Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Colombian Journal of Anestesiology
Print version ISSN 0120-3347
Rev. colomb. anestesiol. vol.39 no.1 Bogotá Jan./Mar. 2011
https://doi.org/10.5554/rca.v39i1.54
Artículo de Revisión
Complex Regional Pain Syndrome
René F. Rodríguez*, Ana María Ángel Isaza**
* Anestesiólogo, docente Universidad Libre Cali. Clínica del Dolor y Cuidados Paliativos de la Clínica Rafael Uribe Uribe. Grupo Investigación CADPAL. Cali, Colombia.
** Universidad Libre Cali. Grupo Investigación Clínica para el Alivio del Dolor y Cuidados Paliativos CADPAL. Cali, Colombia, anaangelmd@hotmail.com.
Recibido: junio 21 de 2010. Enviado para modificaciones: agosto 4 de 2010. Aceptado: agosto 8 de 2010.
SUMMARY
Introduction. Complex Regional Pain Syndrome (CRPS) is a chronic disabling disorder that occurs after an injury. Pain, changes in the color and temperature of the skin, edema and trophic changes are the main characteristics of this syndrome.
Objective. To provide a current literature overview of the CRPS to improve its understanding.
Methodology. An extensive literature search both in indexed journals and pain medicine books was performed. Forty eight articles and 4 book chapters about pain medicine were included.
Conclusion. CRPS is difficult to manage and if it is not treated adequately, functionality of the affected limb can be compromised. Early diagnosis and treatment may reduce the severity and duration of this condition.
Keywords: Complex Regional Pain Syndrome, pain, Causalgia, Peripheral Nerves, Hyperalgesia. (Source: MeSH, NLM).
INTRODUCTION
Complex regional pain syndrome (CRPS) is an entity that appears usually after a trauma. There are 2 types of CRPS: type I previously called reflex sympathetic dystrophy which is characterized because the trauma spares the nerves, like in sprains, some fractures, surgical trauma, some contusions or distentions; and type II previously called causalgia, usually developed after an injury that involves a peripheral nerve (1).
The first description of causalgia goes back to the American Civil War in which Sir Weir Mitchell, one of the fathers of modern neurology, observed that wounded soldiers with major injuries of the nerves in their limbs developed prolonged episodes of burning pain. Mitchell named this causalgia, from the Greek kausos which means heat, and algos which means pain (1-3). The term reflex sympathetic dystrophy was introduced in the literature by Evans in 1946 (4). In 1994, the International Association for the Study of Pain (IASP) introduced the term complex regional pain syndrome to describe this pain entity (5).
DEFINITION
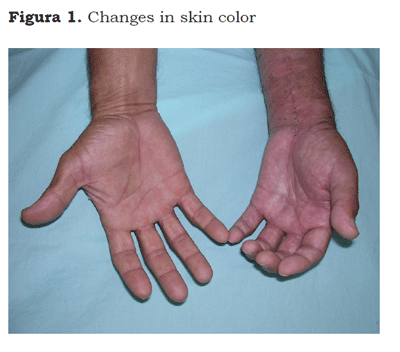
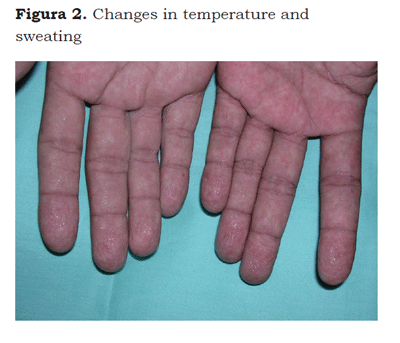
The CRPS describes a variety of pain conditions usually secondary to an injury; which includes regional pain without relationship to individual nervous territories or to the localization of the triggering injury. Pain can be of a burning type, either spontaneous or induced by nonmechani-cal or thermal nociceptive stimuli (allodynia), or by the movement or pressure on joints (deep somatic hyperalgesia). Pain is accompanied by edema, changes in skin color (Figure 1) or temperature, sweating (Figure 2) and can accompany functional abnormalities and muscle weakness. Some patients develop postural tremor, or trophic changes. It differs from other neuropathic syndromes because of the presence of edema, vasomotor changes and sweating (6).
DIAGNOSIS
It is a clinical diagnosis for which it is essential to perform a proper medical history. There are criteria to ease identification of CRPS, however there are controversies regarding the number of criteria that must be met. According to the IASP the characteristics necessary to establish the diagnosis of CRPS are: (5)
1. The presence of a triggering injury.
2. Spontaneous pain or allodynia / hyperalge-sia not limited to the territory of a single peripheral nerve, and out of proportion of the triggering event.
3. Evidence at any time, of edema, changes in skin blood flow, or abnormal sweating in the affected region.
4. Absence of other conditions that in any way can explain the clinical signs. The last 3 criteria are necessary to make a diagnosis of CRPS type I.
