Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista de la Facultad de Medicina
Print version ISSN 0120-0011
rev.fac.med. vol.61 no.1 Bogotá Jan./Mar. 2013
ACTUALIZACIÓN
Rosuvastatin: Role in Cardiovascular High-risk Patient
Papel de la Rosuvastatina en el paciente con alto riesgo cardiovascular
John E. Feliciano-Alfonso1
1 MD. Master in Clinical Epidemiology. Clinical Research Institute School of Medicine. Universidad Nacional de Colombia
Correspondence: jefelicianoa@unal.edu.co
Recibido: 3/12/2012 / Aceptado: 3/03/2013
Summary
Statins are the lipid-lowering drug family of first choice in situations of hypercholesterolemia or mixed dyslipidemia with predominant increase in cholesterol. The evidence shows conclusively that each one of the commercially available statins have proven benefits on outcomes of cardiovascular morbidity and mortality. However, rosuvastatin has certain pharmacokinetic efficacy and cost-effectiveness characteristics that make it an attractive molecule to be the statin of choice in patients at high cardiovascular risk.
Key Words: Hydroxymethylglutaryl-CoA Reductase Inhibitors, cardiovascular diseases, dyslipidemias, Cost-Effectiveness Evaluation (MesH).
Resumen
Las estatinas son la familia farmacológica hipolipemiante de primera opción en situaciones de hipercolesterolemia o dislipiemia mixta con predominio de aumento en el colesterol. La evidencia demuestra contundentemente que cada una de las estatinas disponibles en el mercado ha mostrado beneficios en desenlaces de morbi-mortalidad cardiovascular. Sin embargo, la rosuvastatina presenta ciertas características farmacocinéticas de eficacia y de costo-efectividad que la hacen una molécula atractiva para ser la estatina de elección en pacientes de alto riesgo cardiovascular.
Palabras clave: Inhibidores de Hidroximetilglutaril-CoA Reductasas, enfermedades ardiovasculares, dislipidemias, evaluación de Costo-Efectividad (DeCS).
Introduction
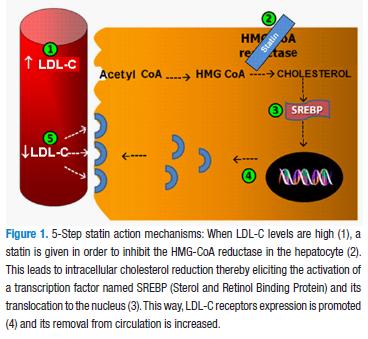
Statins competitively inhibit hidroxi methylglutaryl coenzyme A (HMG CoA) reductase, the enzyme involved in cholesterol endogen production that regulates its formation velocity (2), thereby increasing the availability of cholesterol low density lipoproteins (LDL-C) in the cell membrane and allowing for its levels to decrease (Figure 1).
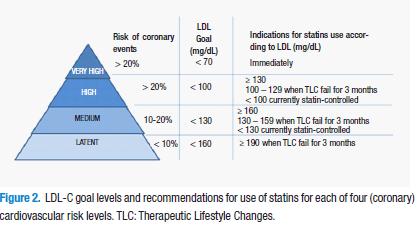
HMG-CoA reductase inhibitors are the first-choice drugs (against other lipid-lowering drugs such as the bile acids sequestering agents or ezetimibe) in hypercholesterolemia and mixed dyslipidemia patients with predominance of increased cholesterol. The decision for use, according to the addenda of NCEP-ATP III (National Cholesterol Educational Program–Adult Treatment Panel III) clinical guidelines recommendations is dependent on the cardiovascular risk (Figure 2), specified in four levels (2,3).
Very high risk
It occurs when there exists a previous cardiovascular episode (myocardial infarction), stable or instable angina, coronary artery procedure such as angioplasty or bypass, of otherwise clinically significantly myocardial ischemia evidence) involving more than one risk factor (e.g. diabetes, hypertension, persistent smoking).
High risk
It occurs under prior coronary disease conditions or its equivalent (peripheral artery disease, aneurism of abdominal aorta, carotid disease (including transient ischemic attack or apoplexy of carotid origin or >50% obstruction of any carotid artery) or primary atherogenic dyslipidemia), as well as in those people which multiple risk factors involve >20% risk of 10 years coronary disease.
Intermediate risk
Occurs in people with metabolic syndrome or which multiple risk factors involve 10 to 20% coronary disease 10 year risk.
Latent risk
Exists in those people which risk factor involve <10% 10-year coronary disease risk.
