Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Químico - Farmacéuticas
Print version ISSN 0034-7418On-line version ISSN 1909-6356
Rev. colomb. cienc. quim. farm. vol.38 no.1 Bogotá Jan./June 2009
Artículo de Investigación
Apoptosis, Shape Change and Reactive Oxygen Species Generation in Human Neutrophils Exposed to Olanzapine, an Atypical Antipsychotic Drug
Apoptosis, cambio estructural y generación reactiva de especies reactivas de oxígeno en neutrófilos humanos expuestos a olanzapine, una droga antipsicótica atípica
F. Vargas1,V. A. Chávez1 B. Betancourt ,2C. León, 2K. Pérez, 2 A. Quezada, 2 L. E. Ojeda, 2 M. Paredes, 2 G. Fraile.2
1 Laboratorio de Fotoquímica, Centro de Química, Instituto Venezolano de Investigaciones Científicas I.V.I.C., Carretera Panamericana km 11, Altos de Pipe, Apartado 20632, Caracas 1020-A, Venezuela. Phone: 0212-5041338, Fax: 0212-5041350, correo electrónico: fvargas@ivic.ve
2 BIOMED-UC, Facultad de Ciencias de la Salud, Universidad de Carabobo-Sede Aragua, Venezuela.
Recibido para evaluación: agosto 10 de 2008. Aceptado para publicación: diciembre 14 de 2008.
ABSTRACT
The effects of olanzapine (OLZ) on the viability and functioning of human polymorphonuclear cells (PMNs) are clearly opposite to those previously reported for clozapine (CLZ). In fact, after 4- or 24-h-treatment with 20-50 µM OLZ, a significant inhibition of the respiratory burst in PMNs activated with opsonized zimosan or phorbol myristate acetate (PMA) was observed, whereas the burst provoked by formyl-methionyl-leucyl-phenylalanine (fMLP) was only inhibited at 50 µM OLZ. Under the same conditions, spontaneous apoptosis was accelerated at 20-50 µM OLZ, while the exogenous addition of H2O2 resulted in the PMN apoptosis being dose-dependently inhibited by OLZ in the entire range of concentrations. However, when H2O2 was intracellularly generated by treatment with PMA, the induced apoptosis was only decreased at 2 µM OLZ. Absorbance scans revealed that OLZ is able to react with equimolar quantities of either H2O2 or HOCl. These results suggest that OLZ inhibits both ROS-induced PMN apoptosis and respiratory burst due to extracellular scavenging of released ROS.
Key words: Olanzapine, neutrophils, apoptosis, respiratory burst, shape change, oxidative stress
RESUMEN
Los efectos de olanzapine (OLZ) sobre la viabilidad y el funcionamiento de células humanas polimorfonucleares (PMN, por sus siglas en inglés) claramente son opuestos a los señalados para la clozapine (CLZ). En efecto, después de 4-24 h de tratamiento con 20-50 µM OLZ, se observó una inhibición significativa del estallido respiratorio en PMN activados con zimosan o con forbol acetato miristato, mientras que la inhibición provocada por el formil-metionil-leucil-fenilalanina fue sólo inhibida a 50 µM de OLZ. En las mismas condiciones, la apoptosis espontánea se aceleró con 20-50 µM OLZ, mientras que la adición exógena de H2O2 dio lugar a la apoptosis de PMN en dosis dependiente inhibida por OLZ en el rango entero de concentraciones. Sin embargo, cuando se generó H2O2 intracelular por tratamiento con PMA, la apoptosis inducida se disminuyó solamente con 2 µM OLZ.
Las exploraciones de los espectros de absorbancia revelaron que OLZ puede reaccionar con cantidades equimolares de H2O2 o de HOCL. Estos resultados sugieren que OLZ inhibe ambos tipos de apoptosis de PMN (la inducida por especies reactivas oxigenadas y por estallido respiratorio debido a atrapadores extracelulares de estas especies reactivas oxigenadas).
Palabras clave: olanzapina, neutrofilos, apoptosis, estallido respiratorio, estrés oxidativo.
