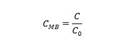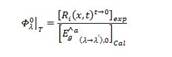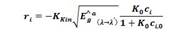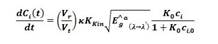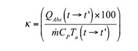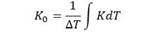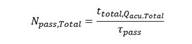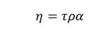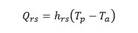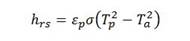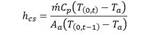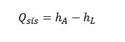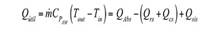1. Introduction
Heterogeneous photocatalysis is an advanced oxidation technology that consists of the absorption of radiant energy by a light-sensitive broadband semiconductor 1. The energy can be converted into heat or transferred to chemical bonds, resulting in electron/hole pairs that are transported and captured by species adsorbed onto the semiconductor surface. This process in turn leads to charge recombination and the formation of hydroxyl radicals, species responsible for degrading contaminant molecules through oxidation-reduction reactions 2. Heterogeneous photocatalysis has been implemented in removing a variety of organic compounds, recalcitrant liquids, and gaseous substances, especially: pesticides, herbicides, pharmaceuticals, inks, and others 3-5.
Because of its complex nature, fundamental research has been focused on laboratory scale, using artificial radiation with UV lamps in differential photocatalytic reactors under strict operating conditions 6, with low thermal energy dissipation by the fluid and the lamps. It has been observed that the temperature is not considered as a variable influencing the reaction rate, and therefore, these systems are assumed to be isothermal 7,8.
However, the effects of heating on the operating conditions and using large-scale solar radiation leads to significant increases in temperature in radiation collection systems, which is the case with CPC reactors. These increases are based on environmental conditions and the amount of incident radiation, in addition to the fluctuations that may occur in the flow of energy from the sun. Some mathematical models have addressed the effects of energy emission corrected with cloudiness factors, the specific month of experimentation and the amount of total global incident radiation 9-11; however, the effects of increased temperature have not been considered in mathematical models of the kinetic law and reaction rate expressions.
This paper presents the experimental evaluation and mathematical modeling of the kinetic behavior of a heterogeneous photocatalytic degradation reaction of a standard substance in a solar CPC reactor at pilot scale by modifying a proposed model based on the Langmuir-Hinshelwood mechanism. Thus, the reaction fluid undergoes changes in temperature due to temporary energy fluxes caused by atmospheric fluctuations in electromagnetic light 12,13. The rate constant is determined by the equation proposed by Arrhenius 14, and the adsorption constant is a variational parameter averaged over the temperature range of operation whereas a directly proportional relationship with the photonics component is kept via the effective quantum yield 10.
Methylene Blue (MB), is widely used in textile industry, is a common water pollutant and it is used as a standard compound due to its ability to accept or donate hydrogen ions in photocatalytic degradation studies 15, testing of photoreactors 16, and optimization of solar operations 17.
2. Methodology
2.1 Materials and equipment
For experimental evaluation, the following materials were considered: commercial grade Methylene Blue (MB), TiO2-P25 Degussa (Evonik 99.8%) with a specific surface area of 50±15 m2/g, HCl (Panreac, 37%), and NaOH (Panreac, 2M). The experiments were carried out in a solar CPC reactor located at the University of Cartagena, Colombia (10°25'32"N 75°32'59"W) coupled with a heat exchanger for regulating the temperature of the reaction fluid. The design parameters and equipment set-up are shown in Figure 1.