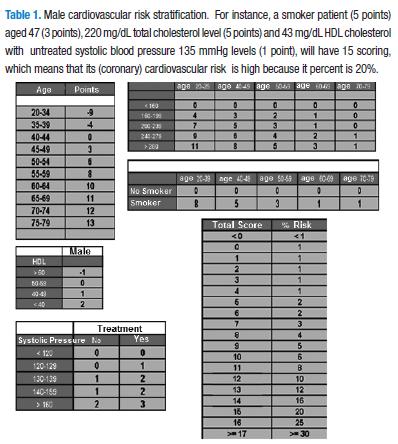
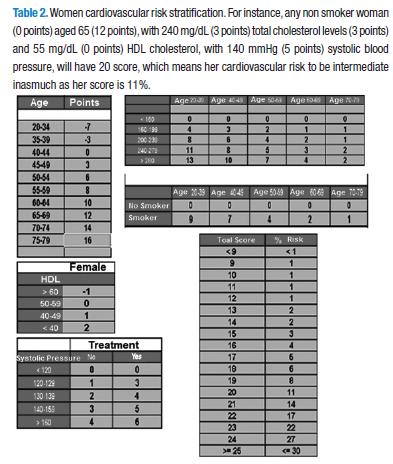
The risk factors, taking into account for cardiovascular risk stratification based on the tables derived from Framingham, study (Tables 1 & 2) are the following: sex (the tables and scores are different for male and female), age (the older age the higher risk score), total cholesterol (which in turn is dependent of the age), HDL cholesterol (which becomes protective, i.e., decreases score when its levels raise to 60 mg/dL), smoking habit (taken in a dichotomy manner: smoking or no-smoking), and systolic blood pressure (the score of which will vary whether or not patient is under pharmacological treatment).
This classification, however, implies some limitations as it only establishes the risk of coronary events (myocardial infarction due to coronary disease) and fails to take into account other significant cardiovascular event, such as cerebrovascular disease. Additionally criticism against the paradigm "to treat until the target" the LDL-C, requesting that the next clinical guides of the ATP IV recommend the treatment with statins in accordance with individual cardiovascular risk independent from LDL cholesterol levels (4).
Rosuvastatin vs other statins
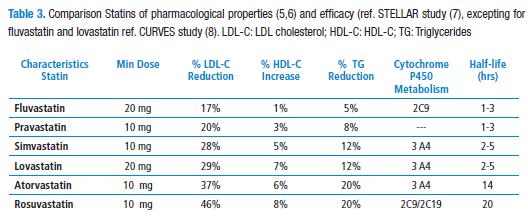
Statins show similar chemical structures as they all show an analogy similar the radical beta-hydroxyl-beta methyl glutaryl (HMG). Rosuvastatin, however, has a methyl-sulfonamide group which allows more interaction with some amino acid residues of the MHG CoA reductase, and this way to have a high affinity for the active site of the enzyme (5). Additionally, rosuvastatin hepatic selectivity shall be taken into account as it is a relatively hydrophilic (the same as pravastatin), compared to other statins, and therefore its uptake by other type of different cells would be limited (6). In fact, classic head-to-head randomized controlled clinical trials (RCT) such as STELLAR (Statin Therapies for Elevated Lipid Levels compared Across doses to Rosuvastatin), have shown rosuvastatin to be the inhibitor of HMGCoA reductase significantly achieving greater LDL-C decreases (Table 3) (7).
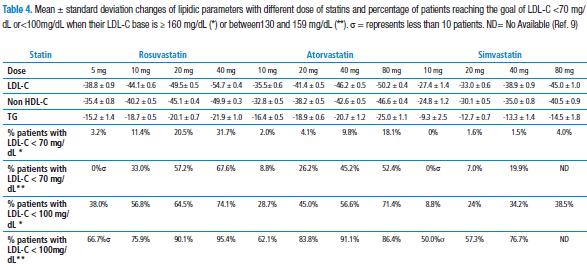
This last assertion was confirmed by a meta analysis (VOYAGER) of data from more than 32000 individual patients derived from 37 studies (9), which determined the ratio between the increment of dosing from three statins frequently used in the clinical practice (rosuvastatin vs. atorvastatin vs. simvastatin) and their capacity to increase atherogenic parameter reduction, as well as the achievement of treatment goals established (see below). It was demonstrated that by duplicating statin dose, a 4% and 7% additional reduction of LDL-C was obtained. In the same way, it was documented that both statin dose and LDL-C level base are predictors to reach treatment goals in high-risk patients (Table 4).
Rosuvastatin ensures HMG CoA reductase sustained inhibition as it has more extended half-life (20 hrs) among statins (Table 3) (6). This characteristic makes it to outstand as a valuable therapeutic option in the intolerance context of statins as described in several case report (10) and retrospective studies (11,12) where up to 72.5% of patients with intolerance resolve their symptoms by delivering rosuvastatin once every other day such dosing (5.6mg mean) reducing LDL cholesterol by 34.5%. In fact, two controlled clinical studies assessed rosuvastatin 10 or 20 mg once every other day versus rosuvastatin 10mg/daily (13,14) during six weeks, resulting in LDL-C reduction up to 48.5%for daily dose and up to 40.9% for 20 mg once every other day (p=0.012).