Validation studies of the criteria of the IASP have demonstrated that they have a great sensitivity with low specificity, which have led to some conditions to be diagnosed as CRPS inappropriately.
Even though the IASP defines the pain as out of proportion of the triggering event, it is common that some patients describe low-level pain, whereas some can even present all the signs and symptoms without any pain, so in these cases the duration of the symptoms would be an out proportion of the clinical course of the trauma sustained, but not in relationship with the severity.
Besides the diagnostic criteria mentioned by the IASP, other authors have suggested some modifications, among which Bruehl (7) and Veldman (8) stand out. The diagnostic criteria by Bruehl also have high sensitivity, these are:
1. Continuous pain out of proportion of the triggering injury.
2. The presentation of at least one symptom in each of the following categories: sensitive (hyperesthesia, hyperalgesia and or al-lodynia); vasomotor (changes in skin temperature and or color); sweating or edema (asymmetrical sweating or edema); motor or trophic (decreased articular motion arcs and or motor dysfunction, weakness, tremor, dystony and / or trophic changes of hair nails and skin).
3. The finding of at least one sign in 2 or more of the following categories: sensitive: evidence of hyperalgesia (pinprick) and or al-lodynia (to soft touch); vasomotor: evidence of temperature asymmetry and / or changes or asymmetry in skin color; sudomotor / edema: evidence of edema and or changes in sweating or asymmetrical sweating; motor / trophic: evidence of decreased motility and/or motor dysfunction (weakness, tremor, dystonia) and/or trophic changes (hair, nails, skin).
In some cases, patients may exhibit a clinical scenario with different vasomotor and sudo-motor changes along with edema, without any pain; for which the diagnostic criteria described by Veldman must be used, as they are the only ones that allow the diagnosis of CRPS in the absence of pain:
1. Four or five of them following aspects: unex-plainable diffuse pain, changes in skin coloring when compared with the other limb, diffuse edema, changes in the temperature of the skin of one limb compared to the other, and decreased articular motion arcs.
2. Appearance or increase of the previous signs after the activity.
3. Presence of the previous signs in an area larger than the initially compromised or inclusion of an area distal to the initially compromised.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis must be made against other neuropathic conditions as diabetes, peripheral neuropathy, entrapment neuropathy, deep venous thrombosis, cellulitis, vascular insufficiency, lymphedema, arthritis, Raynaud's disease, and scleroderma among others (9).
Diagnostic tests: no laboratory or paraclinical test is useful to diagnose CRPS, however they can help with the differential diagnosis (10). Occasionally neurophysiological tests help differentiate a radiculopathy or a peripheral nerve injury from CRPS (3). No type of personality or psychological characteristic in particular has been shown to increase the likelihood of a patient to suffer CRPS (11).
With x-rays, bone demineralization and decreases in calcification can be identified, particularly in the periarticular area, and although this is not a specific change in CRPS, bone demineralization has been linked to limb immobility (12). In some phases of the disease different types of abnormalities are found in bone scans.
There are complementary tests like the sudomotor relex, which is an indirect index of sympathetic activity as it measures in a quantitative way the production of sweat from the affected limb (13). Like this, thermography, the response to electrical conductivity in the skin, and the assessment of skin blood flow with laser Doppler, are tests used in research rather than in clinical practice (3).
The clinical signs of CRPS do not allow to determine whether the sympathetic nervous system is involved in generating and maintaining the pain in CRPS. Local anesthetics, guanethidine, for its noradrenergic effect in the postganglionic sympathetic axons and the depletion of norepinephrine reserves, and phentolamine for its effect of α adrenergic antagonism, are used in different ways like intravenous infusions or regional blocks to determine whether the pain is related to the sympathetic system helps to define its therapy (14).
EPIDEMIOLOGY
In a recent paper, Mos et al. estimated an incidence of CRPS to be 26.2 per hundred thousand people per year. This estimate is about 4 times higher than that previously reported by Sandoni, who found an incidence of 5.46 per hundred thousand persons per year. CRPS is 3 times more common in women than in men between 61 and 70 years old (15, 16). In 65 % of the cases the cause is related with to a trauma (8). There is no correlation between the severity of the injury and the appearance of CRPS (7). CRPS can appear also from a distant process, like brain or spinal injury or myocardial infarction (17).
Kiralp et at. reported that the upper limbs are affected in 61.3 % of the cases whereas the lower limbs are affected in 38.7 % of the cases (18). Both Veldman and Kiralp describe fractures as the most common triggering factor of CRPS (8,18). In about 10 % of the cases there is no triggering event. Psychological factors like stress are potential risk factors that can worsen the severity of symptoms (7).