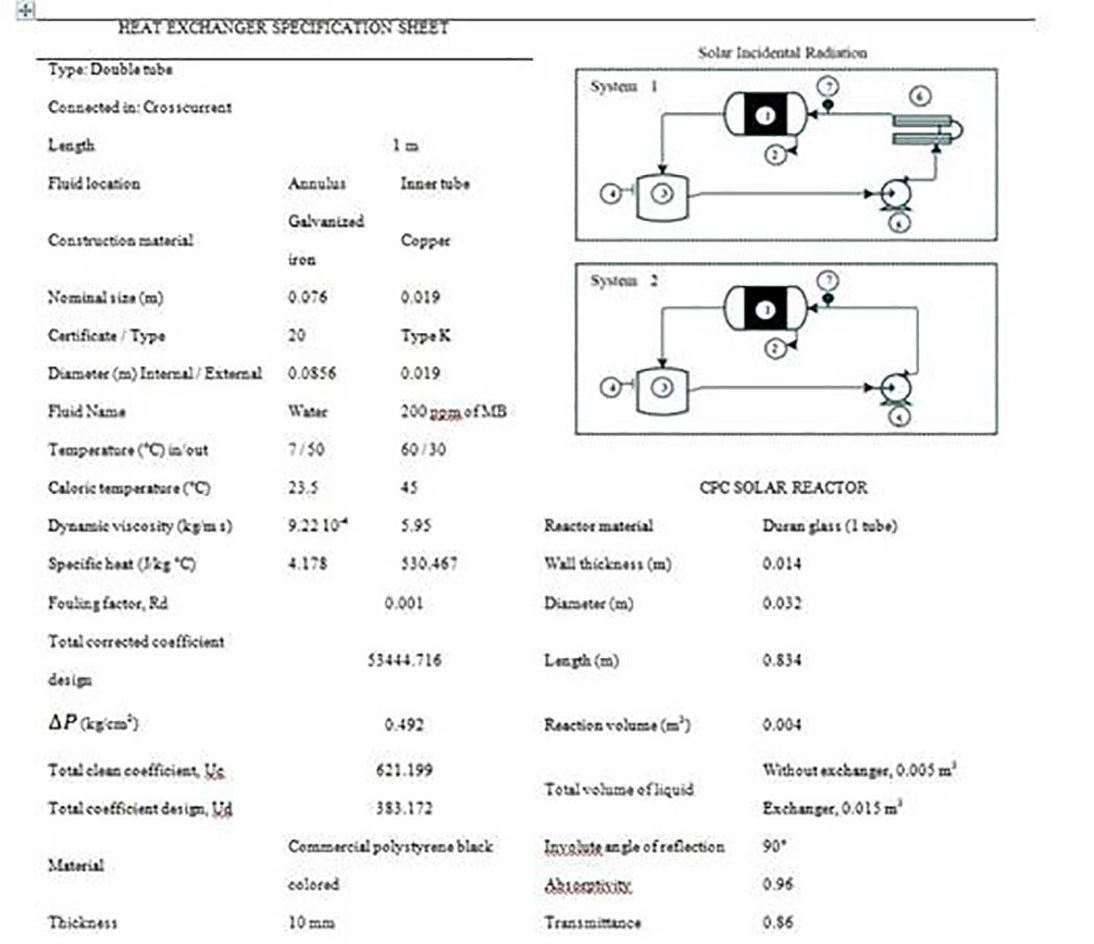
Figure 1 Data Sheet for heat exchanger, reactor systems and study where: 1) CPC reactor, 2) involute, 3) tank, 4) sampling, 5) recirculating pump, 6) heat exchanger, and 7) thermocouple viewfinder.
For this reactor, it was assumed perfect mixing and removal percentages of MB (%Degr) as response variable 19:
The fraction degraded is estimated according to the following equation:
Here, 𝐶 0 is the initial concentration of the substrate, 𝐶 is the concentration of the substrate for each experimental sample, and 𝐶 𝑓 is the final concentration of the substrate when the cumulative energy is 8000 J/m2. The initial quantum yield is given in Equation 3.
where, Ri(x,t) and Êa g (λ→ λ´),0 are the experimental reaction rate and the local volumetric rate of photon absorption, respectively. The applications of solar photocatalytic processes do not consider a uniform radiation field. Therefore, the intensive character of the results is obtained by dividing by the volume of the reactor.
2.2 Experimental evaluation of the effect of temperature
For studying the photocatalytic degradation of MB, molecular absorption tests and four sets of experiments were carried out, as illustrated in Table 1. Two preliminary photolysis tests were carried out under non-preheated, isothermal reaction conditions to confirm that the possible thermal and photolytic reactions are not appreciable. To that end, a general procedure was developed for a photocatalytic test in the absence of a catalyst.
