Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Biosalud
Print version ISSN 1657-9550
Biosalud vol.8 no.1 Manizales Jan./Dec. 2009
MEMBRANE ACYL-CoA TRAFFIC AND REMAINING LEVELS IN RED BLOOD CELLS, PLASMA AND SERUM ANALYSED BY TANDEM MASS SPECTROMETRY
TRÁFICO DE ACYL-CoA DE MEMBRANA Y NIVELES REMANENTES EN GLÓBULOS ROJOS, PLASMA Y SUERO, ANALIZADOS POR ESPECTROMETRÍA DE MASAS EN TANDEM
José Henry Osorio1 y Morteza Pourfarzam2
1 Universidad de Caldas, Laboratorio de Bioquímica Clínica y Patología Molecular, Departamento de Ciencias Básicas de la Salud, Manizales, Colombia.
2 Spence Biochemical Genetics Unit, Royal Victoria Infirmary. Newcastle upon Tyne. England.
Recibido: noviembre 03 de 2009 - Aceptado: noviembre 10 de 2009
ABSTRACT
There has been a permanent question about the ideal fluid for carnitine and acylcarnitine analysis by tandem mass spectrometry. The present study evaluates the percentage of carnitine and acylcarnitines in red blood cells and the relationship with the carnitine and acylcarnitines content in whole blood, plasma, and serum. Human blood samples were centrifuged, plasma or serum extracted, and blood cells were washed with different isotonic solutions. The final pellet was resuspended in PBS for card preparation and tandem mass spectrometry analysis. It was found that carnitine, short-chain, medium-chain and longchain acylcarnitines remain in red blood cells at average percentages of 43.4; 48; 49; and 70% respectively. A significant difference was found between carnitine and acylcarnitine levels in whole blood compare to its levels in plasma or serum (p<0.05). As carnitine and acylcarnitines remained associated with the blood cells, it seems therefore that plasma (or serum) is not the ideal material for the analysis of carnitine and acylcarnitines.
KEY WORDS: carnitine, acylcarnitines, acyl-CoA, tandem mass spectrometry, red blood cells, metabolism.
RESUMEN
Existe un cuestionamiento permanente acerca del fluido ideal para el análisis de carnitina y acilcarnitina por medio de la espectrometría de masas en tándem. El presente estudio evalúa el porcentaje de carnitina y acilcarnitinas en glóbulos rojos y la relación con el contenido de carnitina y acilcarnitina en la sangre, plasma y suero. Se centrifugaron muestras de sangre humana, se extrajeron plasma y suero, y se lavaron los glóbulos rojos con diferentes soluciones isotónicas. Se resuspendió el pellet en PBS para la preparación de tarjetas y análisis por espectrometría de masas en tandem. Se encontró que la carnitina y las acilcarnitinas de cadenas corta, media y larga, permanecen en los glóbulos rojos en porcentajes promedio de 43,4; 48;49; y 70% respectivamente. Se encontró una diferencia significativa entre los niveles de carnitina y acilcarnitina en la sangre comparado con sus niveles en plasma o suero (p<0,05). Dada la asociación de la carnitina y las acilcarnitinas con los glóbulos rojos, parece ser que ni la plasma ni el suero son el material ideal para el análisis de carnitina y acilcarnitinas.
PALABRAS CLAVE: Carnitina, acilcarnitinas, acil-CoA, espectrometría de masas en tándem, glóbulos rojos, metabolismo.
INTRODUCTION
Since carnitine is a vehicle by which the acyl groups can leave the mitochondria and there is equilibrium between acylcarnitines and their respective CoA thioesters, the analysis of carnitine and acylcarnitines in blood is approximately equivalent to the analysis of acyl-CoAs in the mitochondria (1).
Carnitine and acylcarnitine identification in body fluids using tandem mass spectrometry was developed in the late 1980s (1, 2). The method has the potential to screen effectively several disorders (3-7). Some authors suggest that a plasma carnitine and acylcarnitine profile should be performed in all patients presenting an acute episode of hypoketotic hypoglycemia, Reye syndrome, hypertrophic cardiomyopathy, pericardial effusion, cardiac failure or rapid unexpected death in the neonatal period or during infancy, also heart beat disorders during neonatal period, hypotonia with unexplained failure to thrive, retinitis pigmentosa or even muscle pain triggered by exercise (8). The measurement of acylcarnitines using tandem mass spectrometry has been reported in whole blood (9), plasma (10), urine (11), amniotic fluid (12), and bile (13). There has been a permanent question about the ideal fluid for carnitine and acylcarnitine measurement. The present study analyses the carnitine and acylcarnitines content in red blood cells and the possible relationship with its contents in whole blood, plasma, serum and red blood cells trying to establish the recommended fluid for carnitine and acylarnitine analysis by tandem mass spectrometry.