These findings are different from those described in Valle del Cauca, Colombia where the incidence of upper limb CRPS was found to be 1.1 per hundred thousand persons per year, with a male to female ratio of 1:1.1, an average age of 44 years old, bilateral affection in 5 % of the cases, right side compromise in 56 %, and among the symptoms reported, changes in sweating occurred in 96 % of the cases, changes in temperature in 92 %, and changes in skin coloring in 90 % (19).
PATHOPHYSIOLOGY
The pathophysiology of CRPS is still not well understood. Several mechanisms have a role in its appearance and duration. These include neurogenic inflammation, immune mechanisms, and changes in the peripheral and central sympathetic nervous system (20, 21), that are expressed as sensory, motor, sympathetic and inflammatory changes.
According to the study by Schurmann at al., the abnormalities of the sympathetic nervous system in patients with CRPS type I are systemic and not limited to the compromised area (22).
The signs and symptoms that characterize low sympathetic activity (warm limb) usually progress to sympathetic hyperactivity (cold and moist limb), because of the adrenergic sensitivity. The sympathetic activity magnifies vascular abnormalities associated with inflammation. The sympathetic dysfunction seems to be originated in the central nervous system in patients without peripheral nerve injuries (23).
Clinical studies about the autonomic function in patients with CRPS type I have demonstrated abnormalities of the sympathetic and thermoregulatory neurogenic reflexes, as well as in the sweating mechanisms, the skin temperature, and the microcirculatory response both to peripheral reflexes as well as to central autonomic stimuli (24).
Spinal neurons can increase their sensitivity in response to the nociceptive bombardment fueled by the autonomic changes; and in the supraspinal space there can be reorganization of the primary somatosensory cortex, like in the amputated patients, which has been demonstrated with functional magnetic resonance imaging (4). This supports the concept in which it is presumed that in CRPS all the steps in the nociceptive process are implicated (peripheral, medullary, supra medullary and cortical) (25).
It has been suggested that the hemi sensorial deficits observed in some patients can be associated with functional abnormalities in the processing of nociceptive stimuli in the post thalamic ventral nuclei, so the generalized sensory deficits could be the result of subcortical cerebral plasticity in chronic or central inflammatory pain (26-28).
Among the pathophysiological mechanisms, inflammatory and neuro immunological processes are considered, as there are increases in different interleukins, tumoral necrosis factor, and the peptide related to the calcitonin gene in the compromised limb (29,30). Then neurogenic inflammation is involved in the development of edema, vasodilation, and the increases in sweating.
The changes in the motility of the affected limb, the tremor, the decreasing active motor strength, and dystonia, mostly in chronic cases, are probably originated secondary to changes in central motor neurons. Therefore, it can be concluded that in the pathophysiology of CRPS, inflammatory mechanisms are involved in the acute phase, as well as autonomic, sensory and motor mechanisms.
CLINICAL MANIFESTATIONS
The clinical manifestations usually appear after a trauma (fractures, dislocations, sprains, accidents) that involve the distal part of a limb, and are characterized by the presence of pain, autonomic and, trophic changes and functionality of the affected limb.
Pain can be spontaneous or induced, of variable intensity, of stabbing, burning, itching or throbbing type that increases with external stimuli like changes in temperature (thermal allodynia), touch or pressure (mechanical allodynia) or emotional factors like anxiety and stress. It has an orthostatic component with a decrease in intensity when the limb is raised, and an increase in intensity when the limb is kept down (31).
The autonomic signs and symptoms are characterized by vaso and sudomotor changes, asymmetrical skin color changes (blanched, red, cyanotic, spotted, mottled skin) and dynamic changes in temperature that can be influenced by the environmental conditions. Sweat abnormalities are characterized by hyperhidrosis, but dry skin can also occur. These changes vary from patient to patient and even in the same individual during a period of time (32, 33). Trophic changes compromise the skin, nails and hair and could lead to epidermis thinning, glossy skin, thin nails and excess growth of hair.
Motor abnormalities in the affected limb can manifest as weakness, tremors, muscle discoordination, decrease motion capability, muscle spasms, dystonia (7), reduced movements and joint rigidity. Myofascial dysfunction appears by disuse or overuse of the muscle group particularly when it compromises the upper limb (34, 35).
Retrospective studies in patients with CRPS with pain lasting for 3 years report that the symptoms mostly remained stable or tend to improve instead of worsening progressively (36).
TREATMENT
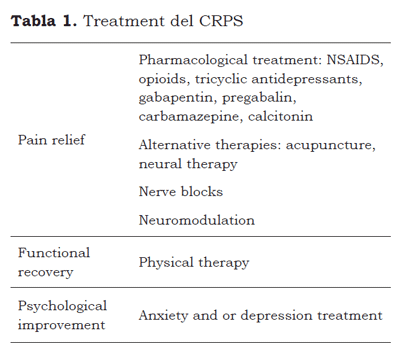
Different treatments have been suggested for management of CRPS, these include pharmacological and non-pharmacological treatments. The goals of the treatment are pain relief, functional recovery, and psychological improvement.