Rosuvastatin also is advantageous because of its minimum metabolism through P450 cytochrome (CTP), especially through CYP2C9 and CYP2C19 isoenzyme CYP3A4, as most of the statins (simvastatin, lovastatin and atorvastatin), which is involved in a broad variety of drug interactions (6). Such drugs usually present in patients under therapy with verapamil, diltiazem, and macrolides, such as erythromycin or clarithromycin, among others (15).
Statins are usually well tolerated. Most common adverse effects include myalgia, constipation, asthenia, abdominal pain, and nausea (16). Several meta-analysis have found all statins to have a similar safety profile (17,18), the most frequent adverse effects occurring with higher doses of statins (19). Someone could believe, however, that rosuvastatin could have difference related to adverse as against other statins. Notwithstanding, a meta-analysis of four pharmacoepidemiological studies conducted on several international databases that evaluated rosuvastatin safety profile versus other statins, evidenced that there was no higher incidence of rare adverse events such as hospitalizations due to myopathies (0.5 episodes per 10000 years-patient; IC95%: -0.6 a 1.6), rhabdomyolysis (0.7 episodes per 10000 years-patient; IC95%: -0.3 a 1.6), acute renal failure (-0.2 episodes per 10000 years-person; IC95%:-2.9 a 2.5) or acute hepatic damage (-0.8 cases per 10000 years-person; IC95%: -1.8 a 0.2) with the use of rosuvastatin (20). What certainly was found is that the therapy with most of statins can impair glycemic control, or slightly increase diabetes mellitus risk by 9% average (OR=1.09; IC95%: 1.02 a 1.17)(18, 21). Due to occurrence, FDA (US Food and Drug Agency) has added up a warning in the labeling from all statins advising that they may increase glycemia and hemoglobin A1c levels, recognizing, however, statins cardiovascular benefits overweight such mildly increases. (22).
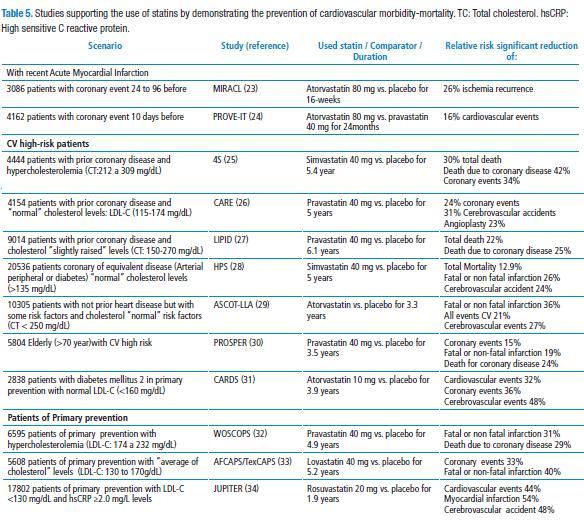
Those benefits are important cardiovascular (CV) morbidity and mortality reductions (CV) evidenced by all and any of the statins in several different scenarios (Table 5).
In fact, a meta-analysis involving some number of studies included in Table 4, determined that a LDL-C reduction by 39 mg/Dl was associated to 21% reduction of the incidence at 5-year of major coronary events, revascularization, and cerebrovascular accident, as well as 12% mortality reduction by all causes, regardless of baseline lipidic values (35) and such benefits are extended to populations with or without coronary disease established (36). Other effects additional to LDL-C reduction by statins include enhancement of endothelial dysfunction, diminution of vascular inflammation, stabilization or regression of atherosclerotic plate and platelet aggregation inhibition (37).
Rosuvastatin: studies in high cardiovascular risk patients
In the scenario where atorvastatin (MIRACL and PROVE IT studies (23,24) was successful, it was determined that rosuvastatin carries more people to the goal: SPACE ROCKET (Secondary Prevention of Acute Coronary Events – Reduction of Cholesterol to Key European Targets) study evaluated the lipid-lowering effects of rosuvastatin 10 mg/day vs. simvastatin 40 mg/day for three months in the emergence context due to an acute myocardial infarction in the previous two weeks (38). A higher number of subjects randomized to rosuvastatin (45%) reached the goal of LDL-C <70mg/dL compared to those subjects with simvastatin therapy (37.8%; p=0.007), with a hepatic, renal and muscular similar profile.
