INTRODUCTION
Organic solvents such as toluene, benzene and xylene pose a great risk to public health as they are widely used in the industry. Solvents are a class of liquid organic chemicals which have high lipophilicity and volatility 1,2. Toluene, also known as toluol, methylbenzene and methacide, is a colorless, fragrant organic hydrocarbon solvent used widely in fabrication of industrial supplies and synthesis of various products. It is an important component of chemical paints, adhesives, coatings, varnishes, printing inks, fuel additives, glues, thinners and plastics 3,4. Toluene is found in liquid form at room temperature, but because of its low vapor pressure, it can be easily volatilized 5,6. As a result of widespread use of toluene, there are both occupational and non-occupational exposures 7. Exposure to toluene can be occured by a variety of ways, such as consumption with drinking water, food, consumer products or occupational exposure and chemical abuse 8. Toluene has lipophilic properties and is rapidly absorbed after being taken orally or by inhalation and tends to accumulate in adipose tissue and lipid-rich organs such as the brain, kidney, liver, intestines, spleen and adrenal glands 5,9. Toluene is rapidly transformed to benzyl alcohol in the liver by the microsomal enzyme system. Then benzyl alcohol turns into benzoic acid. Benzoic acid reacts with glycine or glucuronic acid. It is excreted in the urine as hippuric acid or benzol glucuronides. A small part of the toluene turns into o- and p-cresol 6. When living organisms are exposed to toluene, the formation of free oxygen radicals increases and cellular damage occurs. In physiological conditions of living organisms, reactive oxygen species (ROS) are produced. Antioxidant defense systems reduce these ROS and prevent damage caused by them. While toluene is metabolized in the liver, it generates different ROS including singlet oxygen (O2), superoxide anion (O2 -), hydrogen peroxide (H2O2) and hydroxyl radical (OH-) 10. Free oxygen radicals are molecules containing at least one unpaired electron in its outer orbit. Free oxygen radicals react with nucleic acids, free amino acids, proteins, fats, carbohydrates, causing irreversible damage. Thus, they create pathological changes in the cell membrane, cell organelles and DNA 11,12,13.
p-coumaric acid (p-CA) is a phenolic compound usually found in a variety of vegetables (potatoes, tomatoes, peas), fruits (apples, pears, pineapples), foods and beverages (chocolate, tea, coffee, wine, beer) 14,15. p-CA has been reported to have an antioxidant effect by binding metal ions, cleaning reactive oxygen and reactive nitrogen radicals, rearranging endogenous antioxidant enzymes or repairing oxidative damage in biomolecules 14,16. The aim of this study was to determine the protective effect of p-CA on toluene-induced hepatotoxicity, nephrotoxicity and neurotoxicity in rats.
Materials and Methods
Ethical aspects. The rats used in the present study were obtained from Burdur Mehmet Akif Ersoy University Experimental Animal Production and Experimental Research Center and experimental applications were made in the same place. The research was carried out according to decision at the meeting of the Animal Experiments Local Ethics Committee of Burdur Mehmet Akif Ersoy University, Turkey (Ethics No:126-01/04/2015).
Experimental animals & design. In this study, a total of 32 male 10-12 weeks old Sprague-Dawley rats weighing approximately 200-300 g were divided into four groups of 8 animals each (Table 1). All animals were given ad libitum pellet feed and water. The doses of toluene 5 and p-CA 14,15 to be given to animals were determined in the light of previous studies. The study was terminated at the 25th day. All animals were anesthetized with 2-3% isoflurane (inhalation). The animals were euthanized by cervical dislocation under anesthesia. Blood and tissue samples were collected.
Biochemical estimations. Blood samples were taken into tubes with and without EDTA. They were centrifuged for 10 minutes at 4000 rpm. Aspartate aminotransferase (AST), alanine aminotransferase (ALT) and creatinine in blood were measured in Gesan Chem 200 autoanalyzer (Gesan chem 200 Gesan Production srl, Campobello, Italy). Kinetic UV optimized The International Federation of Clinical Chemistry (IFCC) methods were used in ALT and AST measurements and Jaffè method was used in creatinine measurement 17.