Regarding pain relief, although there are multiple options for its treatment, most have not been tested with controlled studies. Tricyclic antidepressants, gabapentin, pregabalin, and opioids are the treatments commonly used for neuropathic pain even though their effect on CRPS pain has not been completely demonstrated and its use is anecdotal (37).
In a double-blind randomized controlled trial that studied the effect of gabapentin compared to placebo in CRPS, a 43 % pain relief was observed in patients who received gabapentin versus 17 % of pain relief in those who received placebo (38). There is no evidence that suggests that the dose of gabapentin should be different in CRPS compared to the dose is administered in other types of neuropathic pain. Anticonvulsants with sodium channel blocking activity, like carbamazepine, can be of great help in the management of CRPS type II, because the injury of peripheral nerves alters the distribution and expression of sodium channel subtypes in the axonal membrane (37).
Opioids still are the treatment of choice for managing acute pain, although patients with central nervous system injury will not exhibit pain relief with their use. Tramadol has been effective in controlled studies for peripheral neuropathic pain and can be used in patients with CRPS (39, 40). Biphosphonates are powerful inhibitors of bone resorption, and in two studies were these medications were used (41, 42) an improvement in motor function was reported.
Clonidine, and α 2 adrenergic agonist of the pre-synaptic neurons of the primary afferent and of the postsynaptic neurons (43) decreases the release of norepinephrine, the concentration of P substance in C fibers, increases the cholinergic activity in the spinal cord and the concentration of acetylcholine in the cerebrospinal fluid. It has been demonstrated to be effective in CRPS when administered spinally.
The effect of calcitonin has been assessed in 2 studies: in the first one it was compared with placebo; in which a statistically significant decrease of pain was found which however was not clinically relevant, while in the second study there were no statistically significant differences (44, 45). The use of ketamine in patients with CRPS was useful in controlling the allodynia and the hyperalgesia. There are reports about its use epidurally with good results in patients with CRPS (46).
The use of steroidal or nonsteroidal anti-inlam-matory agents (NSAID) is recommended in the early phases of CRPS, to try to block the synthesis of prostaglandins which are responsible of the process of increasing the sensitivity of nociceptors (47). Two randomized studies with simple blinding, reported pain relief which was statistically and clinically significant (48, 49). The collateral effects of their long-term use must be considered, for which their use for extended periods is not recommended.
Regarding medullary electrical stimulation, in a controlled clinical study with patients who did not respond to conventional treatments, compared one group that received medullary electrical stimulation to another that received treatment with physical therapy, observing a statistically significant reduction in pain with the medullary stimulation, even though no benefits were observed in the functional status of the patients (50).
Regarding functional recovery, some authors consider that CRPS type I could be the result of extended immobility and lack of use of the limb involved in an injury, for which they consider early mobilization of the extremity as useful. Therefore intensive physical therapy should be started early, to avoid the functional limitations commonly observed or to prevent their progress (51, 52). Unfortunately, when the pain is severe it is not well tolerated by most patients, so it is necessary to begin an effective analgesic scheme. In some patients psychological therapy is necessary, when anxiety or depression affects the treatment (Table 1).
REFERENCES
1. Wilsey B, Teicheira D, Caneris OA, Fishman SM. A review of sympathetically maintained pain syndromes in the cancer pain population. Pain practice 2001;1:307-23.
2. Mitchell SW, Morehouse GR, Keen WW. Gunshot wounds and other injuries of nerves. Philadelphia: JB Lippincott, 1864, 100-11. (Reprinted in Clin Orthop Relat Res, 1982;163:2-7).
3. Schott GD. Complex? Regional? Pain? Syndrome? Pract Neurol 2007;7:145-57.
4. Díaz PA, Plancarte R, Tamayo AC. Síndrome doloroso regional complejo. Estado actual. Cir Ciruj 2004;72:225-38.
5. Merskey H, Bogduk N. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Second edition. Seattle, Washington: IASP Press;1994:40-2.
6. Cepeda S. Síndrome doloroso regional complejo tipos I-II. En: Rodríguez RF. Medicina del dolor y cuidados paliativos. Cali: Editorial Universidad Libre; 1998; 71-79.
7. Bruehl S, Harden RN, Galer BS, Saltz S, Bertram M, Backonja M, Gayles R, Rudin N, Bhugra MK, Stan-ton-Hicks M: External validation of IASP diagnostic criteria for complex regional pain syndrome and proposed research diagnostic criteria. Pain 1999; 81:147-54
8. Veldman PH, Reynen HM, Arntz IE, Goris RJ. Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients. Lan-cet.1993;342:1012-6.
9. Raja SN, Grabow TS. Complex Regional Pain Syndrome I (Reflex Sympathetic Dystrophy). Anesthesi-ology.2002; 96:1254-60.