2.3 General procedure
Once the equipment was cleaned, systems 1 and 2 (see Figure. 1) were fed with 4 and 15 liters of water, respectively. Independent tests were performed for selection of better amount of catalyst; to this, 0.35 g/L of catalyst (Degussa P-25 TiO2) was added at a low temperature and allowed to recirculate in the absence of light to ensure full mixing and adsorption equilibrium. Tests were performed base in a reaction mixture consisting in 200 mg/L of MB dissolved in natural water, at the pH of this mixture (6.95). Subsequently, the solution was subjected to solar radiation. For decolourization measurements, 10mL of sample was collected at 1000 J/m2 of cumulative energy intervals until reaching a cumulative energy of 8000 J/m2, temperature and adsorbance at 561 nm was measured.
Based on the temperature changes, different tests were carried out adjusting the water temperature in the reaction vessel to the initial temperature using the heat exchanger as shown in Table 1. Once the desired temperature was reached, the general procedure continued as described above. Significantly, the photocatalytic processes are dependent on atmospheric changes, and therefore, it was very important to connect a heat exchanger to maintain a constant temperature for the reaction fluid entering into the reactor during the treatment period.
3. Reactor model
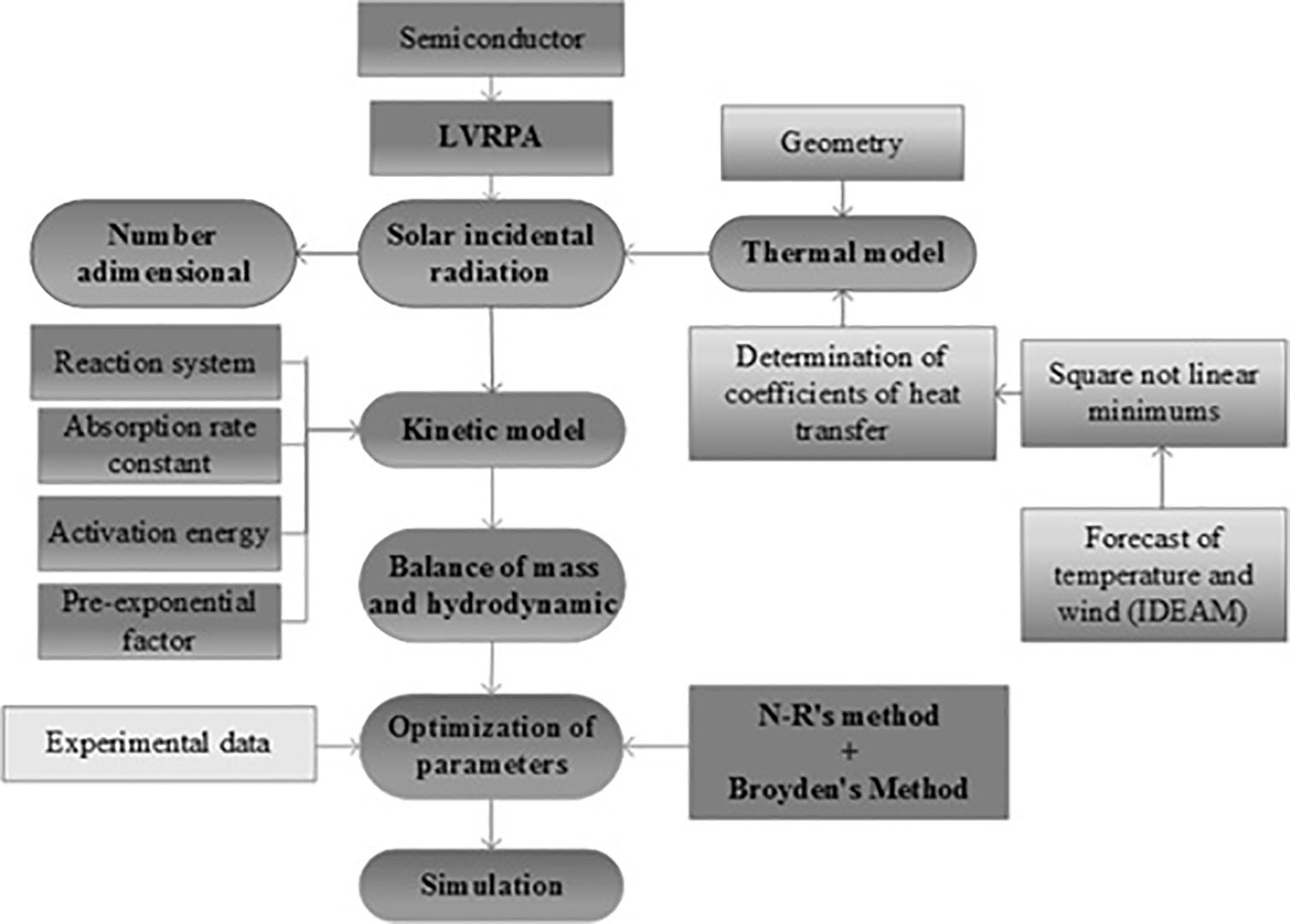
Mathematical modeling and simulation of photocatalytic reactors requires the use of several sub-models to describe the process successfully, including the emission model, the absorption model, the model of global effective quantum yield, and the fluid model dynamic. The reactor mass balance requires a kinetic model that satisfactorily represents substrate oxidation.
