INTRODUCTION
Current guidelines on managing patients with type 2 diabetes recommend starting glucagon-like peptide-1 receptor agonists (GLP-1) as first-line therapy for type 2 diabetes, particularly in individuals with obesity, atherosclerotic disease, or cardiovascular risk. 1,2
GLP-1 analogs came to market in 2005, and since then, the Food and Drug Administration (FDA) has approved six GLP-1s, with semaglutide being the seventh approved in the United States and the second indicated for obesity treatment, regardless of diabetes diagnosis. 3
In patients with type 2 diabetes, GLP-1 concentrations are reduced, and agonists have proven effective in addressing this deficiency. Their use has significantly increased in recent years as newer agents have been effective for improving glucose control and promoting weight loss, in addition to significant cardiovascular and renal benefits. 4,5
A primary mechanism of GLP-1 therapy to lower blood glucose and promote weight loss is slowing gastric emptying 6. In the perioperative context, increased gastric volumes raise concerns about aspiration risk when traditional fasting times are used before a surgical procedure; this mechanism of action has potential implications when considering pre-procedure fasting times and the choice of anesthesia technique.
This delay in gastric emptying can increase the risk of regurgitation and pulmonary aspiration of gastric contents during sedation or general anesthesia. Indeed, several anecdotal reports of regurgitation and aspiration during anesthesia induction have been recently published. 7,8
Given this patient safety concern, the American Society of Anesthesiologists (ASA) recently published consensus-based guidance on the preoperative management of patients on GLP-1RA and its impact on perioperative blood glucose levels. 9
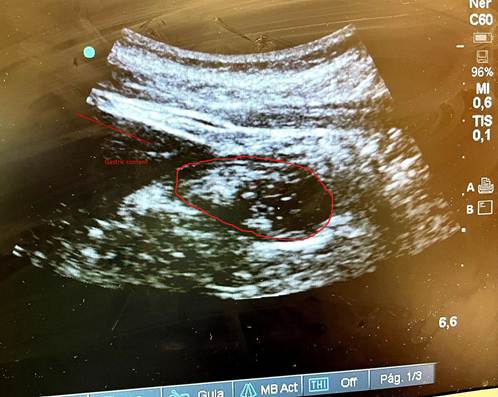
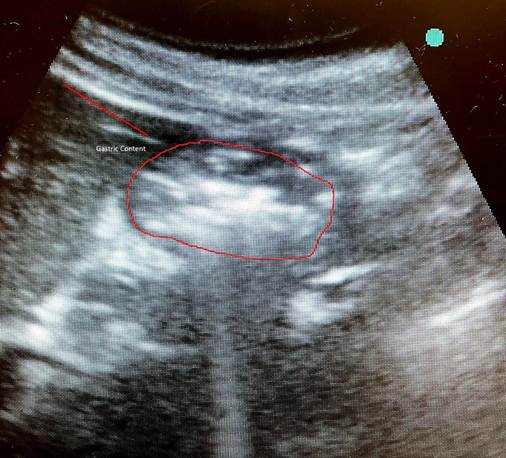
This clinical report presents the case of a patient scheduled for elective surgery, chronically medicated with subcutaneous semaglutide once a week. The patient was instructed to withhold the administration of the drug according to the ASA recommended lead time; however, to ensure safety, a gastric ultrasound was performed, revealing residual gastric content.
Case presentation
A 58-year-old female patient, ASA II E, diagnosed with capsular contracture of breast implants, scheduled for breast implant replacement with mastopexy, with unremarkable family history for the anesthesia plan. History of depression and frontotemporal dementia. Current medications: vortioxetine 10 mg every 24 hours, memantine 10 mg every 24 hours, citicoline 1 g in the morning, and semaglutide 1 mg subcutaneously weekly, last application one week before. Surgical history of cervical spine instrumentation and breast augmentation, with postoperative nausea and vomiting. Physical examination: height 165 cm, weight 62 kg, BMI, Mallampati: I, Patil-AldretI I, wide neck, Bellhouse Dore II. Premedication: metoclopramide 10 mg and omeprazole 40 mg.
The day before surgery, the patient received a telephone call instructing her to refrain from administering the semaglutide and fast for at least 10 hours for solids and liquids. On the day of the procedure, the patient presented with a 12-hour fast for solids and liquids.
The treating anesthesiologists performed the gastric US in the supine and right lateral decubitus positions. The result showed a full stomach with images suggestive of solid and liquid food content (Figure 1).
