Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista colombiana de Gastroenterología
versão impressa ISSN 0120-9957
Rev Col Gastroenterol vol.34 no.4 Bogotá out./dez. 2019
https://doi.org/10.22516/25007440.450
Original articles
Hybrid technique versus standard technique for endoscopic ultrasound guided fine needle aspiration of solid pancreatic lesions
1 Especialista en Gastroenterología y Medicina Interna. Hospital Universitario Nacional de Colombia, Hospital Occidente de Kennedy, Unidad de Gastroenterología y Endoscopia (UGEC). Bogotá, Colombia
2 Especialista en Gastroenterología y Medicina Interna. Universidad San Martín, Hospital Occidente de Kennedy, Hospital Universitario Nacional de Colombia. Bogotá, Colombia
3 Internista, Gastroenterólogo. Universidad Nacional de Colombia. Centro de Enfermedades Digestivas GutMédica. Bogotá, Colombia.
Endoscopic ultrasound (EUS) is widely used to evaluate pancreatobiliary diseases, especially pancreatic masses. EUS has a good ability to detect pancreatic masses, but it is not sufficient for differential diagnoses of various types of lesions. Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) is the diagnostic method of choice for pancreatic masses, but its accuracy is affected by various puncture methods.
Materials and methods:
Our objective was to compare the diagnostic yield of examinations of solid lesions in the pancreas by the standard suction technique (ST) with the yield of the hybrid technique (HT) using a prospective, single blind, randomized, controlled design. Patients diagnosed with solid pancreatic lesions who underwent EUS-FNA from May 2014 to June 2016 were included.
Results:
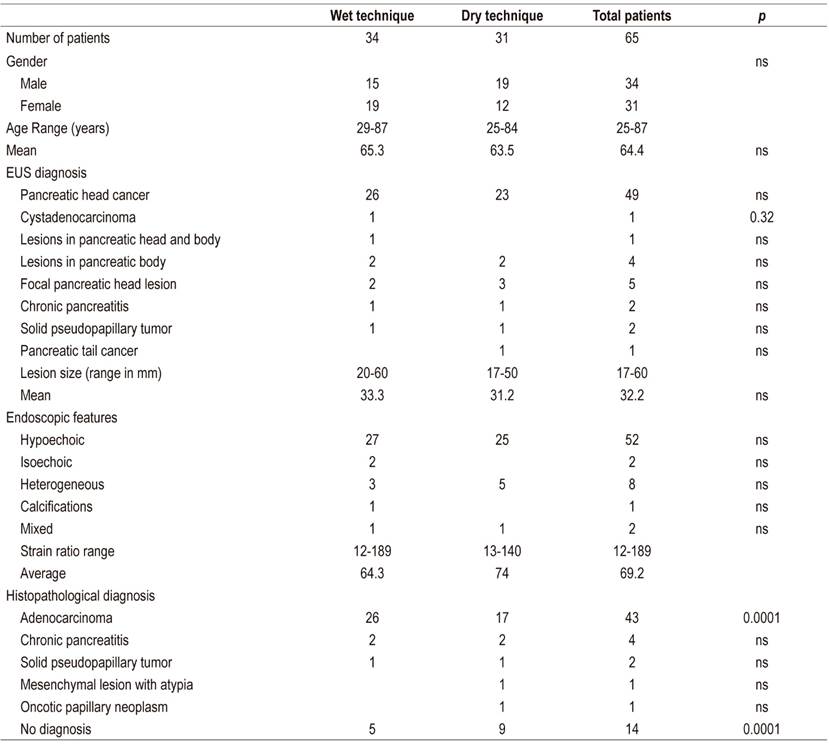
We included 65 patients, 34 of whom (52.3%) were assigned to EUS-FNA with HT, and 31 of whom (47.7%) were assigned to EUS-FNA with TS. We found that the relative frequency that HT successfully obtained an adequate amount of tissue for the cytological diagnosis was 85.2% while ST’s relative frequency of success was 71%. The odds ratio was 2.35 (95% CI; 1.2-4.7) in favor of HT.
Conclusion:
This study suggests that the TH is superior to ST for diagnosis of solid pancreatic lesions. Since implementation of this technique does not increase costs and is very simple, we suggest that it become the technique of choice for EUS-FNA.
Keywords: Endoscopic ultrasound; pancreatic cancer; fine needle puncture; cytology
La ultrasonografía endoscópica (USE) se usa ampliamente para evaluar enfermedades pancreatobiliares, especialmente masas pancreáticas. La USE tiene una buena capacidad para detectar masas pancreáticas, pero no es suficiente para el diagnóstico diferencial de varios tipos de lesiones. La aspiración endoscópica con aguja fina guiada por ultrasonido (USE-PAF) es el método de diagnóstico de elección para masas pancreáticas y su precisión se afecta por diversos métodos de punción.