An expression of the reaction rate has been proposed by deriving it from the analysis of the reaction mechanism proposed by Turchi and Ollis 2, which considers that the molecular absorption of water and hydroxyl ions is the controlling step for the generation of hydroxyl radicals from the attack of the photo-generated holes. Then, the volumetric rate of absorption of radiant energy serves as a correction factor for the effects associated with geometry, operating conditions and process scale. The mathematical methodology adopted by the authors is presented in Figure. 2.
3.1 Mass balance
Mass balance in the illuminated zone: The mass balance in the reactor coupled to the mathematical expression for the reaction rate referring only to the limiting reagent i and the reaction rate is given by Equation (4) and (5) 10:
The progress of the photocatalytic reaction is affected naturally by the solar radiation, causing two main effects: cooling (when the light is reflected) and heating (when the light is absorbed). Therefore, the correction of the reaction rate is proposed through a function that evaluates the energy of the absorbed radiation per unit chemical energy of the wind:
Where λ → λ´ y t → t´ is the heat adsorbed in the reactor by the solar radiation. 𝑚 is the mass flow rate, 𝐶 𝑝 the specific heat capacity of the solution and 𝑇 𝑎 the air temperature, all of this are in the intervals λ → λ´ y t → t´ correspond to the absorption spectrum of the photocatalyst and to the treatment time, respectively.
3.2 Kinetics equation modified with temperature
The calculation of the kinetic constant K kin is performed by using the Arrhenius equation, which relates the changes in temperature via a pre-exponential factor Ka, the activation energy E a , and the adsorption constant K o which is considered a variational parameter averaged over the temperature range of the process:
where, K kin is the reaction rate kinetic constant (mol/m2s), C i is the substrate concentration (mol/m3), Ê a g is the global volumetric rate of photon absorption (Einstein/m3s), and K o is the Langmuir-Hinshelwood kinetic constant (m3/mol).
Mass balance in the dark zone: The reactor outlet concentration for each step is coupled with the inlet concentration that comes from the piping system and the storage tank, known as the recycling zone 10. The mass balance in this area is
The proposed model was incorporated into the mass balance, which is expressed as an equation by reaction step at steady state evaluated at each residence time (pass. In this way, the batch reactor is described as a finite sum of steps N pass,Total 10.
Typically, the interpretation of the experimental results is based on t30w19, the treatment time 20, the incident radiation within the reactor, and the photon flux absorbed by the catalyst. However, these parameters interact and yield erroneous conclusions, which is impractical given that the reactor is comprised of illuminated and non-illuminated areas.
For being consistent with the analysis of the results resulting from the use of two different systems, the temporal distribution of solar radiation incident on the earth's surface 21 is used to represent the evolution of the photocatalytic process according to the energy accumulated by the reactor over time as well as to compare the efficiency of different intrinsic photoreactors by the following equation:
The UV/vis irradiation was measured using a digital radiometer HD 2102.2, and was used to the evaluation of the change in the cumulative energy Q acu with respect to the treatment time t, as illustrated in Figure 3 corresponding to the experimental test period considered in this work. To provide a reproducible conditions, all the experiments were carried out near noon, when the solar radiation was moderately stable.
