INTRODUCTION
In the Americas, Lutzomyia longipalpis (Lutz and Neiva, 1912) is the main vector of Leishmania infantum, the causative agent of visceral leishmaniasis, which is the most severe form of leishmaniasis. Vector control is one of the most important components of disease management, seeking to reduce the exposure of individuals to the infective bite of sandflies, either by reducing the human-vector contact or the density of vectors in specific habitats (Molyneux, 1993). Chemical insecticides are used in different measures, and one of the most important goals is to reduce the age of the natural population of vectors reducing colonization efficiency by an etiologic agent that makes the insect a vector. For L. longipalpis control, different measures have been evaluated, especially chemical measures, and most of them include pyrethroid insecticides and involve spraying of the human dwellings and surfaces of animal shelters (Kelly et al., 1997; Barata et al., 2011), insecticide-treated nets (Courteney et al., 2007), dog collars (David et al., 2001) and pheromone baits (Bray et al., 2010). The efficacy of those measures against L. longipalpis is generally high and spraying over vector resting surfaces with residual action insecticides, including pyrethroids, is recommended in visceral leishmaniasis foci in Colombia (Ministerio de la Protección Social et al., 2012) and Brazil (Ministério da Saúde, 2013). However, baseline information about the toxic activity of pyrethroids in L. longipalpis is limited.
Insect populations have normal response intervals to each insecticide. These intervals are determined by evaluating, under controlled conditions, increasing concentrations of the toxic substance (stimulus), and after a certain exposure time, response variables such as the mortality are evaluated. Based on these concentration-response tests, it is possible to infer the toxic concentration that kills a determinate percentage of exposed insects, for example to 50 % or 95 %, as corresponding to the lethal concentrations 50 (LC50) and 95 (LC95), respectively (Lagunes-Tejeda et al., 2009). Lethal concentrations (with their confidence intervals) are quantitative expressions of the toxicity of an insecticide for a given species allowing to establish comparisons of the toxic activity of two or more insecticides, for which a lower value of LC50 will indicate greater toxicity, and this knowledge may be useful to select insecticides during an intervention. Also, these concentrations are a measure of the susceptibility of a species to an insecticide under experimental conditions (Hubert, 1980).
By the other hand, the susceptibility is the tolerance range in a population to an insecticide compared with 1) a susceptible strain (e.g., Aedes aegypti Rockefeller strain) or 2) baseline susceptibility indicators of this population.
This study aimed to determine the toxicity of the pyrethroids lambda-cyhalothrin, alpha-cypermethrin, and deltamethrin in L. longipalpis through concentration-mortality tests. The first two pyrethroids are recommended for the residual treatment of the outdoor surfaces of dwellings for visceral leishmaniasis vector control, and most of the studies evaluating treated materials against L. longipalpis include one of these two insecticides (Feliciangeli et al., 2003, Romero and Boelaert, 2010). In addition, all three pyrethroids are active ingredients of long-lasting insecticidal nets, and of these, alpha-cypermethrin is the active ingredient of Interceptor® nets, which were mass distributed in 2012 and 2014 for L. longipalpis control in the periurban area of the city of Neiva, Department of Huila, Colombia (Secretaría de Salud de Neiva, unpublished data) because of a recent outbreak of visceral leishmaniasis (Gómez-Romero and Zambrano, 2012).
MATERIALS AND METHODS
For the bioassays, were used L. longipalpis females from the first filial generation of the wild sandflies collected with CDC light traps in the rural area of El Callejón village, municipality of Ricaurte, Cundinamarca, in the Magdalena River basin, Colombia. The specimens were breeding and maintained according to the procedures described by Modi and Tesh, (1983) at the insectary of the Entomology Group (Instituto Nacional de Salud). In the L. longipalpis area of origin, although there is not a regular application of insecticides for vector control made by municipal health authorities, human population apply eventually domestic insecticides for pest control and agricultural use, both with pyrethroids as the active ingredient.
The pyrethroids solutions were prepared in absolute alcohol Merck®. The compounds were acquired as neat grade material purchased from Chem Service® (West Chester, PA, USA): lambda-cyhalothrin (purity = 99.1 %), alpha-cypermethrin (purity = 99.5 %) and deltamethrin (purity = 99.3 %).
The concentration-mortality tests in L. longipalpis were conducted following the guidelines of the WHO, (1970) with two modifications: 1) Plastic tubes lined with impregnated papers at standard concentrations supplied by WHO, were replaced by 250 ml Wheaton glass bottles (with an internal area, including the lid, of 0.0341 m2) treated with 1 ml of insecticide solution in absolute alcohol according to the protocol of the Centers for Disease Control and Prevention, CDC (Brogdon and McAllister, 1998); and 2) WHO kit holding tubes were replaced by observation recipients with plaster moistened especially for the maintenance of Lutzomyia spp. (Santamaría et al., 2002). This observation recipient was used just once and then was discarded.