10. Ribera MV. Síndrome de dolor regional complejo tipo I y II. Dolor. 2003;18:83-84.
11. Neira F, Ortega J. L. El síndrome doloroso regional complejo y medicina basada en la evidencia. Rev. Soc. Esp. Dolor 2007;2:133-46.
12. Gler BS, Scwartz L, Allen R. Síndromes de dolor regional complejo: tipo I (distrofia simpática refleja) y tipo II (causalgia). En: Loeser JD, Butler SH, Chapman CR, et al. Bonica Terapéutica del Dolor. Vol I. 3a ed., México: McGraw-Hill Interamericana; 2003. p. 467-96.
13. Matoses MS. Síndrome del dolor regional complejo. Dolor neuropático periférico. Dolor. 2002;17:78-86.
14. Obata FC, Tsa LL. Síndrome dolorosa complexa regional: Epidemiologia, fisiopatologia, manifestações clínicas, testes diagnósticos e propostas terapêuticas. Rev Bras Anestesiol.2002;52:5:618-27
15. De Mos M, de Bruijn AGJ, Huygen FJPM, Dieleman JP, Stricker BHCh, Sturkenboom MCJM. The incidence of complex regional pain syndrome: A population-based study. Pain.2007;129:12-20.
16. Sandroni P, Benrud-Larson LM, McClelland RL, Low PA. Complex regional pain syndrome type I: incidence and prevalence in Olmsted county, a population-based study. Pain.2003;103:199-207.
17. Pertoldi S, Di Benedetto P. Shoulder-hand syndrome after stroke. A complex regional pain syndrome. Eura Medicophys. 2005;41:283-92.
18. Kiralp ZM, Dinger Ü, Çakar E, Dursun H. Complex regional pain syndrome: epidemiologic features, treatment approaches, workday loss and return to work/disability ratios. Turk J Rheumatol 2009;24:1-5.
19. Rodríguez RF, Angel AM. Epidemiology and clinical characteristics of complex regional pain syndrome of upper limb in Valle-Colombia. In press
20. Janig W, Baron R. Complex regional pain syndrome: mystery explained? Lancet Neurol. 2003;2:687-97.
21. Ghai B, Dureja GP. Complex regional pain syndrome: A review. J Postgrad Med. 2004;50:300-7.
22. Schurmann M, Gradl G, Zaspel J, Kayser M, Lohr P, Andress HJ. Peripheral sympathetic function as a predictor of complex regional pain syndrome type I in patients with radial fracture. Auton Neuros-ci.2000;86:127-34.
23. Drummond DP. Involvement of the Sympathetic Nervous System in Complex Regional Pain Syndrome. Neurology. 2001;9:1296-303.
24. Wasner G, Heckmann K, Maier C, Baron R. Vascular abnormalities in acute reflex sympathetic dystrophy (CRPS I): complete inhibition of sympathetic nerve activity with recovery. Arch Neurol. 1999;56:613-20.
25. Garrido B. Complex regional pain syndrome. An approach between physiopathology and therapeutics. Rev Soc Esp Dolor. 2005;12:227-34.
26. Rommel O, Gehling M, Dertwinkel R, Witscher K, Zenz M, Malin JP, Janig, W. Hemisensory impairment in patients with complex regional pain syndrome. Pain 1999;80:95-101.
27. Rommel O, Thimineur M. Clinical evidence of central sensory disturbances in CRPS. En: Harden RN, Baron R, Janing W, editors. Complex regional pain syndrome. Vol 22.Seattle: IASP Press; 2001;193-208.
28. Sieweki N, Birklein F, Riedl B, Neundorfer B, Handwerker HO. Patterns of hiperalgesia in complex regional pain sindrome. Pain.1999;80:171-7.
29. Weber M, Birklein, B. Neundörfer, M. Schmelz. Facilitated neurogenic inflammation in complex regional pain syndrome. Pain.2001;91(3):251-7.
30. Üceyler N, Eberle T, Rolke R, Birklein F Sommer C. Differential expression patterns of cytokines in complex regional pain syndrome. Pain.2007;132: 195-205.
31. Blumberg H, Janing W. Clinical manifestation of reflex sympathetic dystrophy and sympathetically maintained pain. In: Wall PD, Melzack R (eds). Textbook of pain, 3rd ed. Edinburg: Churchill Livingstone; 1994. p. 685-98.
32. Birklein F, Riedl B, Neundorfer B, Handwerker HO. Sympathetic vasoconstrictor reflex pattern in patients with complex regional pain syndrome. Pain.1998;75:93-100.
33. Wasner G, Heckmann K, Maier C, Baron R. Vascular abnormalities in acute reflex sympathetic dystrophy (CRPS I): Complete inhibition of sympathetic nerve activity with recovery. Arch Neurol. 1999;56:613-20.
34. Allen G, Galer BS, Schwartz L. Epidemiology of complex regional pain syndrome: a retrospective chart review of 134 patients. Pain.1999;80:539-44.