3.3 Thermal balance
Power variations in the reaction fluid are caused both by the energy absorbed through the reactor wall, the optical properties of which remain constant (as does the specific heat of the reaction fluid), and by system components. Thus, it is assumed that the fluid phase does not absorb radiation, the reactor wall does not allow the entry of radiation below 240 nm, and the catalyst does not absorb radiation above 380 nm. In addition, the temperature profile is uniform around the outer wall of the reactor 22,23. Figure 4 shows the control volume of the CPC reactor.
From this, the absorbed solar power is determined as shown below:
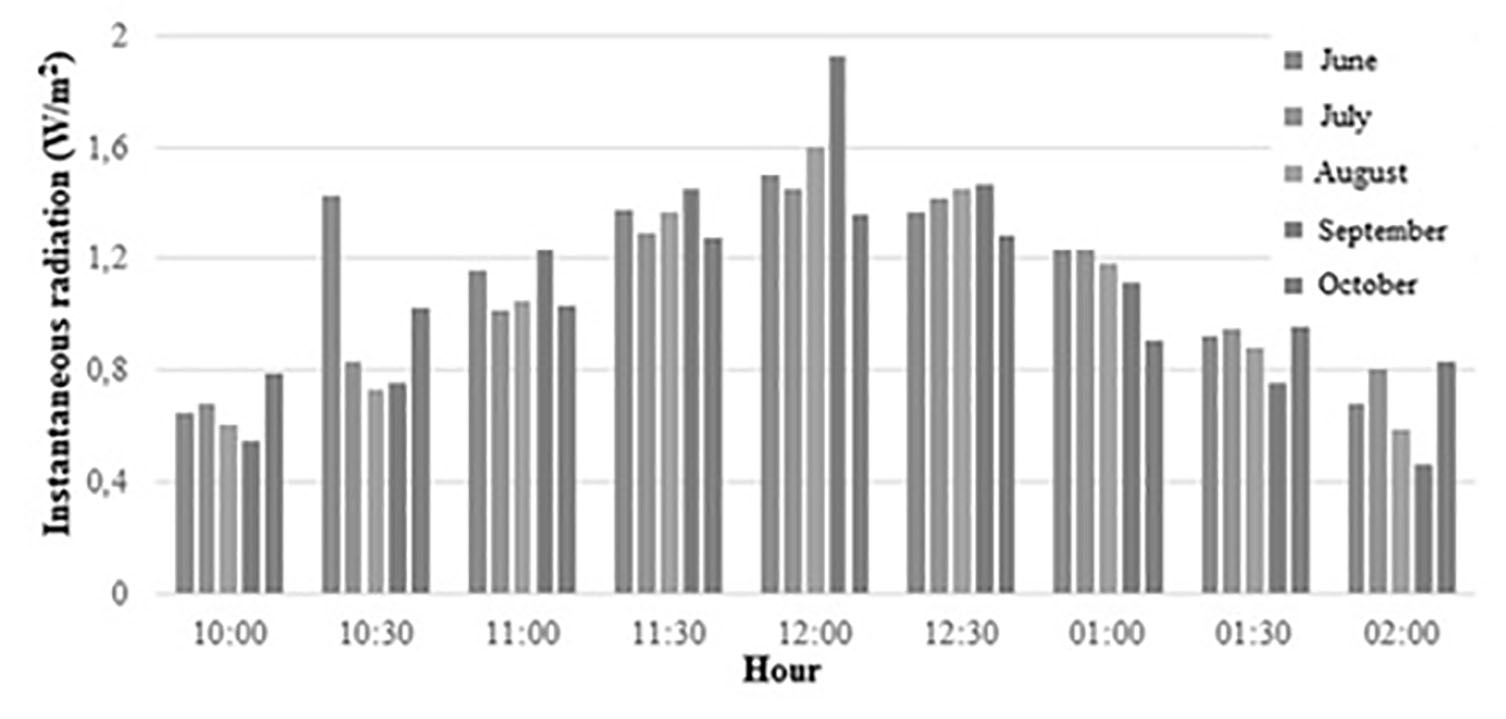
Where (,( and ( and are the transmittance, reflectance and absorptance of borosilicate glass. I is intensity of radiation and A ( is the transversal area of the reactor. In this paper, an empirical model of fitting functions that characterize the behavior of the mass flow of the wind V ( and the temperature T ( is implemented based on data provided by IDEAM in Colombia, 24 according to the following procedure.