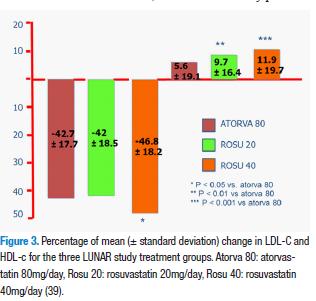
It should be stressed that the LUNAR (Limiting Undertreatment of Lipids in Acute Coronary Syndrome with Rosuvastatin) (39) study which compared head-to-head atorvastatin 80mg/day (atorva 80) peak dose as against rosuvastatin 20 mg/day (rosu 20) and 40 mg/day (rosu 40) in hospitalized patients due to acute coronary syndrome within 48 h of the beginning of ischemic symptoms. In the Figure 3 below, the efficacy of LDL lowering and HDL increase is shown. Adverse effects related to treatment were similar for the three groups 9.4% for rosu20, 14.8% for rosu 40 and 15.6% for atorva 80 and included myalgias, fatigue, and headache, inter alia. Treatment discontinuation due to adverse effect was 3.7% for rosu 20, 6.1% for rosu 40 and 9.3% for atorva 80. Cardiovascular events were infrequent in the three groups (3.4% for rosu20, 1.9% for rosu40 and 2.2% for atorva 80), this way showing higher LDL-C reductions with rosu 40 versus atorva 80, with a similar safety profile.
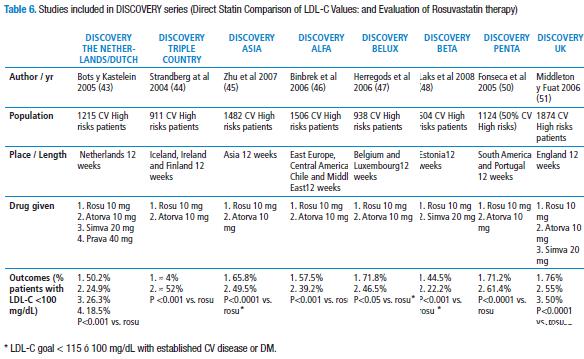
Several RCTs such as PULSAR (Prospective study to evaluate the Use of Low doses of the Statins Atorvastatin and Rosuvastatin) (40), MERCURY II (Measuring Effective Reduction in Cholesterol Using Rosuvastatin therapy II) (41) and POLARIS (Prospective Optimization of Lipids by Atorvastatin or Rosuvastatin Investigated in high risk Subjects with hypercholesterolemia) (42) indicated the superiority of rosuvastatin over other statins to reach the goals of lipidic parameters en patients with cardiovascular risk. We emphasized on the studies of the series DISCOVERY (Direct Statin Comparison of LDL-C Values: an Evaluation of Rosuvastatin therapy), a set of nine independent studies the general purpose of which was to compare the efficacy of rosuvastatin 10 mg/day versus other statins (according to the appropriate initial dose of the one of them) to reach the goals recommended in high cardiovascular risk patients (cardiovascular risk at 10 years>20%, prior myocardial acute infarction or an equivalent atherosclerotic disease) (43-51). In Table 6, a summary of the findings of those studies is found. All of the studies had a safety profile similar among the statins involved. The study DISCOVERY PENTA (50) evaluated specifically South America populations (Brazil, Mexico, Colombia and Venezuela) and Portugal: 1124 patients with hypercholesterolemia (50% out of which were cardiovascular high-risk patients) were authorized to receive rosuvastatin 10mg/day vs. atorvastatin 10mg/day. LDL-C goals, according to NCEP-ATP III were obtained by 71.2% in rosuvastatin group and 61.4% in the other group (p<0.001).
The DISCOVERY BELUX study is worth of especial mention. This study was conducted in Belgium and Luxembourg and its objective was to evaluate how many CV high risk patients reached LDL cholesterol goal (in this event <115 mg/dL, according to the protocol of study and the goals of European Atherosclerosis Society (EAS)by that time) after randomization to rosuvastatin 10 mg/day or atorvastatin 10 mg/day. In the first group 85% of patients reached the goal versus 67% of the second group after 12 weeks of treatment. Interestingly, those patients failing to reach the goal with rosuvastatin 10 mg/day and with atorvastatin 10 mg, were switched to a rosuvastatin dose 20 mg/day and 10 mg/day, respectively, during 12 additional weeks. This resulted that additionally at the end of such period, 57% of the first group and 65% of the second group reached LDL-C goal. This study was the only made in the DISCOVERY series that conducted an economic evaluation from the payer's perspective and found that rosuvastatin 10 mg/day is more cost-effective than atorvastatin 10 mg/day in this scenario (47). The study ECLIPSE (Evaluation to Compare Lipid-lowering effects of rosuvastatin and atorvastatin In forced titrated patients: a Prospective Study of Efficacy and tolerability), on its side, assessed the efficacy of the double rosuvastatin dose 10 mg and atorvastatin 10 mg every 6 weeks, until reaching the peak dose of both two medicaments (40 and 80 mg, respectively) to reach the LDL-C goals (<100 mg/dL) in1036 patients of cardiovascular with hypercholesterolemia high risk. At the end of 24 weeks of treatment, 83.6% of patients randomized to rosuvastatin and 74.6% patients with atorvastatin reached LDL-C goal (p<0.001). In fact, since the first 6 weeks with the initial and peak dosing of rosuvastatin 10 mg/day and atorvastatin 10 mg/dL, the differences between the several percentages of patients who reached the goal with such treatments was remarkable (52.8% vs. 27.6%, respectively; p<0.001). Similarly, upon the completion of 24 weeks of follow-up, in the subgroup of patient in high cardiovascular risk, rosuvastatin carried more patients to the goal <70 mg/dL (38.0%) than atorvastatin (20.2%; p<0.001). Once again, the differences between percent of CV very high risk patients in goal were significant since the first 6 weeks with the minimal dose of rosuvastatin (7.5%) versus atorvastatin (1.8%; p<0.001) (52).