INTRODUCTION
Olanzapine (OLZ) is an atypical anti-schizophrenic drug which is as effective in treating positive symptoms as traditional anti-psychotics but has less severe side effects. This psychotropic agent belongs to the thienobenzodiazepine class. The chemical designation is 2-methyl-4-(4-methyl-1-piperazinyl)-10H-thieno[2,3-b] [1,5]benzodiazepine (Fig. 1). OLZ is used for treating schizophrenia and acute mixed or manic episodes associated with bipolar disorder. It also is used as maintenance therapy for bipolar disorder and for treating agitation due to schizophrenia or bipolar disorder. Although this drug displays antipsychotic efficacy probably by virtue of its dopaminergic blocking properties, the specific mechanisms of action whereby OLZ exerts its therapeutic effects remain unknown. Indeed, in recent years it has been suggested that for many diseases of the central nervous system, the most effective drugs have affinity for many molecular targets. For instance, recent studies have indicated that OLZ protects PC12 pheochromocytoma cells against both N-methyl-4-phenylpyridinium ion- and H2O2-induced apoptosis (Qing et al. 2003, Wei 2003). These findings are in line with the notion that the pathogenesis of schizophrenia may be related to oxidative stress (Herken 2001). Moreover, in a recent report we showed that clozapine, an OLZ-related atypical neuroleptic drug, can rescue PMNs from spontaneous apoptosis in vitro (Vargas et al. 2005). It has been proposed that this type of apoptosis, which represents a classical example of death occurring as a result of the removal of a survival signal, probably ensues as a consequence of the intracellular generation of reactive oxygen species (Scheel-Toellner et al. 2004). To complicate things further, clozapine also induced an increased generation of reactive oxygen species (ROS) after treatment of PMNs with receptor-dependent stimuli, whereas the degree of enhancement was negligible upon subsequent exposure to receptor-independent activators .
Previous reports have shown that the antipsychotic drug olanzapine (OLZ) can protect PC12 pheochromocytoma cells against oxidative stress-induced apoptosis. In addition, the OLZ-structurally related antipsychotic drug clozapine (CLZ) primed the respiratory burst as well as delaying the spontaneous apoptosis of human polymorphonuclear cells (PMNs). These findings suggest that the atypical antipsychotic class of drugs appears to have a generalized protective effect against apoptosis.The in vitro study reported here was carried out to assess whether OLZ at 2-50 µM could alter neutrophil oxidative burst, shape change, viability, and spontaneous- or oxidative stress-induced apoptosis.
MATERIALS AND METHODS
Chemical
Olanzapine (OLZ) 2-methyl-4-(4-methyl-1-piperazinyl)-10H-th>ieno[2,3-b][1,5]benzodiazepine, molecular formula C17H20N4S, with a molecular weight of 312.44 gr/mol, was extracted from the commercial medicine Ziprexa® (Eli Lilly laboratory, United Kingdom) with a soxhlet extractor using ethanol as the solvent, purified by TLC and recrystallized from the same solvent. Purity was determined as 99% by mass spectroscopy and 1H-NMR. Phorbol myristate acetate (PMA), formyl-methionyl-leucyl-phenylalanine (fMLP), glucose oxidase (from Aspergillus niger, 18000 units/g) and luminol, were purchased from Sigma (St Louis, USA). A 0.01 M hydrogen peroxide solution was freshly prepared by a dilution of 30% hydrogen peroxide with distilled water. Hank´s balanced salt solution (HBSS) was prepared by dissolving the commercially available powdered medium (Sigma). Before use, 0.1% (w/v) gelatin was added (HBSS-gel).
Isolation of human PMNs
After informed consent, venous blood was obtained from healthy volunteer donors. Neutrophils were isolated from EDTA-anticoagulated blood following a differential centrifugation method previously described (Eggleton et al. 1989). The separated cells were suspended to 1 x 107/mL of HBSS.
Cell culture and treatment
To evaluate long-term effects (24 h) of OLZ, human PMNs (final concentration: 5 x 106 cells/mL) suspended in a RPMI 1640 medium (pH 7.4) supplemented with 10% fetal calf serum, 2 mM L-glutamine, 100 U/ml penicillin and 100 µg/mL streptomycin, were incubated for different time periods with OLZ (2-50 µM) or solvent (DMSO; final concentration: 1% v/v) at 37°C in an atmosphere containing 5% CO2. In other experiments, OLZ-treated PMNs were incubated with PMA or a glucose-glucose oxidase mixture for the doses and times indicated.