Hourly averages were calculated from 9:00 a.m. until 2:00 p.m. for each month of the experimental test. The data obtained were fitted using non-linear least squares and are reported as their respective correlation coefficients. The main energy flows are described through the mechanisms of heat transfer as follows 25.
Radiation losses from the reactor wall to the environment.
Losses from free or forced convection from the reactor wall to the environment.
The coefficients of heat transfer by radiation and convection between the surface of the reactor wall and the environment are determined by the following equations:
Where ( p is the emissivity and ( is called the Stefan-Boltzmann constant. T p is the average temperature between air and solution temperature. T (0,t) and T (0,t-1) correspond to the temperatures of the reactor wall measured at the outlet and inlet of the reactor, respectively, obtained as an average between the reaction fluid temperature and the ambient temperature for each sampling point. Whereas, the overall energy equation is used to determine the heat added by the system due to pipe friction h L , and the use of the centrifugal pump ℎ 𝐴 , whereby it acquires the following form:
This system of equations takes the following form:
3.4 Calculation of the LVRPA
For complete modeling of reactor, quantification of the volumetric rate of absorption of radiant energy, LVRPA, is performed using the SFM 19:
Where I 0 is the intensity of radiation incident on the wall of the reactor equivalent to 30W/m2, λ corr is the extinction length, ω corr is the scattering albedo coefficient, and γ is the point coordinate in the reaction space 19,26,27. These parameters were estimated through the methodology proposed by Colina-Márquez et al. using the optical properties of the catalyst and the solar radiation conditions in Cartagena, Colombia 28.
4. Results and discussion
Degradation processes under direct photolysis did not contribute to the process of photocatalytic degradation; in the non-preheated condition percentages of degradation of 11% and 9.5% for the isothermal condition at 312 K were obtained. In addition, photolysis can contribute with the degradation mechanism.
Because the phenomena of convection and radiation exert a cooling effect on the reaction fluid throughout the process to prevent any solar power from being exploited as useful heat, it is not possible to develop a study of the thermal behavior of the reactor in a deterministic method. As a result, the treatment of climatological data was performed via nonlinear least squares, thereby obtaining mathematical expressions describing the wind speed in m/s and its temperature (°C) as a function of the time of day during the experiment months with respect to the correlation coefficients as described below:
June
V ( = -0.2198t2 + 5.7025t - 2.6441, R 2 = 0.9994 (21)
T ( = 0.0517t3 - 1.7351t2 + 19.159t - 66.318,R 2 = 0.9851 (22)
July
V ( = -0.2163t2 + 5.537t - 2.0067, R 2 = 0.9963 (23)
T ( = 0.2062t2 - 4.2826t + 24.024,R 2 = 0.9973 (24)
August
V ( = -0.2675t2 + 6.7388t - 9.0823,R 2 = 0.992 (25)
T ( = -0.0169t3 + 0.7279t2 - 9.7893t + 44.147,R 2 = 0.9889 (26)
September
V ( = -0.254t2 + 6.429t - 7.0516,R 2 = 0.9982 (27)
T ( = 0.0494t3 - 1.575t2 + 16.344t - 52.379,R 2 = 0.9864 (28)
October
V ( = -0.2703t2 + 6.7131t - 8.6796,R 2 = 0.9918 (29)
T ( = 0.043t3 - 1.4322t2 + 15.545t - 53.118,R 2 = 0.9823 (30)
By using the structure in Figure 2, it was found that the kinetic parameters K ( , E ( , and K 0 were 2±0.1 mol/ms, -29.1±34 J/mol, and 1.9±0.1 m3/mol, respectively. This equation yields a minimum R2 of 0.938 and a maximum standard deviation of 0.178. Moreover, the energies that systems 1 and 2 added were 8.11 W and 6.11 W, respectively. The results demonstrated that cooling system is not recommended because the radiation causes only moderate increases in temperature that are beneficial to the process (Table 2). This means that the temperature could be important in the catalyst structure under normal operating conditions.