35. Rashiq, S., Galer, B.S. Proximal myofascial dysfunction in complex regional pain syndrome: a retrospective prevalence study. Clin J Pain. 1999;15:151-3.
36. Galer BS, Henderson J, Perander J, Jensen MP. Course of symptoms and quality of life measurement in complex regional pain syndrome: a pilot survey. J Pain Symptom Manage. 2000;20:286-92.
37. Rowbotham MC. Pharmacological management of complex regional pain syndrome. Clin J Pain. 2006;22:425-9.
38. Van de Vusse AC, Stomp van den Berg SG, Kessels AH, Weber WE. Randomised controlled trial of gabapentin in Complex Regional Pain Syndrome type I. BMC Neurology.2004;4:13-22.
39. Sindrup SH, Andersen G, Madsen C, Smith T, Brosen K, Jensen TS. Tramadol relieves pain and allodynia in polyneuropathy: a randomised, double-blind, controlled trial. Pain.1999;83:85-90.
40. Harati Y, Gooch C, Swenson M, et al. Doubleblind randomized trial of tramadol for the treatment of the pain of diabetic neuropathy. Neurol-ogy.1998;50:1842-6.
41. Varenna M, Zucchi F, Ghiringhelli D, Binelli L, Bevi-lacgua M, Bettica P, Sinigaglia L. Intravenous clo-dronate in the treatment of reflex sympathetic dystrophy syndrome. A randomized, double blind, placebo controlled study. J Rheumatol. 2000;27:1477-83.
42. Adami S, Fossaluzza V, Gatti D, Fracassi, E; Braga, V. Bisphosphonate therapy of reflex sympathetic dystrophy syndrome. Ann Rheum Dis. 1997;56:201-4.
43. Cliffort J, Wolf, Mannion RJ. Dolor neuropático, etiología, mecanismos y manejo. Lancet. 1999;353:1959-64.
44. Gobelet C, Waldburger M, Meier JL. The effect of adding calcitonin to physical treatment on reflex sympathetic dystrophy. Pain.1992;48:171-5.
45. Bickerstaff DR, Kanis JA. The use of nasal calcitonin in the treatment of post-traumatic algodystrophy. Br J Rheumatol.1991;30:291-4.
46. Stanton-Hicks M. Complex regional pain syndrome (type-I, RSD; type-II, causalgia): controversies. Clin J Pain. 2000;16:33-40.
47. Kidd BL, Urbán LA. Mecanismos del dolor inflamatorio. British Journal of Anaesthesia.2001;87:3-11.
48. Braus DF, Kraus JK, Strobel J. The shoulder hand syndrome after stroke: a prospective clinical trial. Ann Neurol. 1994;36:728-33.
49. Christensen K, Jensen EM, Noer I. The reflex sympathetic dystrophy syndrome response to treatment with systemic corticosteroids. Acta Chir Scand. 1982;148:653-5.
50. Kemler MA, Barendse GA, van Kleef M, de Vet HC, Rijks CP, Furnee CA, van den Wildenberg FA. Spinal cord stimulation in patients with chronic reflex sympathetic dystrophy. N Engl J Med. 2000;343: 618-24.
51. Galer BS, Butler S, Jensen MP. Case reports and hypothesis: a neglect-like syndrome may be responsible for the motor disturbance in reflex sympathetic dystrophy (CRPS 1). J Pain Symptom Manage. 1995;10:385-91.