Materiales y métodos:
nuestro objetivo fue evaluar el rendimiento diagnóstico de la técnica de succión estándar (TS) versus la técnica húmeda híbrida (TH) en el estudio de lesiones sólidas en páncreas, utilizando un diseño prospectivo, con ocultación única, aleatorizado y controlado, que incluye a pacientes con diagnóstico de lesión sólida en páncreas a los que se realizó USE-PAF desde mayo de 2014 a junio de 2016.
Resultados:
en total incluimos 65 pacientes, 34 (52,3%) se asignaron a USE-PAF con TH y 31 (47,7%) pacientes a USE-PAF con TS. Se encontró que la frecuencia relativa porcentual respecto a la técnica de punción en la USE-PAF en lesiones sólidas de páncreas, que permite obtener la cantidad de tejido adecuado para el diagnóstico citológico, fue de 85,2% para la TH y 71% para la TS, con un OR de 2,35 (IC 95%; 1,2-4,7) a favor de la TH.
Conclusión:
este estudio sugiere que la TH es superior a la TS en el diagnóstico de las lesiones sólidas del páncreas, por lo cual, dado que la implementación de esta técnica no aumenta costos y es muy sencilla, sugerimos que sea la técnica de elección cuando se necesita puncionar una lesión sólida.
Palabras clave: Ultrasonografía endoscópica; cáncer de páncreas; punción aguja fina; citología
Introduction
Solid pancreatic lesions are heterogeneous but can be classified as either neoplastic and non-neoplastic. Neoplastic lesions, the most common, include adenocarcinoma, neuroendocrine tumors, solid pseudopapillary tumors, pancreatoblastomas, lymphomas, metastases, and rare miscellaneous neoplasms. 1 Ductal adenocarcinoma accounts for about 90% of all pancreatic malignancies. 2 It is a significant cause of mortality. Its 5-year survival rate is less than 5% but can reach 20% in selected patients with non-invasive tumors who have undergone surgical resection. The objective is to detect it in early stages. 3 Currently, ultrasound, computed tomography and magnetic resonance imaging are the mainstays used to evaluate 80% to 85% of solid pancreatic lesions. 4 Preoperative diagnosis of solid pancreatic lesions is challenging, despite technological advances in imaging. Endoscopic ultrasound guided fine needle aspiration (EUS-FNA) is the method of choice for detection and diagnosis of these lesions. 5 Its diagnostic yield is highly sensitive and specific, but several factors can affect its performance. Among these are the experience of the echoendoscopist, equipment position, time of day, needle size, technique used, characteristics of lesions, number of passes, whether there is a cytologist in the room, and chronic pancreatitis. 6-16
The two principal techniques developed to address solid pancreatic lesions are dry suction and wet suction. 17. The dry technique (DT) is standard and consists of placing the patient in the optimal position, locating the lesion with endoscopic ultrasound (EUS), insertion of a 22 gauge needle including a removable stylet, selection of the puncture line, puncturing the lesion with the needle, removal of the stylet, suction by vacuum syringe, movement of the needle from side to side, removal of the needle, and ejection of the sample from the needle using the stylet. 18
The wet technique has recently been developed to improve sample quality. Prior to puncturing the lesion, the stylet is removed from the 22 gauge needle and pre-washed with 5 mL of saline solution to replace the air column with liquid. A 10 mL syringe is pre-filled with 3 mL of saline solution and used for aspiration after the lesion is punctured. Once the needle is inside the lesion, it is moved from side to side three times. This maneuver is repeated four times (passes) for a total of 12 movements. When the needle is withdrawn, the aspirate is released onto a slide and air is applied. This technique is safer and more efficient for removing the aspirate than is reinsertion of the stylet. 17,19 A recent metaanalysis has found that patients were more likely to bleed when the stylet was reinserted to remove the aspirate than when the wet technique was used. 20
The hybrid (TH) technique consists of performing the same steps as the initial wet technique except that a the needle containing a pre-assembled vacuum syringe is used. It is activated once the needle is inside the lesion.
The objective of the present work is to determine and compare the diagnostic yields of the standard dry suction technique and the hybrid wet suction technique when used to study solid pancreatic lesions at a third level hospital institution in Bogotá.