The predictive capacity of the model, associated with the use of a correction because of the effect of atmospheric variations over time𝜅, is attractive for considering high substrate concentrations and temperature fluctuations (Figure 5). The calculated kinetic parameters can be used to evaluate the performance of the catalyst at higher temperatures, provided that a tubular or CPC reactor is used 29,30. Similarly, the energy of absorbed radiation per unit chemical energy of wind 𝜅 can be used to evaluate the thermal performance of the reactor. Nevertheless, this is a first pass to interpret the process that appear during the reaction in atmospherics conditions because there is no literature report yet.
Table 2 summarizes the useful heat, the heat absorbed, the dimensionless number, the percentage of degradation, and the initial quantum yield obtained by evaluating the LVRPA at the reactor wall.
Table 2 Kinetic parameters adjustment for photocatalytic degradation AM.
| Condition | Initial T. (K) | Q Abs [W] | Q útil [W] | 100/k | %Degr. | ɸo λ│T[mol/einsten[ |
|---|---|---|---|---|---|---|
| Photolysis | 305 | 0.0365 | 5.0400 | 0.0998 | 11 | --- |
| Isothermal photolysis | 312 | 0.0497 | 7.8124 | 0.1469 | 9.5 | --- |
| Non-preheated | 304 | 0.0556 | 5.6107 | 0.6710 | 32.80 | 2.2573 10-7 |
| 305 | 0.0371 | 9.3752 | 0.0782 | 45.94 | 1.1631 10-9 | |
| Preheated | 323 | 0.0443 | 5.5694 | 0.3890 | 41.80 | 1.9616 10-7 |
| 321 | 0.0576 | 5.9326 | 0.5525 | 38.50 | 2.4446 10-7 | |
| Isothermal | 307 | 0.0615 | 8.0849 | 0.1861 | 13.48 | 3.6472 10-7 |
| 318 | 0.0838 | 7.6748 | 0.2479 | 33.21 | 1.1336 10-6 |
For this study, we considered that the kinetic parameters found point out that the reaction proceeds in two stages. The first stage corresponds to adsorption and the second to the reaction. When the temperature is increased, the reaction stage is favored, increasing the number of effective collisions between molecules and disfavoring the adsorption stage because the equilibrium shifts to the formation of less substrate-semiconductor complex 18. However, the results can be described by first-order kinetics that is slightly favored when the temperature is increased (Figure 5).
For different conditions, the rate of photocatalytic reaction abandons its dependence on the intensity of solar radiation, temperature, and wind speed when the dimensionless parameter assumes values above 0.2, probably because the processes of mass transfer in the midst of the reaction.