52. Hareau J. What makes treatment of reflex sympathetic dystrophy successful? J Hand Ther.1996;9:367-70.
Conflicto de intereses: ninguno declarado
1. Wilsey B, Teicheira D, Caneris OA, Fishman SM. A review of sympathetically maintained pain syndromes in the cancer pain population. Pain practice 2001;1:307-23. [ Links ]
2. Mitchell SW, Morehouse GR, Keen WW. Gunshot wounds and other injuries of nerves. Philadelphia: JB Lippincott, 1864, 100-11. (Reprinted in Clin Orthop Relat Res, 1982;163:2-7). [ Links ]
3. Schott GD. Complex? Regional? Pain? Syndrome? Pract Neurol 2007;7:145-57. [ Links ]
4. Díaz PA, Plancarte R, Tamayo AC. Síndrome doloroso regional complejo. Estado actual. Cir Ciruj 2004;72:225-38. [ Links ]
5. Merskey H, Bogduk N. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Second edition. Seattle, Washington: IASP Press;1994:40-2. [ Links ]
6. Cepeda S. Síndrome doloroso regional complejo tipos I-II. En: Rodríguez RF. Medicina del dolor y cuidados paliativos. Cali: Editorial Universidad Libre; 1998; 71-79. [ Links ]
7. Bruehl S, Harden RN, Galer BS, Saltz S, Bertram M, Backonja M, Gayles R, Rudin N, Bhugra MK, Stan-ton-Hicks M: External validation of IASP diagnostic criteria for complex regional pain syndrome and proposed research diagnostic criteria. Pain 1999; 81:147-54 [ Links ]
8. Veldman PH, Reynen HM, Arntz IE, Goris RJ. Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients. Lan-cet.1993;342:1012-6. [ Links ]
9. Raja SN, Grabow TS. Complex Regional Pain Syndrome I (Reflex Sympathetic Dystrophy). Anesthesi-ology.2002; 96:1254-60. [ Links ]
10. Ribera MV. Síndrome de dolor regional complejo tipo I y II. Dolor. 2003;18:83-84. [ Links ]
11. Neira F, Ortega J. L. El síndrome doloroso regional complejo y medicina basada en la evidencia. Rev. Soc. Esp. Dolor 2007;2:133-46. [ Links ]
12. Gler BS, Scwartz L, Allen R. Síndromes de dolor regional complejo: tipo I (distrofia simpática refleja) y tipo II (causalgia). En: Loeser JD, Butler SH, Chapman CR, et al. Bonica Terapéutica del Dolor. Vol I. 3a ed., México: McGraw-Hill Interamericana; 2003. p. 467-96. [ Links ]
13. Matoses MS. Síndrome del dolor regional complejo. Dolor neuropático periférico. Dolor. 2002;17:78-86. [ Links ]
14. Obata FC, Tsa LL. Síndrome dolorosa complexa regional: Epidemiologia, fisiopatologia, manifestações clínicas, testes diagnósticos e propostas terapêuticas. Rev Bras Anestesiol.2002;52:5:618-27 [ Links ]
15. De Mos M, de Bruijn AGJ, Huygen FJPM, Dieleman JP, Stricker BHCh, Sturkenboom MCJM. The incidence of complex regional pain syndrome: A population-based study. Pain.2007;129:12-20. [ Links ]
16. Sandroni P, Benrud-Larson LM, McClelland RL, Low PA. Complex regional pain syndrome type I: incidence and prevalence in Olmsted county, a population-based study. Pain.2003;103:199-207. [ Links ]
17. Pertoldi S, Di Benedetto P. Shoulder-hand syndrome after stroke. A complex regional pain syndrome. Eura Medicophys. 2005;41:283-92. [ Links ]
18. Kiralp ZM, Dinger Ü, Çakar E, Dursun H. Complex regional pain syndrome: epidemiologic features, treatment approaches, workday loss and return to work/disability ratios. Turk J Rheumatol 2009;24:1-5. [ Links ]
19. Rodríguez RF, Angel AM. Epidemiology and clinical characteristics of complex regional pain syndrome of upper limb in Valle-Colombia. In press [ Links ]
20. Janig W, Baron R. Complex regional pain syndrome: mystery explained? Lancet Neurol. 2003;2:687-97. [ Links ]
21. Ghai B, Dureja GP. Complex regional pain syndrome: A review. J Postgrad Med. 2004;50:300-7. [ Links ]
22. Schurmann M, Gradl G, Zaspel J, Kayser M, Lohr P, Andress HJ. Peripheral sympathetic function as a predictor of complex regional pain syndrome type I in patients with radial fracture. Auton Neuros-ci.2000;86:127-34. [ Links ]
23. Drummond DP. Involvement of the Sympathetic Nervous System in Complex Regional Pain Syndrome. Neurology. 2001;9:1296-303. [ Links ]
24. Wasner G, Heckmann K, Maier C, Baron R. Vascular abnormalities in acute reflex sympathetic dystrophy (CRPS I): complete inhibition of sympathetic nerve activity with recovery. Arch Neurol. 1999;56:613-20. [ Links ]
25. Garrido B. Complex regional pain syndrome. An approach between physiopathology and therapeutics. Rev Soc Esp Dolor. 2005;12:227-34. [ Links ]
26. Rommel O, Gehling M, Dertwinkel R, Witscher K, Zenz M, Malin JP, Janig, W. Hemisensory impairment in patients with complex regional pain syndrome. Pain 1999;80:95-101. [ Links ]