Materials and method
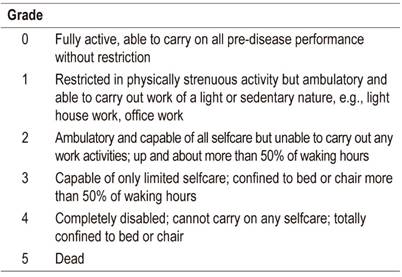
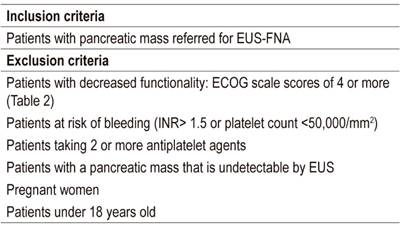
This study presents our experience at a third level hospital in Bogotá. It is a prospective, single-blind, randomized and controlled study of EUS-FNA techniques for obtaining adequate amounts of tissue for pathological diagnosis of solid pancreatic lesions. Patients who had been diagnosed with solid pancreatic lesion and who underwent EUS-FNA between May 2014 and June 2016 were included in the study. The inclusion and exclusion criteria are summarized in Table 1 and Table 2 The procedures were performed in the gastroenterology ward of a third level hospital in Bogotá under anesthesiologist-guided sedation using a combination of propofol and remifentanil. All EUS-FNA procedures used Pentax brand linear EUS equipment and were performed by an endoscopist who had previously performed more than 1000 such procedures.
Table 1 Inclusion and exclusion criteria
ECOG: Eastern Cooperative Oncology Group. INR: international normalized ratio.
FNA 22 gauge (Boston Scientific) needles were used. The hybrid suction technique and the standard dry suction technique (10 mL) with a stylet were used to take biopsies using a total of three passes and four movements within the lesion according to the recommendations described in the literature. 17-19 Samples were spread on slides, fixed in ethyl alcohol, and sent for pathological study by a specialist in cytology of the pancreas who did not know whether the hybrid or dry technique had been used to obtain the samples. Information was recorded in Google Drive. Discrete quantitative variables were obtained and expressed in absolute and relative frequencies from which the risk estimate (odds ratio - OR) was calculated. The capacity of each technique for obtaining a sufficient quantity and sample quality pathological diagnosis was then determined.
Results
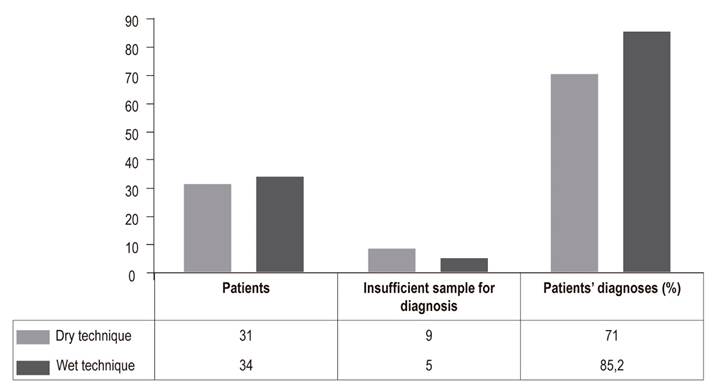
Data were collected from 65 patients who underwent EUS-FNA for diagnosis of solid pancreatic lesions. The hybrid technique was used for 34 patients (52.3%) while the standard dry technique with stylet was used for 31 patients (47.7%). Characteristics are summarized in Table 3. It was found that the relative frequency percentage for EUS-FNA study of solid pancreatic lesions to obtaining adequate amounts of tissue for cytological diagnosis was 85.2% for the hybrid technique and 71% for the dry technique (Figure 1) indicating that the hybrid techniques diagnostic yield is 14.2% higher than that of the standard technique with an OR of 2.35 (95 % CI: 1.2 to 4.7).
