Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Psiquiatría
Print version ISSN 0034-7450
rev.colomb.psiquiatr. vol.36 no.1 Bogotá Jan./Mar. 2007
Artículos originales
Hosam El-Din Matar1 Muhammad Qutayba Almerie1 Ana María Giraldo2 Clive E. Adams3
1 Medical Student, Faculty of Medicine, Damascus University, Syria.
E-mail: hematar@hotmail.co.uk
2 Medical Student, Pontificia Universidad Javeriana, Bogotá.
3 Cochrane Schizophrenia Group, Academic Unit of Psychiatry and Behavioural Sciences, University of Leeds, UK.
Abstract:
Background: Fluphenazine, one of only three antipsychotics on WHO´s list of essential drugs, has been widely available for five decades. Quantitative reviews of its effects compared with placebo are rare and out of date. Methods: We searched for all relevant randomised controlled trials comparing oral administration of fluphenazine with placebo on the Cochrane Schizophrenia Group´s register of trials (October 2006) and in reference lists of included studies. Data were extracted from reliably selected trials. Where possible, we calculated fixed effects relative risk (RR), the number needed to treat (NNT), and their 95% confidence intervals (CI). Results: We found over 1200 electronic records for 415 studies. Ninety papers were acquired; 59 were excluded and the remainder were reports of the seven trials we could include (total participants=349). Compared with placebo, in the short-term, global state outcomes for ‘not improved’ were not significantly different (n=75, 2 RCTs, RR 0.71 CI 0.5 to 1.1). There is evidence that oral fluphenazine, in the short term, increases a person´s chances of experiencing extrapyramidal effects such as akathisia (n=227, 2 RCTs, RR 3.43 CI 1.2 to 9.6, NNH 13 CI 4 to 128) and rigidity (n=227, 2 RCTs, RR 3.54 CI 1.8 to 7.1, NNH 6 CI 3 to 17). We found study attrition to be lower in the oral fluphenazine group, but data were not statistically significant (n=227, 2 RCTs, RR 0.70 CI 0.4 to 1.1). Conclusions: Fluphenazine is an imperfect treatment with surprisingly few data from trials to support its use. If accessible, other inexpensive drugs, less associated with adverse effects, may be a better choice for people with schizophrenia. It is time for the World Health Organisation to revise their list of essential antipsychotic drugs.
Key words: Fluphenazine, schizophrenia, antipsychotics.
Resumen
Introducción: La flufenazina, uno de los tres antipsicóticos de la lista de drogas esenciales de la OMS, está disponible desde hace 5 décadas. Las revisiones cuantitativas de sus efectos vs. placebo son escasas y desactualizadas. Método: Buscamos estudios controlados aleatorizados relevantes que compararan la administración de flufenazina oral contra placebo, en los registros de ensayos de los grupos de esquizofrenia de Cochrane (oct. 2006) y en referencias incluidas en los estudios. Los estudios consistentes y confiables muestran datos de ensayos clínicos aleatorizados (ECA). Calculamos el RR mediante un modelo de efectos fijos, el número necesario por tratar (NNT) y su intervalo de confianza de 95%. Resultados: En flufenazina vs. placebo, a corto plazo, el resultado "no mejoría" no fue muy diferente (n=75, 2 ECA, RR 0,71, IC 0,5 a 1,1). La flufenazina oral, a corto plazo, aumenta el riesgo de manifestaciones extrapiramidales, como la acatisia (n=227, 2 ECA, RR 3,43, IC 1,2 a 9,6, NNT 13, IC 4 a 128) y rigidez (n=227, 2 ECA, RR 3,54, IC 1,8 a 7,1, NNT 6, IC 3 a 17). La deserción fue más baja en el grupo de la flufenazina oral, pero los datos no fueron estadísticamente significativos (n=227, 2 ECA, RR 0,70 CI 0,4 a 1,1). Conclusión: La flufenazina es un tratamiento imperfecto con muy pocos datos que sustenten su uso. Otras drogas de bajo costo y pocos efectos adversos pueden ser mejor opción para pacientes psicóticos. La OMS debe revisar su lista de antipsicóticos esenciales.