In general, the group of studies known as DISCOVERY show that, after 12 weeks follow-up of cardiovascular high risk patients, rosuvastatin 10 mg/day may imply that significantly far much more cardiovascular high-risk patients (between 50 to 75%) obtain LDL-C goal<100 mg/dL, as compared to atorvastatin 10 mg/day (25 to 55%) or simvastatin 20 mg/day (18.5% to 50%). In the same way, according to the results from VOYAGER (9) meta-analysis, it shall be taken into account that the reaching the LDL baseline cholesterol and the dose of stating used (Table 4).
Rosuvastatin: economic evaluation in cardiovascular high-risk patient
Several economic evaluations based on STELLAR study with one year horizon time and under payer's perspective of Canada and the United States (considering the percentage of change of lipidic parameters and the people reaching LDL-C goal), have evidenced that branding rosuvastatin in 10mg/day dosing is more cost-effective than branding atorvastatin (10 and 20 mg/day) and simvastatin (20 and 40 mg/day) and pravastatin generics (20 and 40 mg/day (53-55).
In the same way, other economic evaluations made in Europe and North America have used clustered efficacy data from several rosuvastatin controlled clinical assays compared head-to-head to other statins, concluding once again that rosuvastatin 10 mg/day is more cost-effective than other therapeutic options, such as atorvastatin 10mg/day, from the primary caregivers' perspective in the United Kingdom (56).
It has been determined that for patients with increasingly higher coronary risk, the therapy with statins is more cost-effective (57,58). And the question raised in this connection is whether rosuvastatin is more cost-effective than other statins, specifically in cardiovascular high-risk risk patients. The answer is yes, and it was confirmed by DISCOVERY BELUX study (47), as did as well POLARIS study (42). Additionally, other study used a Markov model to Project the number of CV events and the cost associated to a high-risk population in several pharmacological treatment context, established that using rosuvastatin instead of other statins may reduce cardiovascular events in this type of population and saving cost for several US dollars of United States health systems (59). These results have been confirmed in other RCTs including subjects from other geographic locations, among which PULSAR study (38, 60). For example, in a study using Monte Carlo probabilistic simulation model and based on JUPITER study for long-term cost-effectiveness of rosuvastatin Brand (CRESTOR®) at 20 mg/day versus simvastatin or atorvastatin generics 40 mg/day for CV morbidity-mortality prevention in CV high-risk Sweden population (from Sweden health system payer's perspective and a permanent time-horizon), found that the higher cost-effectiveness and cost-utility of rosuvastatin was basically by the number of CV prevented (60).
Rosuvastatin cost-effectiveness could be determined in the context of Latin America countries, where the cost of medicinal products varies from country to country, using RCT as a basis such as STELLAR or JUPITER studies, as made by other economic evaluations or otherwise, using RCT made in South American population, such as DISCOVERY PENTA study.
In conclusion, it is possible to assert that rosuvastatin is more advantageous than other statins with regard to its pharmacokinetics, LDL-C reduction and percentage of patients reaching a goal, with a similar safety profile. Similarly, rosuvastatin has conclusively demonstrated in the several different economic evaluations to be the most cost-effective compared to other pharmacological options. By taking into account these assertions together with the quality of evidence found in the studies aforementioned; rosuvastatin could be considered as the first-choice for cardiovascular high-risk patients.