The patient received 10 mg of metoclopramide and 40 mg of omeprazole, and the decision was made to repeat the gastric US two hours after this premedication (14 hours of fasting); the result showed a decrease in liquid content but solid contents still present (Figure 2). The surgical procedure was deferred due to high risk of bronchoaspiration and rescheduled after three half-lives of semaglutide.
A gastric US was performed two weeks later (three weeks since the last semaglutide application) which showed an empty gastric antrum; the decision was made to perform the surgical procedure under balanced general anesthesia. The patient was uneventfully discharged the same day.
DISCUSSION
GLP-1 receptor agonists were introduced into clinical practice in the mid-2000s. They include exenatide, lixisenatide, liraglutide, dulaglutide, and semaglutide. The administration regimen is twice daily (standard-release exenatide), once daily (liraglutide, lixisenatide, or oral semaglutide), or once weekly (extended-release exenatide, dulaglutide, or subcutaneous semaglutide). These drugs mimic the action of endogenous GLP-1, which is produced glucose-dependently by L cells in the duodenum and upper gastrointestinal tract in response to the presence of carbohydrates in the intestinal lumen. 10
GLP-1 is an incretin hormone with several actions: it enhances insulin secretion from pancreatic ß-cells, inhibits glucagon secretion from pancreatic a-cells, thereby contributing to limit postprandial glucose excursions, in addition to induce satiety. 11
Since GLP-1 works only in the presence of hyperglycemia, the risk of hypoglycemia while taking a GLP-1RA during fasting is extremely low. However, patients may experience some side effects such as fullness, dyspepsia, bloating, nausea, or vomiting; these symptoms may be the result of loss of appetite due to pharmacological effects on the hypothalamus and delayed gastric emptying. Delayed gastric emptying and ileus can increase residual gastric volume, even with usual preoperative fasting. Therefore, the latest guidelines on perioperative management of diabetic patients have recommended suspending the drug the day before or the day of the procedure. 3,10
The first case of bronchoaspiration was published in March 2023 in a 42-year-old patient on semaglutide for weight reduction. The patient was admitted to the procedure after 18-hour fasting, and endoscopy revealed gastric content. In June 2023, a case was documented of a patient undergoing breast surgery, with no predictors or predispositions for regurgitation, with the only history of semaglutide consumption two days before the procedure; despite a 20-hour fasting time for solids and 8-hour fasting time for liquids, the patient had significant gastric content regurgitation, although it did not evolve to bronchoaspiration. 7
Reviews on the pharmacology of these agents indicate that the effect of gastric emptying depends on the dose and duration of drug use, suggesting possible tachyphylaxis associated with 20 weeks of use. Consequently, adverse gastrointestinal effects attributed to delayed gastric emptying tend to peak at around 12-20 weeks and then progressively decline. 6,10
The ASA working group recommends that patients withhold daily dosed GLP-1RA the day of the procedure and weekly-dosed formulations seven days prior to the procedure. 9
However, these recommendations fail to consider the reason for using the drug; that is, whether the indication is for glycemic control or weight loss. For this reason, Jones et al. suggest holding the GLP-1RA administered for weight loss for at least three half-lives, representing =88% drug elimination. 12
Since the elimination half-life of a long-acting GLP-1RA (e.g., semaglutide) is seven days, this would be approximately three weeks. For patients with diabetes, withholding the drug for such a long time could lead to significant loss of glycemic control. Therefore, the authors of this paper recommend consulting an endocrinologist to assess the risks/benefits of maintaining these medications for at least three half-lives and planning a bridge therapy with hypoglycemic agents or insulin. 2
Currently, no pharmacokinetic studies suggest a tangible correlation between the half-life of a GLP-1RA and gastric emptying. The half-life is a complex and complicated concept; therefore, it is often challenging to incorporate it into clinical practice and use it as a tool for clinical decision-making.
The half-life of daily oral semaglutide differs from the weekly oral administration; therefore, withholding the medication the day before would not significantly reduce plasma concentrations. Moreover, if a patient administers 1 mg of subcutaneous semaglutide and maintains his/her weekly dose, after a week, the plasma concentration would be the same as someone taking 0.5 mg per week and not suspending it. 6
Therefore, the general ASA recommendations may not be consistent with the pharmacokinetics in special cases, as these recommendations fail to differentiate between patients taking a GLP-1RA for diabetes or weight loss. Given the clinical importance of GLP-1 agonists, its key pharmacological characteristics have been listed in Table 1, for a better understanding of their pharmacokinetic differences.