Palabras clave: flufenazina, esquizofrenia, antipsicóticos.
Background
Overall, the antipsychotic drugs, with their anti-dopaminergic effects, are the mainstay treatment for people with schizophrenia (1). They are generally regarded as highly effective, especially in controlling symptoms such as hallucinations and fixed false beliefs (delusions) (2). Fluphenazine, a phenothiazine derivative, is one of the first drugs to be classed as an ‘antipsychotic’. Reports from 1959 and 1960 first indicate its value in psychotic illness (3-4) and it was approved for clinical use in the USA in 1959.
Fluphenazine is thought to elicit its antipsychotic effects via interference with central dopaminergic pathways and blocking receptors, particularly D2, in the mesolimbic zone of the brain (5). Extrapyramidal side effects are a result of interaction with dopaminergic pathways in the basal ganglia. As fluphenazine is not specific to one action within the body it is known to cause adverse effects ranging from orthostatic hypotension as a result of its alpha adrenergic blocking activity to anticholinergic and extrapyramidal symptoms (tardive dyskinesia, pseudo-parkinsonism, dystonia, dyskinesia, akathesia) (6). In addition, the use of fluphenazine has been associated with a potentially fatal disturbance of blood pressure, temperature and muscle control (neuroleptic malignant syndrome)( 7) .
Fluphenazine is still commonly used for people with schizophrenia and is given by mouth or by short-acting injection. Although we have not found precise data on world wide use, fluphenazine, along with only two other antipsychotics (chlorpromazine and haloperidol) is on the World Health Organization´s list of essential drugs (8). In low and middle income countries, where non-proprietary preparations of fluphenazine are inexpensive, it may be one of the only drug treatments available. We, however, know of no up-to-date systematic reviews of the absolute effects of this ‘essential’ antipsychotic.
Methods
Inclusion criteria
The inclusion criteria were de- fined and disseminated for peer review within a Cochrane protocol (9). We included articles if they reported randomized controlled trials where participants had schizophrenia or non-affective serious/chronic mental illness, and where the interventions included oral administration of fluphenazine (any dose) versus placebo or no treatment.
Identification of relevant trials
We identified relevant randomised trials by searching the Cochrane Schizophrenia Group´s Register of trials (Oct 2006) using the phrase: (((fluphen* or flufen* or modec* or moditen* or eutimax* or prolixin* or siqualon* or anaten* or dapotum* or decazate* or decafen* or decentan* or fludecate* or lyogen* or lyoridin* or mirenil*) in title, abstract and index fields inREFERENCE) or (fluphenazin* in interventions field in STUDY))
This register is compiled by regular, systematic, searches of major bibliographic databases, hand searches and conference proceedings. A full description is given in the Group´s module on the Cochrane Library (http://www.cochrane.org/contact/entities.htm#CRGLIST). In addition, the references of all identi- fied studies were inspected for more studies.
Data extraction and study appraisal
All electronic records identified were independently inspected by HEM and MQM, who then obtained full reports of studies of agreed relevance. We assessed the methodological quality of included trials using criteria described in the Cochrane Handbook (10). These criteria are based on the evidence of a strong relationship between allocation concealment and direction of effect (11). Data relating to methods, participants, interventions and outcomes, were extracted and disagreement discussed and documented.
Statistical methods
Dichotomous and continuous data were not used if over half of those randomised were not contributing to the outcome due to early attrition from the study or non-compliance. Dichotomous data were combined using fixed effects Relative Risk (RR) (12). Numbers needed to treat (NNT) (13) were also calculated and I-square tests for heterogeneity were performed (14). Where less than 50% of people were lost to follow-up at the end of a trial, ‘worst case’ intention-to-treat analyses were undertaken by assuming that those who had left a trial early had had a poor outcome. The sensitivity of the final results to this assumption was tested. Continuous data were excluded if derived from scales of unknown validity and if totals or measures of variance were not reported. Summation was not attempted if continuous data were too skewed (15). All estimates of effect are presented with their 95% confidence intervals (CI).