References
1. Russell DW. Cholesterol biosynthesis and metabolism. Cardiovascular Drugs Therap. 1992; 6:103-10. [ Links ]
2. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment on High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002; 106:3143-3421. [ Links ]
3. Grundy SM, Cleeman JI, Merz CNB, Brewer HB, Clark LT, Hunninghake DB, et al. For the Coordinating Committee of the National Cholesterol Education Program. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004; 110:227-39. [ Links ]
4. Hayward RA, Krumholz HM. Three reasons to abandon low density lipoprotein targets: an open letter to the Adult Treatment Panel IV of the National Institutes of Health. Circ Cardiovasc Qual Outcomes. 2012; 5:2-5. [ Links ]
5. Rubba P, Marotta G, Gentile M. Efficacy and safety of rosuvastatin in the management of dyslipidemia. Vasc Health Risk Management. 2009; 5:343-52. [ Links ]
6. McTaggart F. Comparative pharmacology of rosuvastatin. Atheroscl Suppl 2003; 4:9-14. [ Links ]
7. Jones PH, Davidson MH, Stein EA, Bays HE, McKenney JM, Miller E, et al. For the STELLAR Study Group. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin and pravastatin across doses (STELLAR Trial). Am J Cardiol. 2003; 92:152-60. [ Links ]
8. Jones P, Kafonek S, Laurora I, Hunninghake D. For the CURVES Study Investigators. Comparative dose efficacy study of atorvastatin versus simvastatin, pravastatin and fluvastatin in patients with hypercholesterolemia (The CURVES study). Am J Cardiol. 1998; 81:582-7. [ Links ]
9. Nicholls SJ, Brandrup-Wongsen G, Palmer M, Barter PJ. Meta-analysis of comparative efficacy ot increasing dose of atorvastatin versus rosuvastatin versus simvastatin on lowering levels of atherogenic lipids (from VOYAGER). Am J Cardiol. 2010; 105:69-76. [ Links ]
10. Mackie BD, Satija S, Nell C, Miller J. 3rd Wednesday, and Friday dosing of rosuvastatin in patients previously intolerant to statin therapy. Am J Cardiol. 2007; 15:291. [ Links ]
11. Joy T, Hegele RA. Alternate day dosing of rosuvastatin: potential usefulness in statin-intolerant patients. Can J Cardiol. 2009; 25:453. [ Links ]
12. Backes JM, Venero CV, Gibson CA, Ruisinger JF, Howard PA, Thompson PD, et al. Effectiveness and tolerability of every-otherday rosuvastatin dosing in patients with prior statin intolerance. Ann Pharmacother. 2008; 42:341-6. [ Links ]
13. Li JJ, Yang P, Liu J, Jia YJ, Li ZC, Guo YL, et al. Impact of 10 mg rosuvastatin daily or alternate-day on lipid profile and inflammatory markers. Clin Chim Acta. 2012; 413:139-142. [ Links ]
14. Dulay D, LaHaye SA, Lahey KA, Day AG. Efficacy of alternate day versus daily dosing of rosuvastatin. Can J Cardiol. 2009; 25:e28-31. [ Links ]
15. Pasternak RC, Smith SC Jr, Bairey-Merz CN, Grundy SM, Cleeman JI, Lenfant C. ACC/AHA/NHLBI Clinical advisory on the use and safety of statins. Stroke. 2002; 33:2337-41. [ Links ]
16. Astra Zeneca Pharmaceuticals. Crestor® (rosuvastatin calcium) tablets prescribing information. Wilmington, DE. 2005. [ Links ]
17. Wlodarczyk J, Sullivan D, Smith M. Comparison of benefits and risk of rosuvastatin versus atorvastatin from a meta-analysis of head-to-head randomized controlled trials. Am J Cardiol. 2008; 102:1654-62. [ Links ]
18. Alberton M, Wu P, Druyts E, Briel M, Mills EJ. Adverse events associated with individual statin treatments for cardiovascular disease: an indirect comparison meta-analysis. QJMed. 2012; 105:145-57. [ Links ]
19. Silva M, Matthews ML, Jarvis C, Nolan NM, Belliveau P, Malloy M, et al. Meta-analysis of drug induced adverse events associated with intensive-dose statin therapy. Clin Ther. 2007; 29:253-60. [ Links ]
20. García-Rodríguez LA, Herings R, Johansson S. Use of multiple international healthcare databases for the detection of rare drug-associated outcomes: a pharmacoepidemiological programme comparting rosuvastatin with other marketed statins. Pharmacoepidemiol Drug Saf. 2010; 19:1218-24. [ Links ]
21. Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomized statins trials. Lancet. 2010; 375:735-42. [ Links ]
22. Disponible en: http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm. Acceso 28 de febrero de 2012. [ Links ]
23. Schwartz GG, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, Waters D, et al. For the Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) Study Investigators. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA. 2001; 285:1711-8. [ Links ]
24. Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, et al. Intensive versus Moderate Lipid Loweringwith Statins after Acute Coronary Syndromes. N Engl J Med. 2004; 350:1495-1504. [ Links ]
25. Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994; 344:1383-9. [ Links ]
26. Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, et al. For the Cholesterol and Recurrent Events Trial Investigators. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med. 1996; 335:1001-9. [ Links ]
27. Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention ofcardiovascular events and death with pravastatin in patients with coronary heart disease and abroad range of initial cholesterol levels. N Engl J Med. 1998; 339:1349-57. [ Links ]
28. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol loweringwith simvastatin in 20 536 high-risk individuals: a randomised placebo controlled trial. Lancet 2002; 360:7-22. [ Links ]
29. ASCOT Study group: Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower than average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicenter randomizedtrial. Lancet. 2003; 361:1149-58. [ Links ]
30. Shepherd J, Blauw GJ, Murphy MB, Bollen ELEM, Buckley BM, Cobbe SM, et al. Pravastatin in elderly individuals at risk of vascular disease(PROSPER): a randomised controlled trial. Lancet. 2002; 360:1623-30. [ Links ]
31. Colhoun HM, Betteridge J, Durrington PN, Neil HA, Hitman GA, Livinstone SJ, et al. Primary prevention of Cardiovascular disease in type 2diabetes in the Collaborative Aorvastatin Diabetes Study (CARDS): multicentre ramdomised placebo controlledtrial. Lancet. 2004; 364:685-96. [ Links ]
32. Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, Macfarlane PW, et al. For the West of Scotland Coronary Prevention Study Group. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl JMed. 1995; 333:1301-7. [ Links ]
33. Downs JR, Clearfield M, Weis S, Withney E, Shapiro DR, Beere PA, et al. For the AFCAPS/TexCAPS Research Group. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. JAMA. 1998; 279:1615-22. [ Links ]
34. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, et al. For the JUPITER study group. Rosuvastatin to prevent vascular events in men and women with elevated C reactive protein. N Engl J Med 2008; 359:2105-2207. [ Links ]
35. Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T,Peto R, Collins R, Simes R. Cholesterol Treatment Trialists' (CTT) Collaborators: Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials. Lancet. 2008; 371:117-125. [ Links ]
36. Mills EJ, Wu P. Chong G, Ghement I, Singh S, Akl EA. Efficacy and safety of statin treatment for cardiovascular disease: a network meta-analysis of 170 255 patients from 76 randomized trials. QJMed 2011; 104:109-124. [ Links ]
37. Sadowitz B, Maier KG, Gathan V. Statin therapy part I: the pleiotropic effects of statins in cardiovascular disease. Vasc Endovascular Surg. 2010; 44:241-51. [ Links ]
38. Hall AS, Jackson BM, Fairin AJ, Efthymiou M, Barth JH, Copeland J, et al. On behalf of the SPACE ROCKET Trial Group. A randomized, controlled trial os simvastatin vs rosuvastatin in patients with acute myocardial inraction: the secondary prevention of acute coronary events-reduction of cholesterol to key European targets trial. Eur J Cardiovasc Prev Rehabil. 2009; 16:712-21. [ Links ]
39. Pitt B, Loscalzo J, Monyak J, Miller E, Raichlen J. Comparison of lipidmodifying efficacy of rosuvastatin versus atorvastatin in patients with acute coronary syndrome (from the LUNAR Study). Am J Cardiol 2012; 109:1239-46. [ Links ]
40. Clearfield MB, Amerena J, Bassand JP, Hernández-García HR, Miller SS, Sosef FFM, et al. Comparison of the efficacy and safety of rosuvastatin 10 mg and atorvastatin 20 mg in high-risk patients with hypercholesterolemia- Prospective study to evaluate the Use of Low doses of the Statins Atorvastatin and Rosuvastatin (PULSAR). Trials 2006; 7:35. [ Links ]
41. Ballantyne CM, Raichlen JS, Cain VA. Statin therapy alters the relationship between apolipoprotein B and low density lipoprotein cholesterol and non high density lipoprotein cholesterol targets in high risk patients. The MERCURY II (Measuring Effective Reduction in Cholesterol Using Rosuvastatin therapy II) trial. J Am Coll Cardiol. 2008; 52:626-32. [ Links ]