Table 1 Pharmacological characteristics of GLP-1 receptor agonists (GLP-1RA).
| Name | Trade name | Half- life | Withholding times (three elimination half-lives) | Dose | Route of administration |
|---|---|---|---|---|---|
| Lixisenatide | Lyxumia® | 3-4 h | 9-12 h | 10-29 μg daily | Subcutaneous |
| Exenatide | Byetta® | 2.4 h | 7 h | 5-10 μg daily | Subcutaneous |
| Liraglutide | Victoza® | 13 h | 39 h | 0.6-1.8 mg daily | Subcutaneous |
| Dulaglutide | Trulicity® | 90 h | 27 h | 0.75-1.5 mg weekly | Subcutaneous |
| Extended-release Exenatide | Bydureon® | > 7 days | 21 d | 2 mg weekly | Subcutaneous |
| Semaglutide | Ozempic® | 160 h | 20 d | 0.25-1 mg weekly | Subcutaneous |
| Semaglutide (oral formulation) | Rybelsus® | 7 d | 21 d | 3-14 mg daily | Oral |
| Tirzepatide | Mounjaro® | 120 h | 15 d | 2.5 mg weekly | Subcutaneous |
Source: Autores, a partir de Hall et al. 6.
Sherwin et al. conducted a study with 20 volunteers, 10 taking semaglutide and 10 controls. All of the participants underwent gastric US in the supine and then lateral decubitus positions and complied with an eight-hour fasting time. The results showed that in the lateral position, residual gastric contents were observed in 90% of the semaglutide group compared to 20% of the control group. 13 Prolonged fasting is not a recommended alternative for patients taking GLP-1 agonists, due to the negative patient-reported perioperative effects, such as discomfort, anxiety, thirst, hunger, nausea, and omission of other medication doses. 14
Therefore, prolonging fasting duration seems unreasonable, especially given the lack of evidence suggesting a safe fasting duration. So far, there is no evidence of the safety of prolonged fasting in these patients. 3
Withholding the drug for three to five half-lives should be considered in patients taking GLP-1 for weight loss; in the case of semaglutide, this represents three to five weeks. In patients undergoing glycemic control treatment for diabetes, an endocrinologist should be consulted to adjust the treatment and assess the risk-benefit of the intervention. 10
If GLP-1RA has not been suspended as recommended and/or if the patient has significant gastrointestinal symptoms, gastric emptying evaluation by pre-anesthesia or operating room ultrasound should be considered. 15
Ultrasound has been validated to quantify the volume of solid and liquid contents in the stomach. 8,13,16 This tool may help stratify patient risk and facilitate decision-making.
The authors of this article consider that if the stomach is empty, it would be safe to proceed with the usual anesthetic induction. However, if the stomach is full or if the gastric ultrasound is inconclusive or not possible, it is wise to consider delaying the procedure or treating the patient as "full stomach" and managing the case with rapid sequence induction and intubation. 15 The ASA difficult airway guidelines recommend that patients at high risk of aspiration and with documented or suspected disease causing difficult airway should be subject to awake tracheal intubation. 17
Some authors argue that withholding a GLP-1RA and the absence of gastrointestinal symptoms may not be a reliable indication of the absence of risk; consequently, it could be safer to manage patients taking a GLP-1 RA as if they had a "full stomach" at all times.
CONCLUSIONS
People who started GLP-1 analog therapy before surgery could be at risk of delayed gastric emptying, despite withholding the drug as indicated by clinical practice guidelines. Therefore, anesthesiologists should be aware of the possibility of delayed gastric emptying in individuals who have started treatment with GLP-1 receptor agonists and conduct a risk-benefit analysis of perioperative measures.
The necessary precautions and measures to make a decision with regards to each case include performing a gastric ultrasound, using rapid sequence induction and intubation, awake intubation, considering measures for emergence, extubation, and even the possibility of deferring the case in elective surgery.
ETHICAL RESPONSIBILITIES
Protection of people and animals
The authors declare that no experiments were conducted on humans or animals for this research. The authors declare that the procedures followed were in accordance with the ethical standards of the responsible human experimentation committee and in accordance with the World Medical Association and the Declaration of Helsinki.
ACKNOWLEDGMENTS
Author contributions
RSM: Medical management, manuscript writing.
LLF: Medical management, performing the Gastric US, photography, manuscript review.
JACV: Medical management, performing the Gastric US, photography, clinical decision-making.
SB: Medical management, manuscript review.
TA: Medical management, manuscript review and writing.











 text in
text in