Neutrophil chemiluminescence
Samples for neutrophil chemiluminescence determinations were prepared by adding 50 µL of the olanzapine-treated neutrophil suspensions in RPMI to HBSS supplemented with luminol (total volume: 700 µL. Final concentrations: 3.6 x 105 neutrophils/ mL, 52 µM luminol). Chemiluminescence was measured in a Bio-Orbit 1251 luminometer (Labsystems Oy, Helsinki, Finland) using 4-mL polypropylene tubes. The tubes were equilibrated for 5 min at 37 °C after which 50 µL of opsonized zymosan (OZ; final concentration: 0.21 mg/mL), or 2.4 µL of PMA (final concentration: 0.22 µM) were added. The light emission was recorded for 30 s intervals and its intensity was determined by integrating the area under the chemiluminescence curve (in mV.s) during 30 min.
Opsonization of zymosan
Zymosan was boiled in isotonic NaCl for 1 h, washed three times by centrifugation, and suspended (3 mg/mL) in HBSS. Opsonization was carried out by incubating 1 vol of the above suspension with 1 vol of fresh human serum for 30 min at 37°C and then washing three times with HBSS before dividing in aliquots and keeping at -80°C until use (Kroes et al. 1992).
Neutrophil shape change
Neutrophil shape change was assessed by optical microscopy. At 4 or 24 h, neutrophil suspensions treated with OLZ were pelleted, resuspended in RPMI at 5 x 106/mL, and incubated for 15 min in the presence of 0.1 µM fMLP at 37°C and 5% CO2. The cells were then fixed in 2% glutaraldehyde, and assessed morphologically as either "shape changedâ or spherical.
Assessment of neutrophil apoptosis by morphological examination
For morphological assessment, 150 µL of pretreated neutrophils (5 x 106 cells/mL) were spun down (120g, 6 min) onto glass slides using a cyto-centrifuge (Hermle, Z 323 K, Germany). The slides were air-dried and stained with Wright-Giemsa solution for light microscopic evaluation. The percentage of cells with characteristic apoptotic changes (nuclear condensation, vacuolation and blebbing) was assessed by counting at least 300 cells/slide with the use of a 40X objective (Axiolab, Zeiss, Germany).
Flow Cytometric Determination of Apoptosis in Neutrophils by Annexin V/ Propidium Iodide Double Staining
This assay was adapted with some modifications from (McNamee et al. 2005). Briefly, venous blood was collected in a heparinized vacutainer tube (BD Biosciences, San Jose,CA). Whole blood from each donor was diluted 1:10 with RPMI (final leukocyte concentration: 0.5-1.0 x 106 cells/mL) and 1 mL fractions were transferred into 10 mm culture dish. Olanzapine (final concentration: 2-50 µM) was added to whole blood cultures and the cultures were incubated at 37°C and 5% CO2 for 24 h. Then, 1 mL aliquots were removed from each culture dish for analysis by flow citometry. To measure neutrophil apoptosis, 1 mL of diluted blood was added to a flow citometry tube and centrifuged at 250 x g. The supernatant was removed and then 1,5 mL of NH4Cl lysis solution was added to each tube and incubated at room temperature for 15 min. The samples were centrifuged for 6 min at 250 x g and washed two times with 2 mL of PBS. The cells were re-suspended in 100 µL of 1x Annexin V binding buffer (BD Biosciences, San Jose, CA) and were labeled for detection of apoptotic cells by adding 5 µL of Annexin V-FITC (BD Biosciences, San Jose, CA). Immediately before analysis by flow citometry (FACSCanto, BD Biosciences, San Jose, CA), 2 µL of propidium iodide (PI) (1 mg/mL) (BD Biosciences, San Jose, CA) were added to each sample. Neutrophils were identified using forward-/side-scatter. Apoptotic cells were identified as those cells that were Annexin V+
Monitoring of the reaction of OLZ with H2O2 and HOCl
Equimolar solutions (100 µM) of OLA and H2O2 or sodium hypochlorite (NaOCl) were prepared in PBS pH 6.0 and mixed in quartz cells. Repetitive spectra (200-480 nm) were measured every 30 s in a UV-Vis Perkin Elmer Lambda 35 spectrophotometer.
Statistical treatment of results
All values were expressed as the mean ± S.E.M. Data were analyzed using the Student's t-test. Differences were considered to be significant when the p value was <0.05.