Discussion
Endoscopic ultrasound (EUS) offers excellent visualization of the pancreas from the duodenum or stomach. It produces high-resolution images making it one of the most accurate methods for detecting pancreatic focal lesions, especially in patients with tumors measuring 3 cm or less. 22 In addition, EUS-FNA can obtain samples for pathological diagnosis. EUS is currently considered a safe and accurate imaging technique for tissue diagnosis in patients with pancreatic-biliary lesions and is particularly useful for diagnosing pancreatic tumors and guiding therapeutic decisions. 23 For diagnosis of these carcinomas, it has been found to have diagnostic sensitivity of 54% to 96%, specificity of 96% to 98%, and diagnostic accuracy of 83% to 95%. 24-26
Attempts to bring its diagnostic yield closer to 100% have included development of several puncture needles including 19, 22 and 25 gauge needles. The 25 gauge needles are easier to handle, cause fewer complications (bleeding), and are less likely to obtain blood-contaminated specimens than are the 19 and 22 gauge needles. 9,27-29 In addition, 25 gauge needles have been shown to have better diagnostic yields for solid pancreatic tumors than do 22 gauge needles (combined sensitivity: 93% for 25 gauge needles vs. 85% for 22 gauge needles for cytology-based diagnoses). 10
Nevertheless, the four available metaanalyses on this topic have conflicting results. There is consistent evidence that the cytological quality of samples obtained with 22 and 25 gauge needles are similar, and no convincing advantages of either of the two gauges have been demonstrated in terms of technical performance, ease of use, or safety. Consequently, we decided to use 22 gauge needles for both the wet and dry techniques in this study in order to avoid a confounding factor. 8,10,11,18,30,31 Although this technique is considered safe, it is not without complications (0% to 3.4%): the most frequent is mild pancreatitis. 31-33
Target tissue factors such as masses measuring 20 mm or less and endocrine tumors increase the risk of post-puncture complications. 31-34 Also, serious complications such as bleeding (0.2%), ruptured pseudoaneurysms, pancreatic pseudocysts, abscesses, and cancer seeding have been reported even though they are rare. 31,35-37 Infectious complications including bacteremia and sepsis occur in 0% to 1%; of punctures of solid pancreatobiliary lesions, but no documented bleeding or infections were reported in this study. 37,38
The basic principle of this procedure is to use ultrasound to visualize the target lesion. The puncture site is chosen by taking into account the position of the transducer, presence of blood vessels, amount of tissue between the transducer and the lesion and other factors. The needle chosen is advanced to puncture the lesion, the stylet (if used) is removed, and suction is applied. Then, the needle is advanced and withdrawn through the lesion to obtain cellular material. Finally, the needle is withdrawn and the tissue is collected for cytopathological examination. Variations of this technique have been studied to determine how to improve diagnostic yield. Key factors that can vary include selection of the puncture site, choice of needle, use of a stylet, suction, number of punctures and presence of a cytopathologist. 23,31,39
Some modifications have increased diagnostic yields. Positioning is in first place. The procedure can be performed more comfortably way when the echoendoscope is in a stable position with the tip straight. This allows easy passage of the puncture needle. It is generally better achieved from the transgastric position than from the transduodenal position. 18,40 It is important to collect samples from multiple sections of a pancreatic lesion using multiple punctures and the fanning technique. Since neoplastic lesions can be heterogeneous with acellular necrotic centers, it is crucial to focus on multiple areas of the lesion, especially on the periphery. Currently, five punctures using the fanning technique are recommended for solid pancreatic lesions. 13,18,39 This technique consists of intermittently changing the position of the needle angle using the controls and the elevator to take successive samples from multiple areas of the lesion. This increases the amount of tissue collected, so it was included in the protocol of this study. 13,31,41