Results
Search results
Electronic searches identified over 1200 records most of which were ineligible. Full copies of 90 possibly relevant citations were obtained for detailed scrutiny. Of these, fifty-nine papers were excluded and thirty-one reports of the seven randomized trials included (see Table 1). Studies were mainly excluded due to lack of random allocation. Four randomised trials, however, reported irrelevant outcomes, such as critical flicker fusion frequency, the effects of giving ‘phenothiazines’ not broken down by each treatment group, or presented data in such a way as to make the outcomes unintelligible or impossible to use.
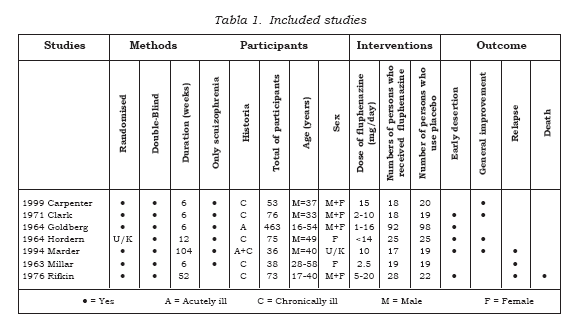
Study quality
All seven included studies either reported use of random allocation or suggested it; one study reported the process used as the groups were matched on age, chronicity and severity of illness (16). Citations to all included and excluded studies are available in the full Cochrane Review (10), otherwise the names and dates cited in this text relate to Table 1. Only two of the seven included studies (29%) adequately describe attempts to double blind (17-18). Other trials indicated that blinding had been made, but gave no description of how this had been done. Often the description of participants who left studies early was inadequate; two of the seven included studies provided details of treatment withdrawals. Three studies reported that withdrawal from treatment had occurred, but gave no further description. Presentation of data was also poor. Trials frequently presented both dichotomous and continuous data in graphs, or reported inexact statistical measures of probability, for example p>0.05. This often made it impossible to extract raw data for synthesis. Continuous scale data were frequently collected in the trials but were frequently poorly reported: two of the seven included trials did not report standard deviations and four other included trials did not present any data from the scales they had used. In this way a lot of potentially informative data were lost.
Study designs
These studies included 814 participants but only 349 of whom were allocated to the specific comparison of interest to this review (oral fluphenazine vs placebo). The great majority of participants in nearly all trials were diagnosed as suffering from schizophrenia. Four of the seven trials described the diagnostic criteria used, or the symptoms required for people to be included. Otherwise entry to most of the included studies was based on a pragmatic diagnosis of schizophrenia. The trials ranged in size from 36 to 190 participants. Most people were hospitalised at the time of the study. Four studies were hospital-based, while three were undertaken in the community. Five studies were conducted in the USA, one in Australia and one in the United Kingdom. All trials compared oral fluphenazine with inactive placebo. The lowest dose of fluphenazine tested was 2.5 mg/day (18) while the highest was 15 mg/day (19). The mean duration of treatment was about 170 days (~6 months), but this was highly skewed (SD 253). The most common study length was six weeks but the range was considerable with the longest being 2 years.
Outcomes
Table 2 presents the main results of this review. These intention- to-treat data are derived by synthesising homogeneous trial findings and results remain essentially unchanged when we only use data from participants who completed studies. These data show no clear pattern indicative of publication bias when sorted by study size and effect (20). None of the included studies attempted to quantify levels of satisfaction or quality of life and there is no evidence of any direct economic evaluation of fluphenazine. We are, however, able to report some data on the absolute effects of oral fluphenazine on aspects of the global and mental state, and adverse effects.