RESULTS AND DISCUSSION
Effects of OLZ on neutrophil viability
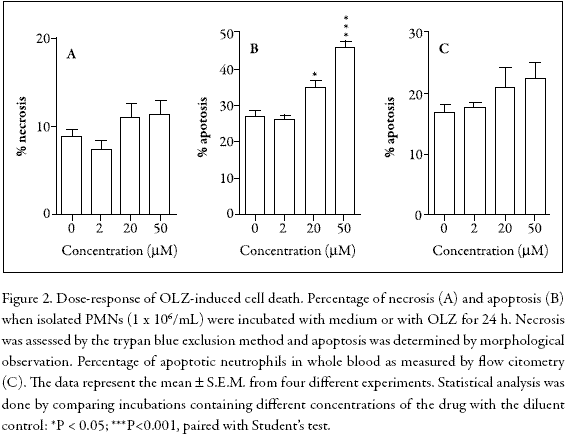
To examine the effects of OLZ on PMN viability, isolated cells were incubated for 24 h and analyzed by trypan blue exclusion (necrosis) or by Wright-Giemsa staining (apoptosis). In addition, to avoid in vitro artifacts related to cell purification and hemolysis, the possibility of apoptosis caused by a flow cytometric analysis in whole blood was investigated. Figure 2 shows that in isolated neutrophils, cell necrosis was not significantly altered (Fig. 2A), whereas spontaneous apoptosis was dose-dependently accelerated (Fig. 2B). In contrast, the flow cytometric analysis did not show any significant effect (Fig. 2C).
Effects of OLZ on functional capabilities of human neutrophils
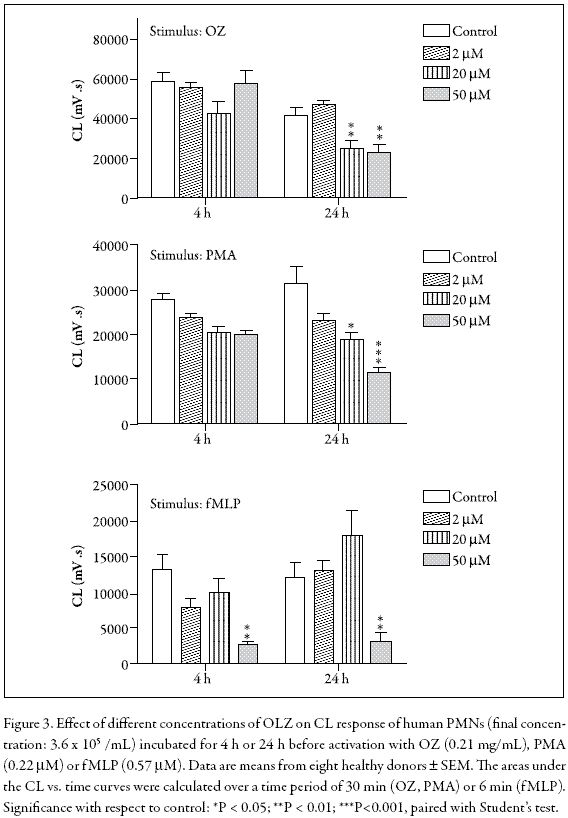
Luminol-enhanced chemiluminescence (LCL) was used to evaluate receptor-dependent (OZ, fMLP)- and receptor-independent (PMA)-induced ROS formation in PMNs treated with OLZ during 4 or 24 h in the 0-50 µM range. These activators were selected because constitutive PMN apoptosis is accompanied by decreased superoxide generation following receptor-dependent stimuli whereas apoptotic PMNs preserve their superoxide production when activated with receptor-independent stimuli (Whyte et al. 1993). Figure 3 shows that in PMNs activated with OZ or PMA after treatment during 4 or 24 h, the LCL was significantly decreased. However, in fMLPactivated PMNs, the LCL was only inhibited at 50 µM OLZ, independently of the incubation times. Besides, the dose-response profile was clearly different from that observed with the other stimuli.