42. Leiter LA, Rosenson RS, Stein E, Reckless J, Schulte KL, Schleman M, et al. On behalf of the POLARIS study investigators. Efficacy and safety of rosuvastatin 40 mg vs atorvastatin 80 mg in high risk patients with hypercholesterolemia: results of the POLARIS study. Atherosclerosis 2007; 194:e154-e164. [ Links ]
43. Bots AFE, Kastelein JJP. Achieving lipid goals in real life: the Dutch DISCOVERY Study. Int J Clin Pract. 2005; 59:1387-94. [ Links ]
44. Strandberg TE, Feely J, Sigurdsson EL. Twelve-week, multicenter, randomized, open-label comparison of the effects of rosuvastatin 10 mg/d and atorvastatin 10 mg/d in high-risk adults: A DISCOVERY study. Clin Ther. 2004; 26:1821-33. [ Links ]
45. Zhu JR, Tomlinson B, Young MR, Sim KH, Lee LT, Sriratanasathavorn C. A randomised study comparing the efficacy and safety of rosuvastatin with atorvastatin for achieving lipid goals in clinical practice in Asian patients at high risk of cardiovascular disease (DISCOVERY-Asia study) Curr Med Res Opin. 2007; 23:3055-3068. [ Links ]
46. Binbrek AS, Elis A, Al-Zaibag M, Eha J, Keber I, Cuevas AM, et al. For the DISCOVERY Alpha Study Group Rosuvastatin versus atorvastatin in achieving lipid goals in patients at high risk for cardiovascular disease in clinical practice: a randomized, open-label, parallel-group, multicenter study (DISCOVERY Alpha Study) Curr Ther Res. 2006; 67:21-43. [ Links ]
47. Herregods MC, Daubresse JC, Michel G, Lamotte M, Vissers E, Vandenhoven G. DISCOVERY BELUX: comparison of rosuvastatin with atorvastatin in hypercholesterolaemia. Acta Cardiol. 2008; 64:493-9. [ Links ]
48. Laks T, Keba E, Leiner M, Merilind E, Petersen M, Reinmets S, et al. Achieving lipids goals with rosuvastatin compared with simvastatin in high risk patients in real clinical practice: a randomized, open label, parallel group, multicenter study: the DISCOVERY Beta study. Vasc Health Risk Manag. 2008; 4:1407-16. [ Links ]
49. Gupta M, Constance C. Direct statin comparison of LDL-C values: an evaluation of rosuvastatin therapy (DISCOVERY - Canada). [abstract] Atheroscler Suppl. 2005; 6:108. [ Links ]
50. Fonseca FAH, Ruiz A, Silva JM, Fuenmayor M, Marotti M. For the DISCOVERY PENTA Investigators. The DISCOVERY PENTA study: a DIrect Statin COmparison of LDL-C Value - an Evaluation of Rosuvastatin therapY compared with atorvastatin. Curr Med Res Opin. 2005; 21:1307-15. [ Links ]
51. Middleton A, Fuat A. Achieving lipid goals in real life: the DISCOVERY-UK study. Br J Cardiol. 2006; 13:72-6. [ Links ]
52. Faergeman O, Hill L, Windler E, Wiklund O, Asmar R, Duffield E, et al. On behalf of the ECLIPSE study investigators. Efficacy and tolerability of rosuvastatin and atorvastatin when force-titrated in patients with primary hypercholesterolemia. Cardiology 2008; 111:219-28. [ Links ]
53. Costa-Scharplatz M, Ramanathan K, Frial T, Beaner B, Gandhi S. Cost effectiveness analysis of rosuvastatin versus atorvastatin, simvastatin and pravastatin from a Canadian Health System perspective. Clin Ther. 2008; 30:1345-57. [ Links ]
54. Tian YBL, Frial T, Miller PSJ. Statin´s cost-effectiveness: a Canadian analysis of commonly prescribed generic and brand name statins. Can J Clin Pharmacol 2007; 14:e205-14. [ Links ]
55. Miller PSJ, Smith DG, Jones P. Cost effectiveness of rosuvastatin in treating patients to low-density lipoprotein goals compared with atorvastatin, pravastatin and simvastatin (a US analysis of the STELLAR trial). Am J Cardiol. 2005; 95:1314-9. [ Links ]
56. Hirsch M, O´Donell J, Olsson A. Rosuvastatin is cost-effective compared with atorvastatin in reaching cholesterol goals. Int J Cardiol 2005; 104:251-6. [ Links ]
57. Huse DM, Russel MN, Miller JD, Kraemer DF, D´Agostino RB, Ellison RC, et al. Cost effectiveness of statins. Am J Cardiol 1998; 82:1357-63. [ Links ]
58. Prosser LA, Stinnett AA, Goldman PA, Williams LW, Hunink MG, Goldman L, et al. Cost-effectiveness of cholesterol-lowering therapies according to selected patients characteristics. Ann Intern Med. 2000; 132:769-79. [ Links ]
59. Huse DM, Song X, Ozminkowski RJ, Maguire J, William SA, Borok GM, et al. Impact of rosuvastatin use on costs and outcomes in patients at high risk for cardiovascular disease in US Managed Care and Medicare populations: a data analysis. Clin Ther. 2006; 28:1425-42. [ Links ]
60. Gandhi SK, Jensen WM, Foz KM, Somolen L, Olsson AG, Paulsson T. Cost-effectiveness of rosuvastatin in comparison wuth generic atorvastatin and simvastatin in a Swedish population at high risk of cardiovascular events. Clinico Economics Outc Res. 2012; 4:1-11. [ Links ]