METHODS
The present study is experimental. All the chemicals used were of analytical grade. Unlabelled acylcarnitine, and deuterated carnitine and acylcarnitines ([d3]C2cn, [d9]C2cn, [d3]C3cn, [d3]C8cn, [d9]C8cn, [d3]C16cn, [d3]C16cn) were obtained from Cambridge isotopes laboratories, (Andover, MA, USA). Butanolic HCL was prepared by passing HCL gas through anhydrous n-butanol (Sigma-aldrich Company, Ltd., Poole, UK) for 30 min. The concentration of the acid was determined by back tritiation and adjusted.
Blood specimens and card preparation: Blood samples used in this study were from ten adult healthy volunteers, who signed written consent. Blood was collected into tubes containing EDTA (23.5 µmol/tube) and into tubes without anticoagulant. Aliquots of 20 µl were spotted on specimen collection filter paper cards (No. 903, 1.88 mm thick; Schleicher & Schuell, Dassel, Germany), dried overnight at room temperature, vacuum sealed and kept in the freezer (-80°C) until analysis. From the same samples, plasma and serum were extracted and cards were prepared as mentioned before. The same procedure was used for analyzing pellets of red blood cells resuspended in PBS (1).
Extraction of acylcarnitines using microtitre plates: spots were punched from the card, (6.35 mm diameter corresponding to 12 µl of samples, and placed into microtitre plates (96 samples each plate). 100 µl of the internal standard (containing the following labeled acylcarnitines in 100 µl methanol: [d3]cn, 360 pmol; [d3]C2cn, 120 pmol; [d3]C3cn, 24 pmol; [d9]C8cn, 12 pmol; [d9]C16cn, 24 pmol) were added, plus 500 µl of methanol to each sample. The plates were placed on an orbital shaker (setting 750 rpm) for 30 min and then sonicated for 15 min (sonic bath. 175SR). The plates were returned to the shaker for a further 2 hours and sonicated again for another 30 min. The filter discs from the card punch were removed and the resulting eluate was evaporated under air at 450C until dry (1).
Derivatization process: 50 µl of 1 M Butanolic HCl was added to each sample and incubated at 60°C for 15 min. Samples were immediately returned to the fume cupboard and evaporated under air at 45°C until dry and re-dissolved in 100 µl of 70% (v/v) acetonitrile in water prior to analysis by ESI-MS/MS (2).
Tandem mass spectrometry (MS/MS) analysis: analysis for acylcarnitines (short-chain, medium-chain and long-chain) in all analyzed samples was performed using the following scan function: parents of m/z 85, scan range 200-500 (m/z), collision energy 25 eV, cone voltage 30V, scan time 2.0 sec, interscan time 0.1 sec, collision gas Argon, collision gas pressure 1.6-2 x 10-3 mBar. All analyses were performed using a Quattro II, triple quadrupole tandem mass spectrometer (Micromass, Manchester, UK) equipped with an ion spray source (ESI) and a micromass MassLynx data system. The samples were introduced into the mass spectrometer source using a Jasco AS980 autosampler and a Jasco PU980 HPLC pump. For this kind of works the use of selected reaction monitoring for each analyte could provide better quantification data, however, analysis for acylcarnitines using parents of m/z 85 is the routine method when analyzing samples from patients, then we adopted this scan function (2).
Analysis of carnitine and acylcarnitine leves in red blood cells: blood samples (2 ml) collected with and without anticoagulant were centrifuged (2300 g x 5 min) and the serum or plasma was retained for acylcarnitine analysis. The pellet was resuspended to the volume of 2 ml in each one of the following isotonic solutions: a) PBS (136.9 nM NaCl, 2.8 mM KCl, 1.47 mM KH2PO4, 8.1 mM Na2PO4), pH 7.4; b) PBS with 50 mg/ml albumin; c) an isotonic glucose solution; d) a solution of 250 mM sucrose, 2 mM HEPES pH 7.4; e) saline solution 0.9% (w/v) NaCl (i.e. 0.9 g/dl). Samples were centrifuged (2300 g x 5 min) and the procedure was repeated twice. The final pellet was resuspended in PBS for card preparation and tandem mass spectrometry analysis. All the samples (starting blood sample, plasma, serum, washed reconstituted blood cells, and pooled wash solution) were extracted and free carnitine and acylcarnitines were analysed. The procedure was performed five times for each sample. Statistical comparisons were performed using one-way ANOVA (SigmaStat version 3.1 statistical software), followed by Dunnett’s test. p<0.05 was considered significant. The study was approved by the correspondent ethical committee.