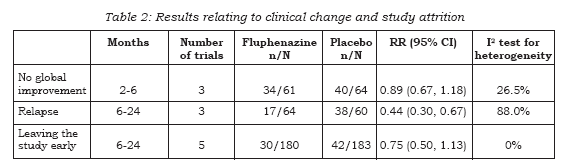
Data on global improvement (not improved or worsened), in the period up to 6 months showed no significant difference between oral fluphenazine and placebo (n=125, 3 RCTs, RR 0.89 CI 0.67 to 1.18) with low heterogeneity (I2 26.5%, Figure 1). One study reported data on relapse up to six weeks (short term assessment) with results indicating a trend favouring fluphenazine (n=38, RR 0.25 CI 0.1 to 1.0). Two other studies reported data for long-term relapse which significantly favoured fluphenazine but data are heterogeneous (I-squared 92%).
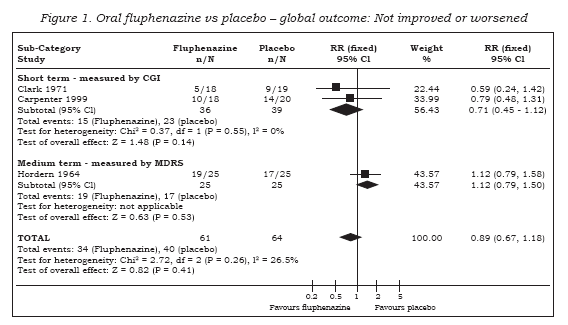
Fluphenazine has many adverse effects (see Table 3). Extrapyramidal symptoms are common. In the short term, there is evidence that fluphenazine increases a person’s chances of experiencing akathisia (n=227, 2 RCTs, RR 3.43 CI 1.2 to 9.6, NNH 13 CI 4 to 128), facial rigidity (n=190, RR 2.77 CI 1.0 to 7.5, NNH 12 CI 4 to 654), ‘loss of associated movements’ (n=190, RR 6.39 CI 2.0 to 21.0, NNH 7 CI 2 to 35), rigidity (n=227, 2 RCTs, RR 3.54 CI 1.8 to 7.1, NNH 6 CI 3 to 17) and tremor (n=227, 2 RCTs, RR 3.19 CI 1.3 to 8.1, NNH 11 CI 4 to 94). We found measures of akinesia, associated movements, dystonia and restlessness/insomnia were not significantly different from those allocated to placebo. Evidence in the medium term indicates that fluphenazine increases the likelihood of having parkinsonism (15) (n=50, RR 5.50 CI 1.4 to 22.3, NNH 3 CI 2 to 35), but risks of akathisia, akinesia and dystonia were equivocal. There were no significant differences between people given placebo and those allocated fluphenazine in the frequency of complaints of gastrointestinal distress, cardiovascular (weakness, faintness, dizziness), lactation and rash.
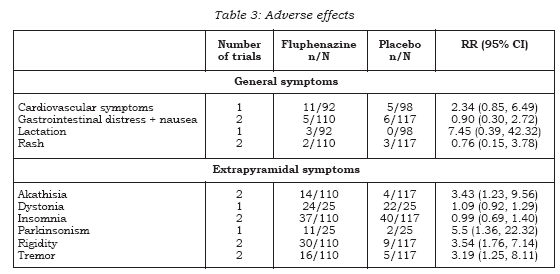
In the short term people allocated to oral fluphenazine did not leave the study any less often than participants who were given placebo (n=227, 2 RCTs, RR 0.70 CI 0.4 to 1.1). This also applied to the medium term (n=50, 1 RCT, RR 5.0. CI 0.3 to 99.2) and long term follow-up (n=86, 2 RCTs, RR 0.69 CI 0.2 to 2.0). Overall, across all time periods, only about 15% of people left these studies early (n=363, 5 RCTs, RR 0.75 CI 0.5 to 1.1). Only one study Rifkin (21-24) reported the outcome of death, with one occurring in the fluphenazine group during longterm follow-up (n=50, RR 2.38 CI 0.1 to 55.7).
