIG-free schemes are still required. In the meantime, many transplant centers worldwide continue to use HBIG as the optimal treatment for preventing reinfection.
This study has several limitations. Among them are its retrospective character and the absence of viral load measurements at the time of transplantation for four patients. Consequently, it was not possible to make associations between pretransplant HBV viral loads and viral loads after relapse. Another limitation was the absence of systematic evaluation of viral loads in the post-transplant period. Nevertheless, extensive follow-up allowed evaluation of graft functioning as well as mortality for more than 3 years after transplantation. Although we did not perform any cost-effectiveness analysis, we can infer that results similar to those reported with the administration of high dosages can be obtained at lower costs through usage of low dosages of HBIG.
While studies of whether HBIG-free prophylaxis can result in sufficient long-term safety continue, low-dose prophylaxis of HBIG will continue to be useful for preventing recurrences of infections following transplantation.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Financial support
This work received no financial support from any entity.
REFERENCES
1. European Association for the Study of the Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57(1):167-85. [ Links ]
2. Cubides VI, Suárez CY, Quintero PA. Epidemiología e historia natural de la hepatitis B. Rev Col Gastroenterol. 2009;24(1):4-12. [ Links ]
3. Tolosa-Pérez N. Protocolo de vigilancia en salud pública. Hepatitis B, C y coinfección hepatitis B-Delta. Bogotá: Instituto Nacional de Salud; 2014. [ Links ]
4. Lok AS. Prevention of recurrent hepatitis B post-liver transplantation. Liver Transplant. 2002;8(10 Supl 1):67-73. [ Links ]
5. Mutimer D. Review article: hepatitis B and liver transplantation. Aliment Pharmacol Ther. 2006;23(8):1031-41. [ Links ]
6. Marzano A, Salizzoni M, Debernardi-Venon W, et al. Prevention of hepatitis B virus recurrence after liver transplantation in cirrhotic patients treated with lamivudine and passive immunoprophylaxis. J Hepatol. 2001;34(6):903-10. [ Links ]
7. Grellier L, Mutimer D, Ahmed M, et al. Lamivudine prophylaxis against reinfection in liver transplantation for hepatitis B cirrhosis. Lancet. 1996;348(9036):1212-5. [ Links ]
8. Mutimer D, Dusheiko G, Barrett C, et al. Lamivudine without HBIg for prevention of graft reinfection by hepatitis B: long-term follow-up. Transplantation. 2000;70(5):809-15. [ Links ]
9. Gane EJ, Angus PW, Strasser S, et al. Lamivudine plus low-dose hepatitis B immunoglobulin to prevent recurrent hepatitis B following liver transplantation. Gastroenterology. 2007;132(3):931-7. [ Links ]
10. Angus PW, McCaughan GW, Gane EJ, et al. Combination low-dose hepatitis B immune globulin and lamivudine therapy provides effective prophylaxis against posttransplantation hepatitis B. Liver Transplant. 2000;6(4):429-33. [ Links ]
11. Markowitz JS, Martin P, Conrad AJ, et al. Prophylaxis against hepatitis B recurrence following liver transplantation using combination lamivudine and hepatitis B immune globulin. Hepatology. 1998;28(2):585-9. [ Links ]
12. Xi ZF, Xia Q. Recent advances in prevention of hepatitis B recurrence after liver transplantation. World J Gastroenterol. 2015;21(3):829-35. [ Links ]
13. Ahn J, Cohen SM. Prevention of hepatitis B recurrence in liver transplant patients using oral antiviral therapy without long-term hepatitis B immunoglobulin. Hepat Mon. 2011;11(8):638-45. [ Links ]
14. McCaughan GW, Spencer J, Koorey D, et al. Lamivudine therapy in patients undergoing liver transplantation for hepatitis B virus precore mutant-associated infection: high resistance rates in treatment of recurrence but universal prevention if used as prophylaxis with very low dose hepatitis B immune globulin. Liver Transplant.1999;5(6):512-9. [ Links ]
15. Togashi J, Akamastu N, Sugawara Y, et al. One-year extended, monthly vaccination prophylaxis combined with hepatitis B immune globulin for hepatitis B after liver transplantation. Hepatol Res. 2016;46(3):E51-9. [ Links ]
16. Varghese J, Sachan D, Reddy MS, et al. Hepatitis B immunoglobulin prophylaxis after liver transplantation: experience in a tertiary transplant centre. J Clin Exp Hepatol. 2014;4(3):209-13. [ Links ]
17. Lim YS, Han S, Heo NY, et al. Mortality, liver transplantation, and hepatocellular carcinoma among patients with chronic hepatitis B treated with entecavir vs lamivudine. Gastroenterology. 2014;147(1):152-61. [ Links ]
18. Teperman LW, Poordad F, Bzowej N, et al. Randomized trial of emtricitabine/tenofovir disoproxil fumarate after hepatitis B immunoglobulin withdrawal after liver transplantation. Liver Transplant. 2013;19(6):594-601. [ Links ]
19. Choudhary NS, Saraf N, Saigal S, et al. Low-dose short-term hepatitis B immunoglobulin with high genetic barrier antivirals: the ideal post-transplant hepatitis B virus prophylaxis? Transpl Infect Dis. 2015;17(3):329-33. [ Links ]
20. Tanaka T, Renner EL, Selzner N, et al. One year of hepatitis B immunoglobulin plus tenofovir therapy is safe and effective in preventing recurrent hepatitis B post-liver transplantation. Can J Gastroenterol Hepatol. 2014;28(1):41-4. [ Links ]
21. Fung J, Cheung C, Chan SC, et al. Entecavir monotherapy is effective in suppressing hepatitis B virus after liver transplantation. Gastroenterology. 2011;141(4):1212-9. [ Links ]
22. Jeong SW, Choi Y, Kim JW. Management of viral hepatitis in liver transplant recipients. Clin Mol Hepatol. 2014;20(4):338-44. [ Links ]











 texto em
texto em