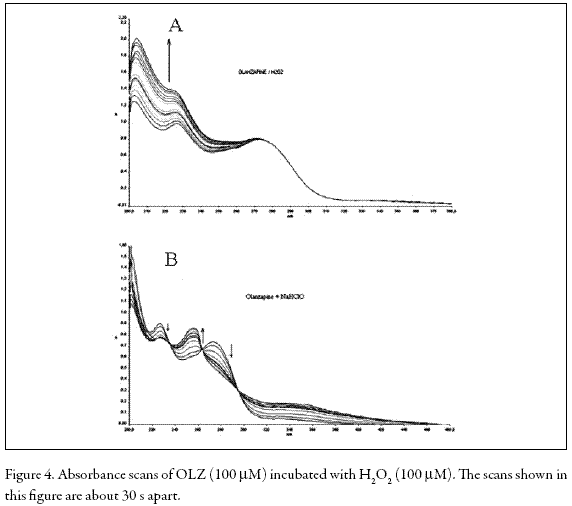
In ROS-producing cells such as PMNs, superoxide (O2 -) is generated by a NADPH oxidase from dioxygen and dismutated to H2O2 by superoxide dismutase (SOD). Hydrogen peroxide can be metabolized to hypochlorite (OCl-) by myeloperoxidase (MPO) or converted to hydroxyl radical (HO.) by Haber-Weiss or Fenton reactions (Fialkow et al. 2007). These active oxygen species enhance LCL (Dahlgren and Karlsson 1999). On the other hand, it has been shown that fMLP-stimulated LCL of PMNs predominantly reflects the generation of hydroxyl radical (HO.) while PMA- and OZstimulated LCL mostly reflects the formation of hypochlorite (OCl-) and/or singlet oxygen (1O2) (Takahashi et al. 1991). Additionally, the attenuation of LCL in PMNs stimulated by either OZ or PMA suggests that a direct reaction of OLZ and H2O2 or OCl- may occur. We examined this possibility by recording the UV spectra during the OLZ-H2O2 and OLZ-OCl- reactions. Figure 4 shows that the addition of H2O2 or OCl- caused marked changes in the absorption spectrum of OLZ, a result which is indicative of the formation of new products.
In a previous report we noted the interesting result that using OZ- or fMLP as stimuli, there was a substantially enhanced LCL response in PMNs treated with 20-50 µM clozapine, whereas the degree of enhancement was negligible upon subsequent exposure to PMA (Vargas et al. 2005). It is worth noting that, in the absence of an activating peroxidase, clozapine was not oxidized after mixture of the drug with H2O2, suggesting there was no direct chemical reaction between the two substances (Gardner et al. 1998).
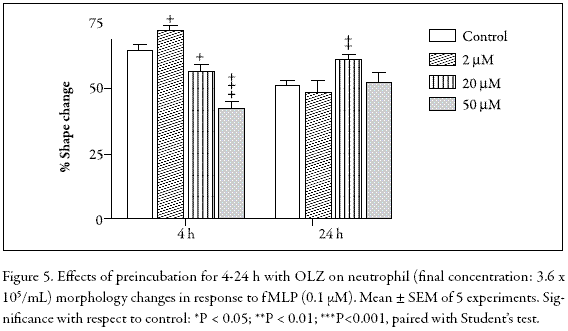
Neutrophil polarization is a characteristic change in cell shape in response to a chemotactic stimulus such as fMLP. Figure 4 shows that the OLZ-treated PMNs generate complex responses when stimulated with 10 µM fMLP. For instance, in the 4 h-incubation assay, 2 µM OLZ induced a slight increment in cell polarization whereas in the 20-50 µM range, a dose-dependent inhibition was evident. In contrast, in the 24 h-incubation, no inhibition was observed. Indeed, at 20 µM OLZ, a statistically significant stimulation occurred, suggesting a protective effect. Although not significant, this effect was also observed at 20 µM OLZ in the fMLP-stimulated chemiluminescence (Fig. 5). Clearly, when OLZ-treated PMNs are activated by fMLP, the results obtained, whether in chemiluminescence or in shape change assays, are rather puzzling and difficult to interpret.
