Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Ingeniería e Investigación
Print version ISSN 0120-5609
Ing. Investig. vol.33 no.1 Bogotá Jan./Apr. 2013
G. Buitrago Hurtado1, W. A. Villamil Porras2, D. J. Vargas Sepúlveda3, A. Otálvaro Alvarez4 and G. Y. Flórez5
1 Gustavo Buitrago Hurtado. M.Sc. Ingeniería Ambiental, Ingeniero Químico, Universidad Nacional de Colombia, Colombia. Affiliation: Institututo de Biotecnología, Universidad Nacional de Colombia. Colombia. E-mail: gbuitragoh@unal.edu.co
2 Withney Andrea Villamil Porras. Laboratorista Clínico, Bacterióloga, Universidad Colegio Mayor de Cundinamarca, Colombia. Affiliation: Clínica El Bosque. Colombia. E-mail: withney132003@yahoo.com
3 Daissy Jasbeydi Vargas Sepúlveda. Laboratorista Clínico, Bacterióloga, Universidad Colegio Mayor de Cundinamarca, Colombia. Affiliation: Centro de Medicina Diagnóstica. Colombia. E-mail: daissyjasbeydi@gmail.com
4 ángela Otálvaro Alvarez. Dr. Ingeniería Química, M.Sc. Ingeniería Química, Ingeniera Química, Universidad Nacional de Colombia. Affiliation: Universidad de La Salle. Colombia. E-mail: amotalvaro@unisalle.edu.co
5 Glaether Yohn Flórez. M.Sc. Microbiología, Ingeniero de Alimentos, Universidad Nacional de Colombia. Affiliation: Instituto de Biotecnología, Universidad Nacional de Colombia. Colombia. E-mail: gyflorez@unal.edu.co
How to cite: Aristizábal, J., Villamil Porras, W. A., Vargas Sepúlveda, D. J., Otálvaro Alvarez, A., Flórez, G. Y., Evaluating the effect of the number of generations in IBUN 91.2.98 Leuconostoc mesenteroides cultures on enzyme extract production., Ingeniería e Investigación. Vol. 33, No. 1. April 2013, pp. 66-70.
ABSTRACT
This work studied the effect of the number of generations of the IBUN 91.2.98 Leuconostoc mesenteroides strain on enzyme complex production. The subculturing technique was used on a medium which had been designed specifically for this organism for producing an enzyme complex. The effect was indirectly determined by monitoring microorganism growth and measuring the glucosyltransferase and hydrolytic activity of an enzyme extract obtained from such culture. There were 40 subcultures, representing 196 generations of IBUN 91.2.98 Leuconostoc mesenteroides. The results led to establishing that the extract's higher enzymatic activity (from 4 to 6 U/mL) was reached at the end of the culture's exponential phase and that this activity was stable during subculturing, confirming that there was no variation in strain regarding enzyme extract production until such number of generations had occurred, thereby not being limited to scaling-up to 8,000 litres.
Keywords: Leuconostoc mesenteroides, generation number, scale-up, glucosyltransferase activity.
RESUMEN
Por medio de este trabajo de investigación se estudió el efecto del número de generaciones de la cepa IBUN 91.2.98 de Leuconostoc mesenteroides sobre la producción de un complejo enzimático. Para ello se empleó la técnica de subcultivos en un medio diseñado específicamente para este microorganismo, con fines de producción de un complejo enzimático. La estabilidad se determinó, de manera indirecta, por medio del seguimiento del crecimiento del microorganismo y la medición de las actividades glucosiltransferasa e hidrolítica de un extracto enzimático obtenido del cultivo. En total se realizaron 40 subcultivos, correspondientes a 196 generaciones de Leuconostoc mesenteroides IBUN 91.2.98. Los resultados obtenidos permiten establecer que la mayor actividad del extracto enzimático (entre 4 y 6 U/mL) se alcanza al final de la fase exponencial del cultivo y que dicha actividad se mantiene estable durante los subcultivos, confirmando que hasta ese número de generaciones no se presenta una variación de la cepa en la producción del extracto enzimático, por lo que no constituye límite para escalar hasta 8.000 litros.
Palabras clave: Leuconostoc mesenteroides, número de generaciones, escalado, actividad glucosiltransferasa.