Groups with ten L. longipalpis females without access to a blood meal, from 1 to 3 days old, that was previously supplied with water and 30 % sugar solution ad libitum were exposed to different concentrations of the active ingredients of pyrethroids in the treated bottles.
After 1 h of exposure, the females were moved to the observation recipients with water, and saturated sugar solutions supplied idem (embedded in cotton balls) and kept in polystyrene boxes. The mortality was recorded 24 h after exposure, monitoring the observation recipient under the stereomicroscope. A female fallen were considered dead if, after a soft touch with an entomological needle, it did not move (Marceló et al., 2014, Denlinger et al., 2015) or if it responded with some type of movement, but was not able to fly (WHO, 2013) and return to an upright position.
For each insecticide, different concentrations (between five and six) that caused between 0 % and 100 % mortality in the exposed groups of females were tested, using the median lethal concentrations of pyrethroids reported in other species of sandflies as a reference (Tetreault et al., 2001; Álvarez et al., 2006). The concentrations determined were used for subsequent tests. Each set of tests included seven bottles with two different concentrations of each pyrethroid and a bottle treated just with the solvent (control). The sets of tests were conducted on different days. Each concentration was repeated between five and six times.
Each treated bottle was used a maximum of three times. Between tests, the bottles remained capped, covered with foil and refrigerated at 4 °C. During the tests, the temperature ranged between 23 and 25 °C, and the relative humidity was between 60 and 70 %. A probit analysis (concentration - mortality regression) was performed using the Finney method with the BioStat v.5 (2009) program.
RESULTS
In bioassays with concentrations defined for each insecticide, between five and six repetitions per concentration, a total of 1,090 L. longipalpis females were used. The mortality in the control bottles was low: 1.2 % (3 of 252 exposed females). Table 1 shows for each pyrethroid, the concentrations (g/ml) that caused between 0 % and 100 % mortality in L. longipalpis.Table 2 shows the results of the probit analysis with the lethal concentrations for each pyrethroid, with their 95 % confidence intervals for L. longipalpis.
Table 1 Pyrethroids concentrations ((g /ml) causing mortalities between 0 and 100% in Lutzomyia longipalpis
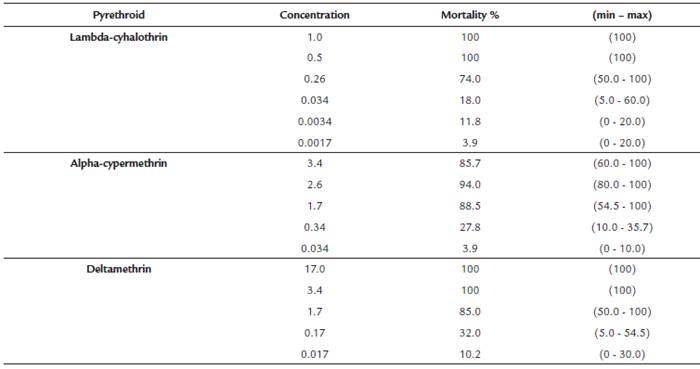
Table 2 Lethal concentrations ((g /ml) for three pyrethroids in Lutzomyia
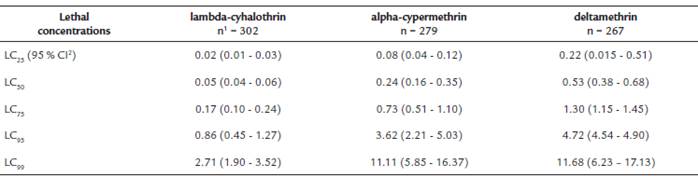
1Total number of L. longipalpis females exposed
2Confidence intervals
Comparing the median lethal concentrations of the three pyrethroids, lambda-cyhalothrin showed the highest degree of toxicity in L. longipalpis (the lowest LC50), followed by alpha-cypermethrin and deltamethrin. The LC50 of lambda-cyhalothrin was 10.6 times less than the LC50 found for deltamethrin and 4.8 times less than the LC50 of alpha-cypermethrin. The LC50 of alpha-cypermethrin was 2.2 times lower than the one of deltamethrin.