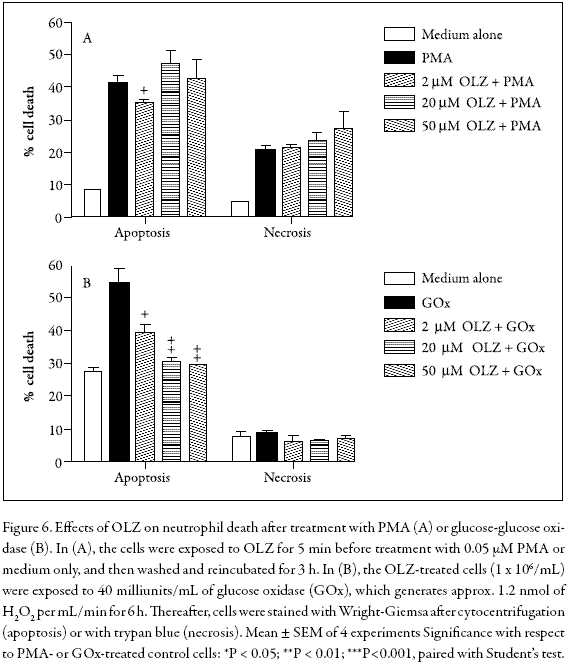
Effects of OLZ on apoptosis in PMA- or H2O2-treated neutrophils
To determine whether OLZ could alter neutrophil apoptosis induced by intracellularly generated hydrogen peroxide, we used a protocol that includes a brief period (30 min) of incubation with PMA followed by washing with medium and re-incubation for 180 min. It had previously been demonstrated that treatment with PMA leads to intracellular H2O2 production, whereas non-apoptotic death was minimized through washing with culture medium (Lundqvist-Gustafsson and Bengtsson 1999). Figure 6A indicates that PMA induced a marked apoptotic effect on PMNs when compared with cells treated with medium alone (P<0.001, N = 5), whereas in cells co-incubated with 2 µM OLZ, a modest inhibition of apoptosis was observed. In addition, parallel experiments carried out by the trypan blue exclusion method showed that OLZ did not affect the percentage of cell that died.
To elucidate the exact mechanism by which some drugs such as clozapine and OLZ induce neutropenia or lethal agranulocytosis, many experiments have been conducted to study their oxidation behavior in the presence of an added activating system (horseradish peroxidase or myeloperoxidase) plus H2O2 or NaOCl (Gardner et al. 1998, Kohara et al. 2002). However, in our study we were interested in examining how susceptible the PMNs are to H2O2 in the absence of peroxidases. Thus, to evaluate the effects of OLZ on PMNs treated with exogenous H2O2, a bolus of H2O2 was added directly to the cells. However, a considerable degree of variability with respect to PMN viability was observed (data not shown). In contrast, when the cells were exposed to a low but continuous supply of H2O2 through its enzymatic generation by glucose and glucose oxidase, the results were much easier to reproduce. Figure 6B shows that this treatment induced a significant apoptotic effect on PMNs when compared with medium alone (P<0.001, N = 5) whereas pre-incubation with 2-50 µM OLZ inhibited this effect in a dose-dependent fashion, without affecting cell membrane integrity. Again, the attenuation of H2O2-induced apoptosis in human neutrophils by OLZ may at least partly result from its ROS-scavenging effects. Similar results were obtained when H2O2-treated PC12 cells were exposed to OLZ for 24 h at concentrations of 100 µM or more (Wei et al. 2003), further suggesting that the OLZ-H2O2 reaction does not generate toxic products.
In the presence of OLZ and isolated neutrophils, cell necrosis was not significantly altered, whereas spontaneous apoptosis was dose-dependently accelerated, but the flow cytometric analysis did not show any significant effect.
The disparate findings (Figure 2) may be attributed to the use of purified PMNs versus the use of a whole blood assay with the added presence of various cytokines and other cell types. Alternatively, it is possible that the presence of albumin or other competitive agents in the whole blood may explain the absence of OLZ effects on neutrophil apoptosis. Indeed, an early report showed that in vivo, OLZ is predominantly bound to albumin and alpha-1-acid glycoprotein (Callaghan et al. 1999).
The results obtained from both chemiluminescence assays and absorbance scans suggest that OLZ inhibits neutrophil apoptosis only extracellularly. A direct chemical reaction between H2O2 and OLZ could account for these findings. Besides, the effects of OLZ on both spontaneous neutrophil apoptosis and the respiratory burst clearly contrasts with those previously reported for clozapine. At this point, we do not know whether these contrasting effects may be related to the differences in therapeutic and toxic properties of olanzapine and also clozapine.
PMA induced a marked apoptotic effect on PMNs when compared with cells treated with medium alone, whereas in cells co-incubated with 2 µM OLZ, a modest inhibition of apoptosis was observed. Further studies on biological systems will contribute to elucidating this behaviour.
CONCLUSIONOur results indicate that OLZ is able to inhibit neutrophil apoptosis mediated by extracellularly added H2O2 but not by intracellularly generated H2O2. Further investigations are necessary to determine the effects of the presence of OLZ, applied to other PMN functions such as chemotaxis, degranulation and phagocytosis and also on other biological systems. In conclusion, OLZ is an interesting anti-schizophrenic drug that may act through mechanisms unique to itself to exert its apoptotic action on human polymorphonuclear cells.