RESULTS
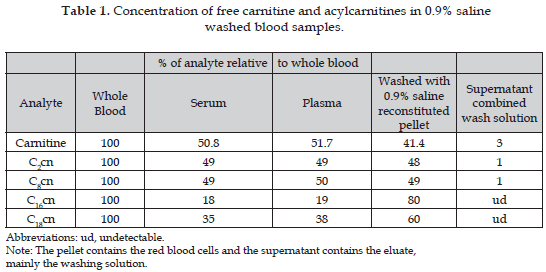
The results from whole blood reconstituted pellet obtained using the different isotonic solutions: a) PBS (136.9 nM NaCl, 2.8 mM KCl, 1.47 mM KH2PO4, 8.1 mM Na2PO4), pH 7.4; b) PBS with 50 mg/ml albumin; c) an isotonic glucose solution; d) a solution of 250 mM sucrose, 2 mM HEPES pH 7.4; e) saline solution 0.9% (w/v) NaCl (i.e. 0.9 g/dl) were not significant different, therefore the analysis was performed using only the 0,9% NaCl solution (data no shown). It was found that carnitine, short-chain (C2cn), mediumchain (C8cn) and long-chain (C16cn, and C18cn) acylcarnitines remain in red blood cells after washing whole blood at average percentages of 43,4; 48; 49; and 70% respectively. The highest percentage was for hexadecanoylcarnitine (80%) (Table 1).
DISCUSSION
We found acylcarnitines remaining in RBC after washing whole blood (C2cn, C8cn, C16cn, and C18cn) (Table 1). This is unexpected for RBC due to the absence of mitochondria and because they are energetically independent of fatty acid oxidation, with no demand for classical carnitine-mediated fatty acid transport. However, there have been reports of the activity of carnitine palmitoyltransferase as the essential enzyme for the physiological expression of deacylation-reacylation process, within the phospholipid fatty acid membrane of human erythrocytes (14, 15). The results however seem to have a far more important diagnostic implication in that although free carnitine and short-chain acylcarnitine are distributed equally between plasma and blood cells long-chain acylcarnitines are more associated with the latter. Serum carnitine concentrations reflect less than 0.5% of the total body carnitine pool, of which 98% is represented by the muscle mass, the remaining 1.5% being distributed between the different organ systems and blood cells (16). The contribution of red blood cell to whole blood level of carnitine increased significantly at delivery (17). However according to Mares- Perlman et al. (18) carnitine content found in red blood cells represents 73.6±4.0% of whole-blood carnitine by human preterm neonates at birth but declined to 42.2±14.1% by day 14. This finding agrees with the percentage found in the present study, and then it is possible to postulate that after this day this percentage remains constant even to adulthood.
There is a close correlation between the plasma and muscle carnitine levels, but carnitine in red blood cells seems to represent a carnitine compartment of its own. Carnitine level in red blood cells is probably less related with fatty acid metabolism of the mitochondrial than with cell membrane stabilization or buffer function for Na-K-ATPase (19). The role of the carnitine system is to maintain homeostasis in the acyl-CoA pools of the cell, keeping the acyl-CoA/CoA pool constant even under conditions of very high turnover of the acyl-CoA (20). The enzyme carnitine palmitoyltransferase (CPT) properties and locations are consistent with this (21). Above all, the carnitine derivatives can be moved across intracellular barriers, so the carnitine system provides a shuttle mechanism between microsomes, peroxisomes and mitochondria for complex lipid-synthetic and breakdown pathways (22). However it was also demonstrated that the acyl-carnitine pool could act as a source of acyl groups, via the CoA pool, for the incorporation into lipids when energy, required to activate free fatty acids, is limited (23).
CONCLUSION
Carnitine and acylcarnitines remained associated with the blood cells. It seems therefore that plasma (or serum) is not the ideal material for the analysis of carnitine and acylcarnitines for the investigation of inherited metabolic defects and whole blood should be used for this purpose.