Preparation of tissue samples. The tissue samples were prepared for homogenization by washing with 0.9% ice-cold isotonic saline. Buffer solution was prepared as follows: 140 mM KCl, 10 mM NaHCO3, 3 mM KH2PO4 and 2 mM K2HPO4; dissolved in 950 ml of deionized water and adjusted to pH 7.2 with NaOH (5N) and completed to 1000 ml. The tissue samples were homogenized with the buffer (1/10 w/v). For 45 minutes at 15000 rpm and 4°C, homogenates were centrifuged, supernatants were separated and kept at -20°C until analysis.
Estimations of antioxidant/oxidant parameters in brain, kidney and liver tissues. Glutathione (GSH) levels were measured using the method reported by Sedlak and Lindsay 18 and expressed in µmol/g protein. Glutathione peroxidase (GSH-Px) activities were measured according to the method reported by Paglia and Valentine 19 and expressed in U/g protein. Malondialdehyde (MDA) levels were measured according to the method reported by Ohkawa et al 20 and expressed in µmol/g protein. Determination of total protein was performed based on the Biuret method reported by Gornall et al 21.
Statistical evaluation. Data of the study were evaluated using the "SPSS 22.0" program (IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY). Results are expressed with arithmetic mean ± standard error of the mean (SEM). To determine the comparisons of means between groups, one-way analysis of variance (ANOVA) was applied. For determination of the differences between the groups, Tukey's post hoc test was used. p<0.05 was considered as statistically significant.
RESULTS
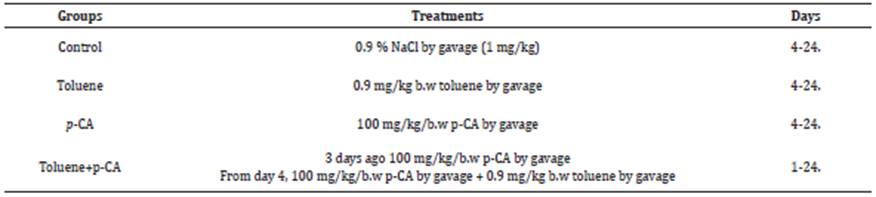
Significant (p<0.05) increases in serum AST and ALT activities, and creatinine level were detected in toluene group compared to the control. In the toluene+p-CA group, significant (p<0.05) decreases in the activities of AST, ALT and level of creatinine, which were increased when toluene was administered alone, were determined. No significant changes were found in the group where p-CA was administered alone compared to the control (Table 2).
Table 2 Serum AST, ALT and creatinine activities of the groups.
* Values are expressed as arithmetic mean ± SEM.
** (a b c) shows differences between groups in the same line, p<0.05
p-CA: p-coumaric acid; AST: Aspartate aminotransferase (U/L); ALT: Alanine aminotransferase (mg/dL); Creatinine: mg/dL
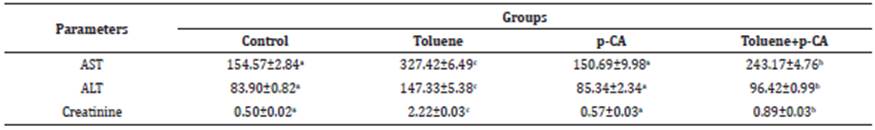
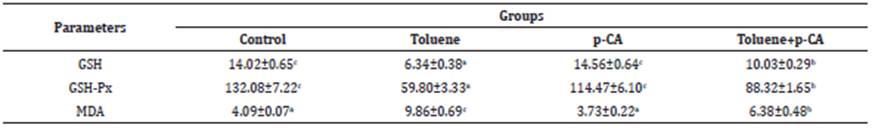
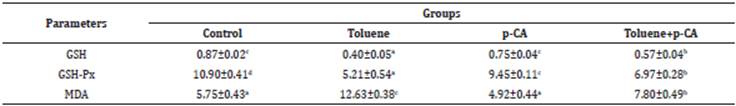
Significant (p<0.05) increases in the levels of MDA and significant (p<0.05) decreases in the levels of GSH and the activities of GSH-Px were detected in the brain, kidney and liver tissues in the toluene group, compared to the control group. In the toluene+p-CA group, significant (p<0.05) decreases in the levels of MDA and significant (p<0.05) increases in the levels of GSH and the activities of GSH-Px were detected in the brain, kidney and liver tissues compared to toluene group (Table 3,4,5).