A growing amount of evidence also supports the use of Rapid On-site Evaluation (ROSE) with EUS-FNA. ROSE requires the presence of a cytopathologist in the endoscopy room. Using an optical microscope in the endoscopy room, the cytopathologist evaluates the smears and provides the endosonographer with immediate feedback about the quality of the samples for diagnosis and whether additional samples are required. 15,18,31,39,41
Numerous studies have confirmed that ROSE increases diagnostic yield by limiting the number of passages and decreasing the number of inappropriate samples. 15,18,40-45 Unfortunately, in our setting, the possibility of having a cytopathologist in the endoscopy room is quite limited given the high cost. For this reason, we did not adopt this practice in our study. We decided to perform five punctures and use the fanning technique as recommended by the European Society for Gastrointestinal Endoscopy for puncture protocol. 18
It is currently known that EUS-FNA commonly fails to result in diagnosis when the cellularity of aspirates obtained is low. This leads to repeated procedures, increased costs and delays in diagnosis which in turn delay early adaptation of treatment strategies. The consequences are higher rates of morbidity and mortality for patients. 44,45 Initially, wet and dry suction techniques were developed to improve the diagnostic yields of FNA of intra-abdominal solid lesions or those located in the mediastinum. The hybrid technique has not yet been recommended as the overall standard for EUS-FNA. When the dry technique is used, tissue samples have greater cellularity, but there may be more blood contamination which affects the overall quality of the sample. 20,46
The wet technique’s theoretical superiority is based on a dynamic three-dimensional computational fluid model. Because water is less compressible than air, a needle filled with water should be superior to a needle filled with air since it allows a faster aspiration of the material at the distal end of the needle. 17 The results of our study show that the samples obtained with the wet technique were sufficient to obtain a pathological diagnosis in 85.2% of the cases for which the wet technique was used but in only 71% of the cases in which the dry suction technique was used. The wet technique’s diagnostic yield was 14.2 % higher than that of the standard dry technique. These results correlate with the findings of Attam et al. They compared the wet suction technique with the dry technique in 117 patients and found that the wet suction technique significantly increased the acquisition of tissue and had better diagnostic yield: 85.5% versus 75.2% (P <0.035). There was no difference in the amount of blood contamination between the two techniques. 19
Another pilot study comparing wet, hybrid and dry EUS-FNA techniques in 15 patients with solid lesions was conducted by Berzosa et al. Their objectives were to determine the appropriate sample needed to reach a final pathological diagnosis and to determine the volume of material aspirated and the diagnostic yield (malignant or non-malignant) for each technique. 47 No significant differences were found among the hybrid, wet and dry techniques (87%, 87% and 67%, respectively), but this may be explained by the study’s low statistical power. 17
Although the exact reason why the wet technique provides greater cellularity in the samples obtained is not yet known, theories based on computer models show that a needle filled with water is superior to a needle filled with air since it allows faster aspiration of the material at the distal end of the needle which allows better transmission of the suction applied than that of an air column inside the needle. The saline solution can coat the inner lining of the needle, therefore changing the properties of the surface. This facilitates the movement of the aspirate towards the needle. In addition, the saline column can act as a stylet, potentially reducing tissue contamination during puncture of a lesion while also preventing the needle from clogging. 17,18,47
In addition, the wet technique’s saline solution changes the properties of the internal surface of the hollow needle which can reduce friction between the tissue aspirate and the needle wall thereby allowing greater movement into the needle channel. 17 Given the conditions in which this study was conducted, we consider that its main limitation was our inability to have a cytopathologist use ROSE in the endoscopy room. However, we consider that this is not feasible in most endoscopy centers in Colombia even though its absence is likely to result in larger numbers of inadequate samples and therefore lower diagnostic yields. 48 Another limitation is the small sample size although it is much larger than the sample used in the study by Barsa et al. Also, it would be important for the volume of material in each group to have been measured, even though our goal was diagnostic sensitivity.