27. Rommel O, Thimineur M. Clinical evidence of central sensory disturbances in CRPS. En: Harden RN, Baron R, Janing W, editors. Complex regional pain syndrome. Vol 22.Seattle: IASP Press; 2001;193-208. [ Links ]
28. Sieweki N, Birklein F, Riedl B, Neundorfer B, Handwerker HO. Patterns of hiperalgesia in complex regional pain sindrome. Pain.1999;80:171-7. [ Links ]
29. Weber M, Birklein, B. Neundörfer, M. Schmelz. Facilitated neurogenic inflammation in complex regional pain syndrome. Pain.2001;91(3):251-7. [ Links ]
30. Üceyler N, Eberle T, Rolke R, Birklein F Sommer C. Differential expression patterns of cytokines in complex regional pain syndrome. Pain.2007;132: 195-205. [ Links ]
31. Blumberg H, Janing W. Clinical manifestation of reflex sympathetic dystrophy and sympathetically maintained pain. In: Wall PD, Melzack R (eds). Textbook of pain, 3rd ed. Edinburg: Churchill Livingstone; 1994. p. 685-98. [ Links ]
32. Birklein F, Riedl B, Neundorfer B, Handwerker HO. Sympathetic vasoconstrictor reflex pattern in patients with complex regional pain syndrome. Pain.1998;75:93-100. [ Links ]
33. Wasner G, Heckmann K, Maier C, Baron R. Vascular abnormalities in acute reflex sympathetic dystrophy (CRPS I): Complete inhibition of sympathetic nerve activity with recovery. Arch Neurol. 1999;56:613-20. [ Links ]
34. Allen G, Galer BS, Schwartz L. Epidemiology of complex regional pain syndrome: a retrospective chart review of 134 patients. Pain.1999;80:539-44. [ Links ]
35. Rashiq, S., Galer, B.S. Proximal myofascial dysfunction in complex regional pain syndrome: a retrospective prevalence study. Clin J Pain. 1999;15:151-3. [ Links ]
36. Galer BS, Henderson J, Perander J, Jensen MP. Course of symptoms and quality of life measurement in complex regional pain syndrome: a pilot survey. J Pain Symptom Manage. 2000;20:286-92. [ Links ]
37. Rowbotham MC. Pharmacological management of complex regional pain syndrome. Clin J Pain. 2006;22:425-9. [ Links ]
38. Van de Vusse AC, Stomp van den Berg SG, Kessels AH, Weber WE. Randomised controlled trial of gabapentin in Complex Regional Pain Syndrome type I. BMC Neurology.2004;4:13-22. [ Links ]
39. Sindrup SH, Andersen G, Madsen C, Smith T, Brosen K, Jensen TS. Tramadol relieves pain and allodynia in polyneuropathy: a randomised, double-blind, controlled trial. Pain.1999;83:85-90. [ Links ]
40. Harati Y, Gooch C, Swenson M, et al. Doubleblind randomized trial of tramadol for the treatment of the pain of diabetic neuropathy. Neurol-ogy.1998;50:1842-6. [ Links ]
41. Varenna M, Zucchi F, Ghiringhelli D, Binelli L, Bevi-lacgua M, Bettica P, Sinigaglia L. Intravenous clo-dronate in the treatment of reflex sympathetic dystrophy syndrome. A randomized, double blind, placebo controlled study. J Rheumatol. 2000;27:1477-83. [ Links ]
42. Adami S, Fossaluzza V, Gatti D, Fracassi, E; Braga, V. Bisphosphonate therapy of reflex sympathetic dystrophy syndrome. Ann Rheum Dis. 1997;56:201-4. [ Links ]
43. Cliffort J, Wolf, Mannion RJ. Dolor neuropático, etiología, mecanismos y manejo. Lancet. 1999;353:1959-64. [ Links ]
44. Gobelet C, Waldburger M, Meier JL. The effect of adding calcitonin to physical treatment on reflex sympathetic dystrophy. Pain.1992;48:171-5. [ Links ]
45. Bickerstaff DR, Kanis JA. The use of nasal calcitonin in the treatment of post-traumatic algodystrophy. Br J Rheumatol.1991;30:291-4. [ Links ]
46. Stanton-Hicks M. Complex regional pain syndrome (type-I, RSD; type-II, causalgia): controversies. Clin J Pain. 2000;16:33-40. [ Links ]
47. Kidd BL, Urbán LA. Mecanismos del dolor inflamatorio. British Journal of Anaesthesia.2001;87:3-11. [ Links ]
48. Braus DF, Kraus JK, Strobel J. The shoulder hand syndrome after stroke: a prospective clinical trial. Ann Neurol. 1994;36:728-33. [ Links ]
49. Christensen K, Jensen EM, Noer I. The reflex sympathetic dystrophy syndrome response to treatment with systemic corticosteroids. Acta Chir Scand. 1982;148:653-5. [ Links ]
50. Kemler MA, Barendse GA, van Kleef M, de Vet HC, Rijks CP, Furnee CA, van den Wildenberg FA. Spinal cord stimulation in patients with chronic reflex sympathetic dystrophy. N Engl J Med. 2000;343: 618-24. [ Links ]
51. Galer BS, Butler S, Jensen MP. Case reports and hypothesis: a neglect-like syndrome may be responsible for the motor disturbance in reflex sympathetic dystrophy (CRPS 1). J Pain Symptom Manage. 1995;10:385-91. [ Links ]
52. Hareau J. What makes treatment of reflex sympathetic dystrophy successful? J Hand Ther.1996;9:367-70. [ Links ]











 text in
text in 