REFERENCES
1. Millington DS, Kodo N, Norwood DL, Roe CR. Tandem Mass Spectrometry: A new method for acylcarnitine profiling with potential for neonatal screening for inborn errors of metabolism. J Inher Metab Dis 1990;13:321-324. [ Links ]
2. Millington DS, Kodo N, Terada N, Roe D, Chace DH. The analysis of diagnostic markers of genetic disorders in human blood and urine using tandem mass spectrometry with liquid secondary ion mass spectrometry. Int J Mass Spect Ion Proc 1999; 111:211-228. [ Links ]
3. Levy PA. Inborn errors of metabolism: part 1: overview. Pediatr Rev 2009;30(4):131-137. [ Links ]
4. Sahai I, Marsden D. Newborn screening. Crit Rev Clin Lab Sci 2009;46(2):55-82. [ Links ]
5. Chalcraft KR, Britz-McKibbin P. Newborn screening of inborn errors of metabolism by capillary electrophoresis-electrospray ionization-mass spectrometry: a second-tier method with improved specificity and sensitivity. Anal Chem 2009;81(1):307-314. [ Links ]
6. Artuch Iriberri R, Moreno J, Puig R, Quintana M, Montero R, Ormazábal A, Vilaseca M. Laboratory diagnosis of rare diseases. An Sist Sanit Navar 2008;31(Suppl 2):91-103. [ Links ]
7. Agarwal RL, Muranjan MN. Diagnostic practice for organic acidemias: barriers to early diagnosis. Arch Dis Child 2008;93(11):1000. [ Links ]
8. Vianey-Saban, N. Guffon, F. Delolne, P. Guibaud, M. Mathieu, P. Divry. Diagnosis of inborn errors of metabolism by acylcarnitine profiling in blood using tandem mass spectrometry. J Inher Metab Dis 1997;20:411-444. [ Links ]
9. Johnson JW, Lee MS, Lee MR, Yost T. Triple Quadrupole MS/MS. In: Mass Spectrometry in Biomedical Research. London; 1986. pp. 459-457. [ Links ]
10. Millington DS, Chace DH. Carnitine and acylcarnitines in metabolic disease diagnosis and management. In: Desiderio DM, ed. Mass spectrometry: clinical and biomedical applications, vol. I. New York: Plenum Press; 1992. pp. 299-316. [ Links ]
11. Libert R, Van Hoof F, Thillaye M, Vincent MF, Nassogne MC, Stroobant V, et al. Identification of new medium-chain acylcarnitines present in urine of a patient with medium-chain acyl-CoA dehydrogenase deficiency. J Inher Metab Dis 1999;22:9-18. [ Links ]
12. Y. Shigmatsu Y, Hata I, Nakai A, Kikawa Y, Sudo M, Tanaka Y, et al. Prenatal Diagnosis of Organic Acidemias Based on Amniotic Fluid Levels of Acylcarnitines. Pediatr Res 1996;39:680-684. [ Links ]
13. M.S. Rashed MS, Ozand PT, Bennet MJ, Barnard JJ, Govindaraza DR, Rinaldo P. Inborn errors of metabolism diagnosed in sudden death cases by acylcarnitine analysis of postmorten bile. Clin Chem 1995;41:1109-1114. [ Links ]
14. Arduini A, Mancinelli G, Radtti GL, Dottori S, Molajoni F, Ramsay RR. Role of carnitine and carnitine palmitoyltransferase as integral components of the pathway for membrane phospholipid fatty acid turnover in intact human erythrocytes. J Biol Chem 1992;267:12673-12681. [ Links ]
15. Arduini A, Tyurin V, Tyuruna Y, Arrigoni-Martinelli E, Molajoni F, Dottori S, et al. Acyl-trafficking in membrane phospholipid fatty acid turnover: the transfer of fatty acid from the acyl-L-carnitine pool to membrane phospholipid in intact human erythrocytes. Biochem Biophys Res Commun 1992;187:353-358. [ Links ]
16. Borum PJ, York CM, Bennet SG. Carnitine concentration of red blood cells. Am J Clin Nutr 1985;41:653-656. [ Links ]
17. Schoderbeck M, Auer B, Legenstein E, Genger H, Sevelda P, Salzer H, et al. Pregnancy-related changes of carnitine and acylcarnitine concentrations of plasma and erythrocytes. J Perinat Med 1995;23:477-485. [ Links ]
18. Mares-Perlman JA, Farrel PM, Gutcher GR. Changes in erythrocyte and plasma carnitine concentrations in preterm neonates. Am J Clin Nutr 1986;43:77-84. [ Links ]
19. Reichmann H, van Lindeneiner N. Carnitine analysis in normal human red blood cells, plasma and muscle tissue. Europ Neurol 1994;34:40-43. [ Links ]
20. Vessey DA, Chen WW, Ramsay RR. Effect of carboxylic acid xenobiotics and their metabolites on the activity of carnitine acyltransferases. Biochem J 1991;279(Pt 3):895-897. [ Links ]
21. Ramsay RR, Arduini A. The carnitine acyltransferases and their role in modulating acyl-CoA pools. Arch Biochem Biophys 1993;302:307-314. [ Links ]
22. Igal RA, Wang P, Coleman RA. Triacsin C blocks synthesis of glycerolipids and cholesterol esters but not recycling of fatty acid into phospholidpid: evidence for functionally separate pools of acyl-CoA. Biochem J 1997;324:529-534. [ Links ]
23. Ramsay RR. The carnitine acyltransferases: modulators of acyl-CoA-dependent reactions. Biochem Soc Trans 2000;28:182-186. [ Links ]