Table 3 GSH and MDA levels and GSH-Px activities in liver tissues of the groups.
* Values are expressed as arithmetic mean ± SEM.
** (a, b, c) shows differences between groups in the same line, p<0.05
p-CA: p-coumaric acid; GSH: Glutathione (µmol/g protein); GSH-Px: Glutathione peroxidase (U/g protein); MDA: Malondialdehyde (µmol/g protein)
Table 4 GSH and MDA levels and GSH-Px activities in kidney tissues of the groups.
* Values are expressed as arithmetic mean ± SEM.
** (a, b, c, d) shows differences between groups in the same line, p<0.05
p-CA: p-coumaric acid; GSH: μmol/g protein; GSH-Px: U/g protein; MDA: μmol/g protein
DISCUSSION
The findings of the present study revealed that p-CA has a protective effect aganist toluene-induced tisssue damage. In this study, it was determined that toluene given to rats at a dose of 0.9 mg/kg b.w for 21 days significantly changed the oxidant/antioxidant balance and increased aminotranferases activities and creatinine level. Results of this study showed that toluene could induce oxidative stress in the brain, liver and kidney tissues. On the other hand, p-CA revealed protective effects by improving the antioxidant system defence, suppressing oxidative stress in tissues.
The liver is the primary organ in which drugs and xenobiotics are primarily metabolized. In the present study, significant (p<0.05) decreases in GSH level and GSH-Px activity; a significant (p<0.05) increase in MDA level were detected in liver samples of toluene group compared to control. In previous studies 22,23,24,25 it was emphasized that toluene causes decrease in the efficiency of the antioxidant systems and increases lipid peroxidation in liver tissues. Mattia et al 22,23 administered toluene (1.5 mg/kg intraperitoneally) to rats and detected a significant (p<0.05) decrease in GSH level and increase in MDA level in the liver tissues. El-Nabi Kamel and Shehata 24 gave toluene (650 mg/kg single dose) to rats and found a significant (p<0.05) increase in MDA level and decrease in GSH level in the toluene group. Tas et al 25 reported that liver MDA level in the toluene group were significantly (p<0.05) higher than in control group. In the present study, in liver samples significant (p<0.05) increases in GSH-Px activity and GSH level and decrease in MDA level were detected in toluene+p-CA compared to toluene group. There are studies in which p-CA is used as a protector against oxidative damage induced by different agents. Similar to the current study, p-CA in liver tissues increased GSH levels and GSH-Px activities, and decreased MDA levels in previous studies 26,27,28.
The kidney is the primary excretion organ for most xenobiotics. In this study, MDA level was significantly (p<0.05) increased and GSH-Px activity and GSH level were significantly (p<0.05) decreased in toluene group when compared to the control group in the kidney tissues. El-Nabi Kamel and Shehata 24 administered toluene (650 mg/kg b.w) to rats and found a significant (p<0.05) increase in MDA level and a significant (p<0.05) decrease in GSH level in the toluene group. Ahmadizadeh et al 8 reported that rats administered to rats determined a significant (p<0.05) decrease in GSH level in kidney tissues compared to the control. Afravy et al 3 gave toluene (300, 600 and 900 mg/kg intraperitoneally) to the rats and investigated on toluene-induced kidney damage. In the toluene group compared to the control, the researchers stated that there was a significant (p<0.05) dose-dependant increase in MDA level. The results of the present study are agreed with those of previous studies. In the present study, in kidney samples significant (p<0.05) increases in GSH-Px activity and GSH level and decrease in MDA level were detected in the toluene+p-CA group compared to toluene group. Similar to the present study, p-CA in kidney tissues increased GSH levels and GSH-Px activities and decreased in MDA levels in previous studies 26,27,28,29,30.