Conclusion
Our findings suggest that the hybrid wet technique significantly increases cellularity in samples obtained from solid pancreatic lesions above those obtained by the conventional technique. Moreover, the technique is easy to apply in the context of the absence of a cytopathologist in the endoscopy. In addition, the implementation of this has no additional costs. Since this study and another smaller international one suggest the technique’s superiority, it should become the technique of choice for EUS-FNA of solid pancreatic lesions.
Referencias
1. Low G, Panu A, Millo N, Leen E. Multimodality imaging of neoplastic and nonneoplastic solid lesions of the pancreas. Radiographics. 2011;31:993-1015. doi: https://doi.org/10.1148/rg.314105731. [ Links ]
2. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-86. doi: https://doi.org/10.1002/ijc.29210. [ Links ]
3. Liles J, Katz M. Pancreaticoduodenectomy with vascular resection for pancreatic head adenocarcinoma. Expert Rev Anticancer Ther. 2014;14:919-29. doi: https://doi.org/10.1586/14737140.2014.919860. [ Links ]
4. Scialpi M, Reginelli A, D’Andrea A, Gravante S, Falcone G, Baccari P, et al. Pancreatic tumors imaging: an update. Int J Surg. 2016;(1):S142-55. doi: https://doi.org/10.1016/j.ijsu.2015.12.053. [ Links ]
5. Wang W, Shpaner A, Krishna S, Ross W, Bhutani M, Tamm EP, et al. Use of EUS-FNA in diagnosing pancreatic neoplasm without a definitive mas son CT. Gastrointest Endosc. 2013;78:73-80. doi: https://doi.org/10.1016/j.gie.2013.01.040. [ Links ]
6. Hewitt M, McPhail M, Possamai L, Dhar A, Vlavianos P, Monahan K. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc. 2012;75:319-31. doi: https://doi.org/10.1016/j.gie.2011.08.049. [ Links ]
7. Eloubeidi M, Tamhane A. EUS-guided FNA of solid pancreatic masses: a learning curve with 300 consecutive procedures. Gastrointest Endosc. 2005;61:700-8. doi: https://doi.org/10.1016/S0016-5107(05)00363-9. [ Links ]
8. Korenblit J, Tholey D, Tolin J, Loren D, Kowalski T, Adler DG, et al. Effect of the time of day and queue position in the endoscopic schedule on the performance characteristics of endoscopic ultrasound-guided fine-needle aspiration for diagnosing pancreatic malignancies. Endosc Ultrasound. 2016;5:78-84. doi: https://doi.org/10.4103/2303-9027.180470. [ Links ]
9. Ramesh J, Bang J, Hebert-Magee S, Trevino J, Eltoum I, Frost A, et al. Randomized trial comparing the flexible 19G and 25G needle for endoscopic ultrasound-guided fine needle aspiration of solid pancreatic mass lesions. Pancreas. 2015;44:128-33. doi: https://doi.org/10.1097/MPA.0000000000000217. [ Links ]
10. Madhoun M, Wani S, Rastogi A, Early D, Gaddam S, Tierney W, et al. The diagnostic accuracy of 22-gauge and 25-gauge needles in endoscopic ultrasound-guided fine needle aspiration of solid pancreatic lesions: a meta-analysis. Endoscopy. 2013;45:86-92. doi: https://doi.org/10.1055/s-0032-1325992. [ Links ]
11. Kamata K, Kitano M, Yasukawa S, Kudo M, Chiba Y, Ogura T, et al. Histologic diagnosis of pancreatic masses using 25-gauge endoscopic ultrasound needles with and without core trap: a multicenter randomized trial. Endoscopy. 2016;48:632-8. doi: https://doi.org/10.1055/s-0042-106294. [ Links ]
12. Nakai Y, Isayama H, Chang K, Yamamoto N, Hamada T, Uchino R, et al. Slow pull versus suction in endoscopic ultrasound-guided fine-needle aspiration of pancreatic solid masses. Dig Dis Sci. 2014;59:1578-85. doi: https://doi.org/10.1007/s10620-013-3019-9. [ Links ]
13. Bang J, Magee S, Ramesh J, Trevino J, Varadarajulu S. Randomized trial comparing fanning with standard technique for endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic mass lesions. Endoscopy . 2013;45:445-50. doi: https://doi.org/10.1055/s-0032-1326268. [ Links ]
14. Suzuki R, Irisawa A, Bhutani M, Hikichi T, Takagi T, Sato A, et al. Prospective evaluation of the optimal number of 25-gauge needle passes for endoscopic ultrasound-guided fine-needle aspiration biopsy of solid pancreatic lesions in the absence of an onsite cytopathologist. Dig Endosc. 2012;24:452-6. doi: https://doi.org/10.1111/j.1443-1661.2012.01311.x. [ Links ]