ACKNOWLEDGMENTS
This research was supported by a grant from the Fondo Nacional de Investigaciones Científicas y Tecnológicasâ FONACIT- Venezuela (S1-2502, S1-96001724), the German Embassy in Venezuela and the Fundación Polar.
REFERENCES
1. J. T. Callaghan, R.F. Bergstrom, L.R. Ptak y 1. C.M. Beasley, Olanzapine. Pharmacokinetic and pharmacodynamic profile, Clin. Pharmacokinet., 37, 177 (1999). [ Links ]
2. C. Dahlgren y A. Karlsson, Respiratory burst in human neutrophils, J. Immunol. Methods., 232, 3 (1999). [ Links ]
3. P. Eggleton, R. Gargan y D. Fisher, Rapid method for the isolation of neutrophils in high yield without the use of dextran or density gradient polymers, J. Immunol. Methods., 121, 105 (1989). [ Links ]
4. L. Fialkow, Y. Wang y G.P. Downey, Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function, Free Rad. Biol. Med., 42, 153 (2007). [ Links ]
5. I. Gardner, N. Zahid, D. MacCrimmon y J.P. Uetrecht, A comparison of the oxidation of clozapine and olanzapine to reactive metabolites and the toxicity of these metabolites to human leukocutes, Mol. Pharmacol., 53, 991 (1998). [ Links ]
6. H. Herken, E. Uz, H. Ozyurt, S. Sogut, O. Virit y O. Akyol, Evidence that the activities of erythrocyte free radical scavenging enzyme and the products of lipid peroxidation are increased in different forms of schizophrenia, Mol. Psychiatry., 6, 66 (2001). [ Links ]
7. T. Kohara, T. Koyama, M. Fujimura, H. Tanaka, J. Maeda, T. Fujimoto, I. Yamamoto y M. Arita, Y-931, a novel atypical antipsychotic drug, is less sensitive to oxidative phenomena, Chem. Pharm. Bull. (Tokyo), 50, 818 (2002). [ Links ]
8. B.H. Kroes, A.J. van den Berg, H.C. Quarles van Ufford, H. van Dijk y R.P. Labadie, Anti-inflammatory activity of gallic acid, Planta Med., 58, 499 (1992). [ Links ]
9. H. Lundqvist-Gustafsson y T. Bengtsson, Activation of the granule pool of the NADPH oxidase accelerates apoptosis in human neutrophils, J. Leukoc. Biol., 65, 196 (1999). [ Links ]
10. J.P. McNamee, P.V. Bellier, B.C. Kutzner y R.C. Wilkins, Effect of proinflammatory cytokines on spontaneous apoptosis in leukocyte sub-sets within a whole blood culture, Cytokine., 31, 161 (2005). [ Links ]
11. H. Qing, K. Xu, Z. Wei, K. Gibson y X.M. Li, The ability of atypical antipsychotic drugs vs. haloperidol to protect PC12 cells against MPP+-induced apoptosis, Eur. J. Neurosci., 17, 1563 (2003). [ Links ]
12. D. Scheel-Toellner, K.Q. Wang, P.R. Webb, S.H. Wong, R. Craddock, L.K. Assi, M. Salmon y J.M. Lord, Early events in spontaneous neutrophil apoptosis, Biochem. Soc. Trans., 32, 461 (2004). [ Links ]
13. R. Takahashi, K. Edashige, E.F. Sato, M. Inoue, T. Matsuno y K. Utsumi, Luminol chemiluminescence and active oxygen generation by activated neutrophils, Arch. Biochem. Biophys., 285, 325 (1991). [ Links ]
14. F. Vargas, C. Rivas, H. Perdomo, A. Rivas, L.E. Ojeda, M. Velásquez, H. Correia, A. Hernández y G. Fraile, Clozapine prevents apoptosis and enhances receptordependent respiratory burst in human neutrophils, Pharmazie, 60, 364 (2005). [ Links ]
15. Z. Wei, O. Bai, J.S. Richardson, D.D. Mousseau y X.M. Li, Olanzapine protects PC12 cells from oxidative stress induced by hydrogen peroxide, J. Neurosci. Res., 73, 364 (2003). [ Links ]
16. M.K. Whyte, L.C. Meagher, J. MacDermot y C. Haslett, Impairment of function in aging neutrophils is associated with apoptosis, J. Immunol., 150, 5124 (1993). [ Links ]