DISCUSSION
The three pyrethroids tested were toxic to L. longipalpis at very low concentrations. For L. longipalpis, there are only three studies about concentration-mortality, one was conducted for the deltamethrin pyrethroid, in which the median lethal concentration reported was 2.5 mg/m2 (Falcao et al., 1988). In the second study, the LC50 for lambda-cyhalothrin and deltamethrin were 0.23 μg/bottle and 0.92 μg/bottle, respectively (Denlinger et al., 2015).
In the third study, the LC50 for alpha-cypermethrin was 0.78 mg/m2 (Pessoa et al., 2015). However, a direct comparison with the results of this study cannot be made, because of the crucial differences between methodologies.
As concerns; in the first study, they used blood-fed females for the bioassays, in the second one, they used 1000 ml or 1892 ml glass bottles as test chambers also used L. longipalpis females and males in the same proportion; and in the third study the authors do not report any information about the sex of the exposed sandflies, or about the device where exposure was performed, in which there may be areas not treated with the insecticide such as WHO exposure tube.
The WHO recommended dosages for indoor residual treatment against mosquitoes (WHO, 2006) and sandflies (WHO, 2010) for these pyrethroids (between 20 and 30 mg active ingredient/m2) as well as other control measures, are approximately 253.2, 60.6 and 58.8 times greater than the LC99 obtained in L. longipalpis for lambda-cyhalothrin, alpha-cypermethrin and deltamethrin, respectively (using an internal area of the bottle and its lid of 0.0341 m2 as a reference, for the calculation of LC99 in mg/m2). Importantly, the lethal concentrations obtained under experimental conditions cannot be compared directly with field application dosages, because a laboratory determination does not consider insecticide losses produced by drag, photolysis, the surface where the insecticide is applied, and the potential contact time of the insect with the treated surface (Lagunes-Tejeda et al., 2009).
Because the recommended operating level concentrations for sandflies are extrapolated from vector control of malaria, it would be advisable to study the effect of the pyrethroids at both, the recommended dosages and the LC found in this study on L. longipalpis because such high doses could have distinct results to those expected. For example, instead of observing an impact on mortality, high contact irritancy, may produce that the insect moves away before acquiring the lethal dose.
In another vector, knowledge of effects that cause lethal and sub-lethal concentrations of pyrethroids in the vector has been applied. Recently, a successful strategy was evaluated in Aedes aegypti resting sites within the home, they were focally-treated using the minimum effective concentration of the pyrethroid in the vector (<LD90), which was equivalent to half of the WHO-recommended field application dosages (Manda et al., 2013), which showed a greater efficacy in the measure, with less amount of insecticide.
Another context, in which the intervals of the toxic response to pyrethroids are useful, is in the comparison of the susceptibility between two populations of the same species. This data, especially the LC50 could be used in the comparison of the susceptibility of the population of L. longipalpis from the Magdalena River basin, with 1) other populations of L. longipalpis subjected to continuous pressure with different chemical control measures, 2) the same population in a longitudinal study to track changes over time in response to pyrethroids after an eventual intervention. The comparison can be made through the calculation of the resistance ratio of 50 % (RR50) (Mazzarri et al., 1997; Pessoa et al., 2015).
In L. longipalpis, indicators of susceptibility to lambda-cyhalothrin, deltamethrin y alpha-cypermethrin have been estimated before, either with the method used by the Centers for Disease Control and Prevention (Marceló et al., 2014), or by establishing lethal times at fixed concentrations of insecticides (Mazzari et al., 1997; Alexander et al., 2009) or lethal concentrations at fixed times, according to the method proposed by WHO (Pessoa et al., 2015) or with significant modifications (Denlinger et al., 2015). The method used to estimate lethal concentrations in this study was the one reported by the WHO, but the standard impregnated papers were not used. These papers have disadvantages, such as the high cost of shipping and short expiration. Further, in the WHO device for insecticide exposure, the top and bottom bases of the cylinder are not covered by impregnated paper, so the insects may take different insecticide amounts in each replicate, which can lead to errors in the results. The glass bottle treated according to CDC guidelines is more versatile, it can be treated at any concentration with absolute alcohol and technical grade insecticides, and the entire surface available for insect exposure is treated.
CONCLUSION
In conclusion, through the traditional method of WHO, but using the glass bottle as an exposure chamber, concentrations of three pyrethroids that kill 25, 50, 75, 95 and 99 % of females exposed were established for L. longipalpis, which comes from an area with sporadic application of insecticides (domestic or agricultural use). These data could be mainly useful in studies on the effects of sub-lethal concentrations of these pyrethroids in the target population and other L. longipalpis populations from Magdalena river valley in Colombia, because of its genetic similarities (Hoyos et al., 2012).