15. Hebert-Magee S, Bae S, Varadarajulu S, Ramesh J, Frost R, Eloubeidi MA, et al. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: a meta-analysis. Cytopathology. 2013;24:159-71. doi: https://doi.org/10.1111/cyt.12071. [ Links ]
16. Varadarajulu S, Tamhane A, Eloubeidi M. Yield of EUS-guided FNA of pancreatic masses in the presence or the absence of chronic pancreatitis. Gastrointest Endosc. 2005;62:728-36. doi: https://doi.org/10.1016/j.gie.2005.06.051. [ Links ]
17. Villa N, Berzosa M, Wallace M, Raijman I. Endoscopic ultrasound-guided fine needle aspiration: the wet suction technique. Endosc Ultrasound. 2016;5:17-20. doi: https://doi.org/10.4103/2303-9027.175877. [ Links ]
18. Polkowski M, Jenssen C, Kaye P, Carrara S, Deprez P, Gines A, et al. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline - March 2017. Endoscopy . 2017;49:989-1006. doi: https://doi.org/10.1055/s-0043-119219. [ Links ]
19. Attam R, Arain M, Bloechl S, Trikudanathan G, Munigala S, Bakman Y, et al. “Wet suction technique (WEST)”: a novel way to enhance the quality of EUS-FNA aspirate. Results of a prospective, single-blind, randomized, controlled trial using a 22-gauge needle for EUS-FNA of solids lesions. Gastrointest Endosc. 2015;81:1401-7. doi: https://doi.org/10.1016/j.gie.2014.11.023. [ Links ]
20. Lee J, Choi J, Lee K, Kim K, Shin J, Lee JK, et al. A prospective, comparative trial to optimize sampling techniques in EUS- guided FNA of solid pancreatic masses. Gastrointest Endosc. 2013;77:745-51. doi: https://doi.org/10.1016/j.gie.2012.12.009. [ Links ]
21. Skelton W, Parekh H, Starr J, Trevino J, Cioffi J, Hughes S, et al. Clinical factors as a component of the personalized treatment approach to advanced pancreatic cancer: a systematic literature review. J Gastrointest Cancer. 2018;49(1):1-8. doi: 10.1007/s12029-017-0021-z. [ Links ]
22. Lee E, Lee J. Imaging diagnosis of pancreatic cancer: a state-of-the-art review. World J Gastroenterol. 2014;20:7864-77. doi: https://doi.org/10.3748/wjg.v20.i24.7864. [ Links ]
23. Han J, Chang K. Endoscopic ultrasound-guided direct intervention for solid pancreatic tumors. Clin Endosc. 2017;50:126-37. doi: https://doi.org/10.5946/ce.2017.034. [ Links ]
24. Chen G, Liu S, Zhao Y, Dai M, Zhang T. Diagnostic accuracy of endoscopic ultrasound-guided ne-needle aspiration for pancreatic cancer: a meta-analysis. Pancreatology. 2013;13:298-304. doi: https://doi.org/10.1016/j.pan.2013.01.013. [ Links ]
25. Puli S, Bechtold M, Buxbaum J, Eloubeidi M. How good is endoscopic ultrasound-guided ne-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass?: a meta-analysis and systematic review. Pancreas . 2013;42:20-6. doi: https://doi.org/10.1097/MPA.0b013e3182546e79. [ Links ]
26. Banafea O, Mghanga F, Zhao J, Zhao R, Zhu L. Endoscopic ultrasonography with fine-needle aspiration for histological diagnosis of solid pancreatic masses: a meta-analysis of diagnostic accuracy studies. BMC Gastroenterol. 2016;16:108. doi: https://doi.org/10.1186/s12876-016-0519-z. [ Links ]
27. Varadarajulu S, Bang J, Holt B, Hasan M, Logue A, Hawes R, et al. The 25-gauge EUS-FNA needle: Good for on-site but poor for off-site evaluation? Results of a randomized trial. Gastrointest Endosc . 2014;80:1056-63. doi: https://doi.org/10.1016/j.gie.2014.05.304. [ Links ]
28. Rastogi A, Wani S, Gupta N, Singh V, Gaddam S, Reddymasu S, et al. A prospective, single-blind, randomized, controlled trial of EUS-guided FNA with and without a stylet. Gastrointest Endosc . 2011;74:58-64. doi: https://doi.org/10.1016/j.gie.2011.02.015. [ Links ]
29. Kamata K, Kitano M, Omoto S, Kadosaka K, Miyata T, Minaga K, et al. New endoscopic ultrasonography techniques for pancreaticobiliary diseases. Ultrasonography. 2016;35:169-79. doi: https://doi.org/10.14366/usg.15042. [ Links ]
30. Larghi A, Iglesias-García J, Poley J, Monges G, Petrone M, Rindi G, et al. Feasibility and yield of a novel 22-gauge histology EUS needle in patients with pancreatic masses: a multicenter prospective cohort study. Surg Endosc. 2013;27:3733-8. doi: https://doi.org/10.1007/s00464-013-2957-9. [ Links ]
31. Matsubayashi H, Matsui T, Yabuuchi Y, Imai K, Tanaka M, Kakushima N, et al. Endoscopic ultrasonography guided-fine needle aspiration for the diagnosis of solid pancreaticobiliary lesions: Clinical aspects to improve the diagnosis. World J Gastroenterol . 2016;22:628-40. doi: https://doi.org/10.3748/wjg.v22.i2.628. [ Links ]
32. Yasuda I, Iwashita T, Doi S. Tips for endoscopic ultrasound-guided fine needle aspiration of various pancreatic lesions. J Hepatobiliary Pancreat Sci. 2014;21:E29-33. doi: https://doi.org/10.1002/jhbp.60. [ Links ]