Brain is one of the target organs of toluene-induced toxicity due to its lipid-rich characteristics 31. In this study, in brain samples significant (p<0.05) increase in MDA level and significant (p<0.05) decreases in GSH-Px activity and GSH level were detected in toluene group compared to the control group. Mattia et al 22 administered toluene (a single intraperitoneal dose of 1.5 mg/kg b.w) to rats and determined a significant (p<0.05) decrease in GSH levels in brain tissues (hippocampus, cerebellum and striatum). Coskun et al 32 gave toluene (3000 ppm by inhalation for 16 weeks, 8 hour/day, 6 day/week) to rats and detected significant (p<0.05) decrease in GSH-Px activity and significant (p<0.001) increase in MDA level in sciatic nerve tissues of rats. El-Nabi Kamel and Shehata 24 administered toluene (650 mg/kg a single dose) to rats and found a significant (p<0.05) decrease in GSH level and a significant (p<0.05) increase in MDA level depending on time in the toluene group. Kodavanti et al 33 treated rats with toluene (4, 12 and 24 months old rats with 0, 0.65 or 1 g/kg b.w single oral) and stated that there was a significant (p<0.001) decrease in GSH-Px activities in striatum tissues of 24-month-old rats and hippocampus tissues of toluene-treated 4-month-old rats. Abdel-Salam et al. 31 administered toluene (900 mg/kg b.w/day intraperitoneally) to rats and determined a significant (p<0.05) decrease in GSH level and a significant (p<0.05) increase in MDA level compared to control group. Montes et al. 34 treated mice with toluene (0 or 4000 ppm) for 4 weeks and 30 min in a day by inhalation. They indicated that there was a significant (p<0.05) decrease in GSH/GSSG level in both hippocampus and prefrontal cortex tissues of toluene-treated mice. In the present study, in brain samples significant (p<0.05) decrease in MDA level and significant (p<0.05) increases in GSH-Px activity and GSH level were detected in the toluene+p-CA group compared to toluene group. Similar to the present study, p-CA in brain tissues increased GSH levels and GSH-Px activities and decreased in MDA levels in previous studies 35,36.
In organ damage, significant changes occur in biochemical parameters. Increases in serum ALT and AST enzyme levels indicate liver damage. Creatinine and urea are markers of kidney damage or failure. Serum aminotransferase levels increase due to hepatitis, cirrhosis, liver cancer, and toxicity of drugs and various xenobiotics 37. In this study, in the toluene group compared to the control, significant (p<0.05) increases in serum ALT and AST activities and creatinine level were detected. Bae and Yoon 38 stated that toluene caused a significant (p<0.01) increase in ALT and AST levels in rat liver tissues. Tas et al. 25 administered 3000 ppm/hour/day toluene with inhalation to male rats for 4 weeks. It is stated that levels of serum ALT and AST were significantly (p<0.05) high in toluene-treated group. Moro et al 39 found that ALT and AST values were significantly (p<0.05 and p<0.001, respectively) higher in subjects exposed to toluene compared to control. Meydan et al 40 stated that there was a significant (p<0.001) increase in serum creatinine levels in toluene group compared to control. Afravy et al 3 applied different doses of toluene to rats (600 and 900) and reported a significant (p<0.05) dose-dependant increase in creatinine levels of toluene group. Meydan et al 37 reported that in serum ALT and AST levels there was a significant (p<0.001) increase in toluene group compared to the control group. These studies are in line with our results. In the present study, ALT and AST activities in the toluene+p-CA group were significantly (p<0.05) higher than in the toluene group. Similar to the present study, p-CA in serum samples decreased ALT and AST activities, and cretainine level 41,42,43,44.
In this study, it was found that the organs most sensitive to lipid peroxidation are brain, kidney and liver, respectively. This shows that toluene, which is a quite lipophilic solvent, accumulates primarily in lipid-rich tissues. The results of this study showed that toluene caused oxidative stress in brain, kidney and liver tissues at the dose administered and the period indicated. Also, it was found that p-CA has not any toxic effects on these tissues. As a result, it has been determined that p-CA (at the indicated dose and period) has a protective effect against toluene-induced hepatotoxicity, nephrotoxicity and neurotoxicity.