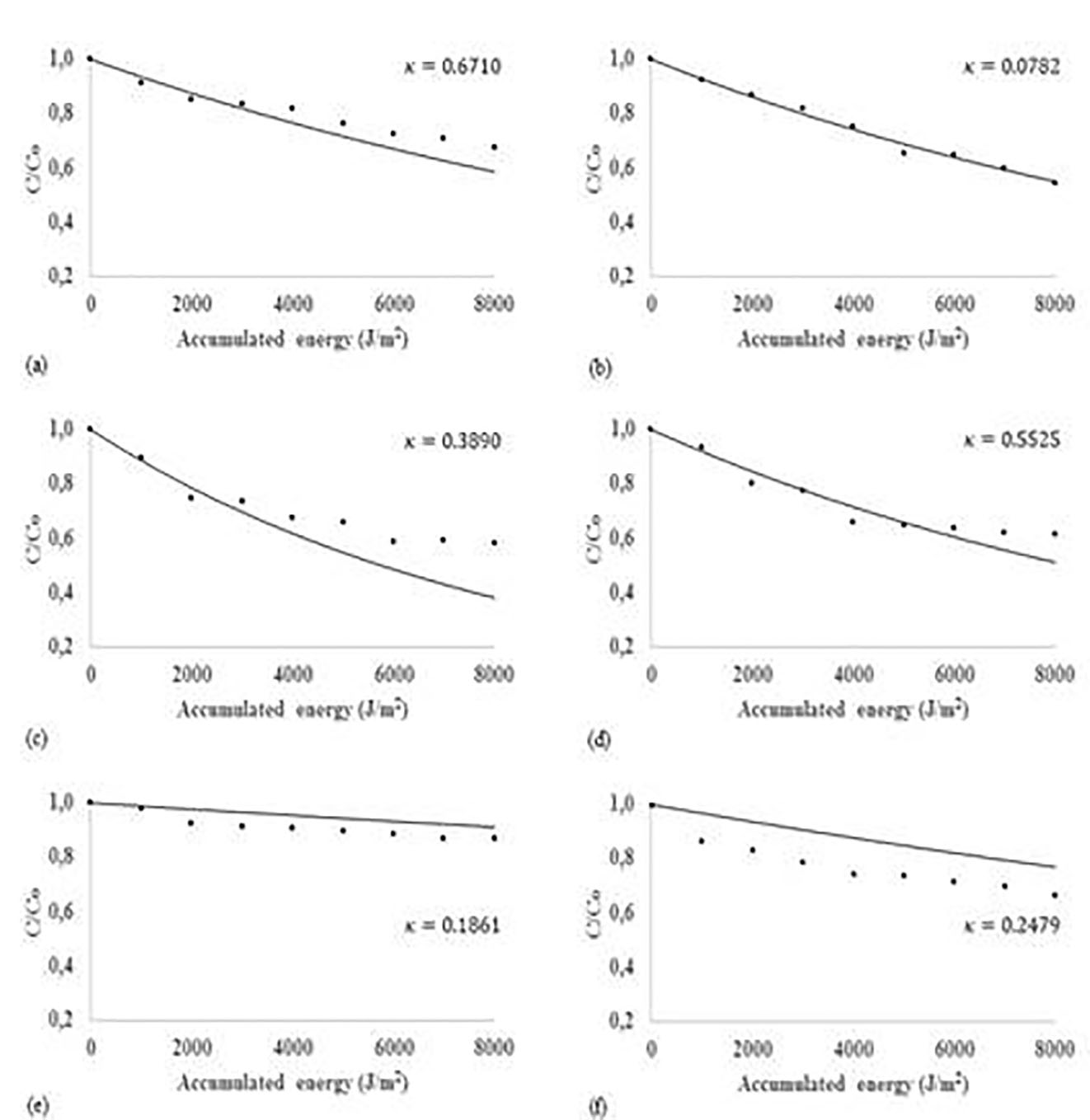
Figure 5 Adjusting experimental kinetic data for the solar heterogeneous photocatalytic degradation CPC reactor: (a) not preheated to 306 K, (b) not preheated to 305 K, (c) preheated to 323 K, (d) preheated to 321 K, (e) isothermal at 307 K and (f) at isothermal at 318 K.. Experimental data (●), Reaction rate model (-). The model reaction rate includes the effect of temperature.
5. Conclusions
A new model for the description of the heterogeneous photodegradation of Methylene Blue using TiO2 was formulated from a mechanism based on the attack of hydroxyl radicals and considering the atmospheric changes that cause heating of the process fluid through a balance of matter and energy. The resulting model has a simple mathematical structure with a correction term for the effects of variation of the radiation intensity, temperature, and wind speed, i.e., as a function of weather and reaction geometry.
For the non-preheated reactor condition, the model adequately describes the processes of charge transfer, unlike the preheated and isothermal reactor conditions. The kinetic parameters obtained from this model are associated strictly with the radiant field configuration and the nature of the substrate. Consequently, the proposed model can be generalized and used in the treatment of organic pollutants by heterogeneous solar photocatalysis. In addition, since our experiments, a detailed reaction mechanism could be presented considering the electronic properties of the catalyst.
The experimental evaluation shown that the temperature effects over the system affected the performance, when the temperature is increased the reaction stage is favored, the first-order kinetics that is slightly favored too and disfavoring the adsorption stage.