33. Haba S, Yamao K, Bhatia V, Mizuno N, Hara K, Hijioka S, et al. Diagnostic ability and factors affecting accuracy of endoscopic ultrasound-guided fine needle aspiration for pancreatic solid lesions: Japanese large single center experience. J Gastroenterol. 2013;48:973-81. doi: https://doi.org/10.1007/s00535-012-0695-8. [ Links ]
34. Puri R, Vilmann P, Săftoiu A, Skov B, Linnemann D, Hassan H, et al. Randomized controlled trial of endoscopic ultrasound-guided fine-needle sampling with or without suction for better cytological diagnosis. Scand J Gastroenterol. 2009;44:499-504. doi: https://doi.org/10.1080/00365520802647392. [ Links ]
35. Hamada T, Yasunaga H, Nakai Y, Isayama H, Horiguchi H, Matsuda S, et al. Severe bleeding and perforation are rare complications of endoscopic ultrasound-guided fine needle aspiration for pancreatic masses: an analysis of 3,090 patients from 212 hospitals. Gut Liver. 2014;8:215-8. doi: https://doi.org/10.5009/gnl.2014.8.2.215. [ Links ]
36. Wang J, Zhao S, Chen Y, Jia R, Zhang X. Endoscopic ultrasound guided fine needle aspiration versus endoscopic ultrasound guided fine needle biopsy in sampling pancreatic masses: a meta-analysis. Medicine (Baltimore). 2017;96:e7452. doi: https://doi.org/10.1097/MD.0000000000007452. [ Links ]
37. Katanuma A, Maguchi H, Hashigo S, Kaneko M, Kin T, Yane K, et al. Tumor seeding after endoscopic ultrasound-guided fine-needle aspiration of cancer in the body of the pancreas. Endoscopy . 2012;44:E160-1. doi: https://doi.org/10.1055/s-0031-1291716. [ Links ]
38. Fisher L, Segarajasingam D, Stewart C, Deboer W, Yusoff I. Endoscopic ultrasound guided fine needle aspiration of solid pancreatic lesions: Performance and outcomes. J Gastroenterol Hepatol. 2009;24:90-6. doi: https://doi.org/10.1111/j.1440-1746.2008.05569.x. [ Links ]
39. Storm A, Lee L. Endoscopic ultrasound-guided techniques for diagnosing pancreatic mass lesions: Can we do better? World J Gastroenterol . 2016;22:8658-69. doi: https://doi.org/10.3748/wjg.v22.i39.8658. [ Links ]
40. Artifon E, Guedes H, Cheng S. Maximizing the diagnostic yield of endoscopic ultrasound-guided fine-needle aspiration biopsy. Gastroenterology. 2017;153:881-5. doi: https://doi.org/10.1053/j.gastro.2017.08.058. [ Links ]
41. Holt B, Varadarajulu S, Hébert-Magee S. High-quality endoscopic ultrasound-guided fine needle aspiration tissue acquisition. Adv Ther. 2014;31:696-707. doi: https://doi.org/10.1007/s12325-014-0129-5. [ Links ]
42. Bhatia V, Varadarajulu S. Endoscopic ultrasonography- guided tissue acquisition: how to achieve excellence. Dig Endosc. 2017;29:417-30. doi: https://doi.org/10.1111/den.12823. [ Links ]
43. Yamabe A, Irisawa A, Bhutani M, Shibukawa G, Fujisawa M, Sato A, et al. Efforts to improve the diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for pancreatic tumors. Endosc Ultrasound. 2016;5:225-32. doi: https://doi.org/10.4103/2303-9027.187862. [ Links ]
44. Wani S. Basic techniques in endoscopic ultrasound-guided fine-needle aspiration: role of a stylet and suction. Endosc Ultrasound. 2014;3:17-21. doi: https://doi.org/10.4103/2303-9027.123008. [ Links ]
45. Costache M, Iordache S, Karstensen J, Săftoiu A, Vilmann P. Endoscopic ultrasound-guided fine needle aspiration: from the past to the future. Endosc Ultrasound. 2013;2:77-85. doi: https://doi.org/10.4103/2303-9027.117691. [ Links ]
46. Wang Y, Chen Q, Wang J, Wu X, Duan Y, Yin P, et al. Comparison of modified wet suction technique and dry suction technique in endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) for solid lesions: study protocol for a randomized controlled trial. Trials. 2018;19:45. doi: https://doi.org/10.1186/s13063-017-2380-y. [ Links ]
47. Berzosa M, Villa N, Bartel M, Wallace M, Tau J, Trang T, et al. Pilot study comparing hybrid vs. wet vs. dry suction techniques for EUS-FNA of solid lesions. Gastrointest Endos. 2014;79:AB430. doi: https://doi.org/10.1016/j.gie.2014.02.597. [ Links ]
48. Iwashita T, Yasuda I, Mukai T, Doi S, Nakashima M, Uemura S, et al. Macroscopic on-site quality evaluation of biopsy specimens to improve the diagnostic accuracy during EUS-guided FNA using a 19-gauge needle for solid lesions: a single-center prospective pilot study (MOSE study) Gastrointest Endosc . 2015;81:177-85. doi: https://doi.org/10.1016/j.gie.2014.08.040. [ Links ]
Received: March 25, 2018; Accepted: August 29, 2018











 texto em
texto em