No study reported service outcomes such as discharge from hospital, or levels of satisfaction and quality of life, nor could we identify any direct economic evaluation of fluphenazine.
Discussion
It is surprising that there are so few trial-based data for the absolute effects of fluphenazine. It is feasible that we have failed to identify some trials, but we think it unlikely that we have missed any large studies. Fluphenazine may well be antipsychotic, but data in this review are not convincing. Even in the short term, it is a drug prone to cause a variety of extrapyramidal and anticholinergic effects. Fluphenazine is widely available and inexpensive and for that reason alone it is understandable that it remains one of the many drugs used for treating people with serious mental illnesses. However, with weak evidence for its positive effects and some adverse effects that could be expensive in terms of human suffering and cost of treatment, it could prove better to use another inexpensive drug supported by more favourable data (25-26).
Even though this drug has been used as an antipsychotic drug for decades, the fluphenazine story is incomplete. Questions remain regarding the effect of this drug on global and mental state, quality of life and satisfaction and the consequences of the adverse effects. One or more large, methodologically sound randomised, placebo-controlled trials could help answer these questions. However, with the advent of widely available moderately effective antipsychotic drugs, the day for studies comparing oral fluphenazine with placebo has passed.
Conclusions
This review includes studies that span nearly four decades of evaluative trials within psychiatry. There is some empirical evidence that the quality of schizophrenia trial reporting has not changed over time (27). We have found no timerelated differences in reporting of studies within this review and no suggestion of a change of effect sizes across the decades.
The seven included studies in this review include people with schizophrenia who would be recognisable in everyday practice. There are those with strictly diagnosed illnesses, very likely to suffer from schizophrenia, and people whose illness was diagnosed using less rigorous criteria. The dose of fluphenazine in the studies included in this review could be considered standard (mean 8.2 mg/day SD 3.9). Although the outcomes that have been used in this review are accessible to both clinicians and recipients of care, generalising to treatment in community settings, may be problematic.
The strength of this review is that it presents up-to-date quantitative data for a benchmark treatment for schizophrenia which is considered one of the essential antipsychotics. Data, however, were often inadequately reported and this rendered many outcomes unusable. Most trials report only six to twelve week outcomes for an illness that is mostly life-long. No studies reported on service utilisation, economic outcomes, or on satisfaction with care. It is not for us to judge past recommendations by standards of today. Now, however, it would seem prudent for WHO´s essential list of antipsychotics to be revised to include equally inexpensive and potentially accessible drugs, but with better data on positive outcomes and more gentle profiles of adverse effects (26-27)
Acknowledgements
Thanks to Judith Wright (Leeds, UK) for doing the electronic search, Tessa Grant (Leeds, UK) Cochrane Schizophrenia Group coordinator.
References
1. Dencker SJ, Lepp M, Malm U. Do schizophrenics well adapted in the community need neuroleptics? A depot neuroleptic withdrawal study. [ Links ]
2. Kane JM, Woerner M, Sarantakos S. Depot neuroleptics: A comparison review of standard, intermediate and low-dose regimens. Journal of Clinical Psychiatry. 1986;47(suppl 5):30-3. [ Links ]
3. Darling HF. Fluphenazine: a preliminary study. Disease of the Nervous System. 1959;20(4):167-70. [ Links ]
4. Holt JP, Wright ER. Preliminary results with fluphenazine (prolixin) in chronic psychotic patients. American Journal of Psychiatry. 1960;117:157-9. [ Links ]
5. Coirini H, Kallstrom L, Wiesel FA, Johnson AE. Modulation of basal ganglia neurotransmission by the classical antipsychotic fluphenazine due in part to the blockade of dopamine D1-receptors. Brain research. Molecular brain research. 1997;49(1-2):197-210. [ Links ]
6. BNF 48 (British National Formulary) by the British Medical Association. 2004: p181 [ Links ]
7. Deng MZ, Chen GQ, Phillips MR. Neuroleptic malignant syndrome in 12 of 9,792 Chinese inpatients exposed to neuroleptics: a prospective study. American Journal of Psychiatry. 1990;147(9):1149-1155 [ Links ]
8. Essential Medicines. WHO Model List. 14th Edition.http://whqlibdoc.who.int/hq/2005/a87017_eng.pdf. [ Links ]
9. Matar HE, AlMerie MQ. Oral fluphenazine versus placebo for schizophrenia. Cochrane Database of Systematic Reviews 2007, Issue 1. Art. No.: CD006352. DOI: 10.1002/14651858.CD006352. [ Links ]
10. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 (updated May 2005). The Cochrane Library. 2005;(3). [ Links ]
11. Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408-12. [ Links ]
12. DerSimonian R, Laird N: Meta-analysis in clinical trials. Control Clin Trials. 1986, 7: 177-188. [ Links ]
13. Cook RJ, Sackett DL: The number needed to treat: a clinically useful measure of treatment effect. BMJ. 1995, 310: 452-454. [ Links ]
14. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327: 557-60. [ Links ]
15. Altman DG, Bland JM: Detecting skewness from summary information. BMJ. 1996, 313: 1200. [ Links ]
16. Hordern A, King A, Holt NF, Collins J, Toussaint J. Thioproperazine in chronic schizophrenia. British Journal of Psychiatry. 1964;110:531-9. [ Links ]
17. Clark ML, Huber WK, Charalampous KD, Serafetinides EA, Trousdale W, Colmore JP. Drug treatment in newly admitted schizophrenic patients. Archives of General Psychiatry. 1971;25(5):404-9. [ Links ]
18. Millar J. A trial of fluphenazine in schizophrenia. British Journal of Psychiatry. 1963;109:428-32. [ Links ]
19. Carpenter WT Jr, Buchanan RW, Kirkpatrick B, Lann HD, Breier AF, Summerfelt AT. Comparative effectiveness of fluphenazine decanoate injections every 2 weeks versus every 6 weeks. American Journal of Psychiatry. 1999;156(3):412-8. [ Links ]
20. Egger M, Davey Smith G, Schneider M, Minder C: Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997, 315: 629-634. [ Links ]
21. Rifkin A, Quitkin F, Kane J, Klein DF, Ross D. The effect of fluphenazine upon social and vocational functioning in remitted schizophrenics. Biological Psychiatry 1979;14(3):499-508. [ Links ]
22. Rifkin A, Quitkin F, Kane J, Klein DF. Fluphenazine decanoate, oral fluphenazine, and placebo in the treatment of remitted schizophrenics. II. Rating scale data. Psychopharmacology Bulletin 1977;13(2):40-50. [ Links ]
23. Rifkin A, Quitkin F, Klein DF. Fluphenazine decanoate, oral fluphenazine, and placebo in treatment of remitted schizophrenics. II. Rating scale data. Archives of General Psychiatry 1977;34(10):15-9. [ Links ]
24. Rifkin A, Quitkin F, Rabiner CJ, Klein DF. Comparison of fluphenazine decanoate, oral fluphenazine, and placebo in remitted outpatient schizophrenics. Psychopharmacology Bulletin. 1976;12(2):24-6. [ Links ]
25. Soares BG, Fenton M, Chue P. Sulpiride for schizophrenia. Cochrane Database Syst Rev. 2000;CD001162.) [ Links ]
26. Rifkin A, Quitkin F, Rabiner CJ, Klein DF. Fluphenazine decanoate, fluphenazine hydrochloride given orally, and placebo in remitted schizophrenics. I. Relapse rates after one year. Arch Gen Psychiatry. 1977;34(1):43-7. [ Links ]
27. Thornley B, Adams C. Content and quality of 2000 controlled trials in schizophrenia over 50 years. BMJ. 1998;317:1181-4. [ Links ]
Received to evaluation: January 18, 2007 Approved to publish: February 26, 2007