Received: June 2th 2011 Accepted: March 11th 2013
Introduction
A desired biological activity's stability which can be affected during different process stages lies in the possible limitation on the strains used in biotechnological processes: i.e. storage, propagation and fermentation. Such stability can also be altered by changes in the scale of culture, affecting the performance thereof (Thiry and Cingolani, 2002; Garcia et al., 2006; Kaneko et al., 2010). Currently, about 96 dextransucrase producing-bacterial strains have been identified which have been classified according to their dextran producing properties and structure. These characteristics affect dextran applications which are basically linked to the pharmaceutical, food and agricultural industries (Purama et al., 2008).
This paper studies the Leuconostoc mesenteroides strain (listed as IBUN 91.2.98) which produces an extracellular enzyme complex having dextransucrase activity which synthesises a dextran from sucrose. Dextransucrase (DS) is produced by batch submerged culture and corresponds to a primary metabolite. DS enzymes (enzyme commission numbers, EC 2.4.1.5.) catalyse α-D-glucopyranose residue transfer from sucrose (S) to acceptor molecules (mainly sugars), resulting in gluco-oligosaccharide (GOS) synthesis and fructose (F) release as a residual product, following the reaction (Dols et al., 1997; Dols et al., 1998):
These reactions are carried out without cofactors and the energy required for the reaction is supplied by sucrose glycosidic bond hydrolysis (Moulis et al., 2006). DS has two enzymatic activities: hydrolytic activity characterised by hydrolysing the glycosidic bond of the sucrose molecule that acts as a substrate generating a free fructose molecule (F) and transfer activity which is responsible for glucosyl groups transfer to the acceptor molecule (generally glucose) synthesising dextran (Breton et al., 2006; Moulis et al., 2006).
This study was aimed at using successive cultures for evaluating the effect of the number of generations of Leuconostoc mesenteroides IBUN 91.2.98 on the production of an enzyme complex having dextransucrase activity. The results suggested that cultures could be developed up to 8,000 litres by using this microorganism, without changes in enzymatic activity occurring.
Experimental development
Maintaining stock
The organism used was a native Colombian Leuconostoc mesenteroides strain, listed as 91.2.98 IBUN, which had been isolated from sugarcane (Saenz and Prado, 1998). A strainbank was constructed, wherein the original strain was cryopreserved at -70oC in 20% glycerol. Some subcultures were originally made from this strain to ensure its maintenance. The experimentation material was produced from one of these subcultures; the rest was isolated and protected from contamination (ISO 17025).
Culture medium composition
The culture medium used in this study was a modification of the medium described by Tsuchiya et al. (1956). It had the following composition in g/L: 60 sucrose (Carlo Erba ≥ 99.9%), 10 yeast extract (Pronadisa), 0.14 CaCl2.2H2O (JT Baker ≥ 99.9%), 0.04 MgSO4.7H2O (Merck ≥ 99.5%), 0.04 FeSO4.7H2O (Merck ≥ 99.5%), 0.02 MnSO4.H2O (Merck ≥ 98%), 0.01 NaCl (≥ 99.9% Carlo Erba), 5.7 H3PO4 (JT Baker ≥ 85%) and pH was adjusted to 7.0 with a concentrated KOH solution (86.8% ≥ JT Baker).
Strain activation and inoculum preparation
Initial activation of the strain involved incubating it in a 1 ml vial filled with the medium described in 2.2 for 12 hours at 30oC, stirred at 150 rpm. An activated sample from this strain (2% inoculums per volume to be prepared - 200μL) was mixed with the culture medium and incubated at 30oC for 6 hours.
Growth curve development
The strain's growth curve was developed because biomass production is associated with strain stability; Leuconostoc mesenteroides IBUN 91.2.98 was thus cultured in the medium described in 2.2 (9.8 mL) at 30oC, 150 rpm, monitoring the suspension's optical density every hour for 6 hours as an indirect biomass measurement. A 2% inoculum of the initial volume was used in the test. Biomass quantification involved using a calibration curve relating dried biomass to absorbance at 600 nm (0.99 correlation coefficient). The equation used to relate these variables was:
where:
B: dry biomass (mg/mL) and
ABS: absorbance at 600 nm
Calculating the number of generations
The number of generations in subcultures was experimentally determined by using initial and final cell numbers (before incubation and after 6 hours' incubation) using equation 2:
where n was the number of generations, Nf the end number of cells and No the initial number of cells. The calibration curve relating absorbance at 600 nm with cell number by counting in Petri dishes containing sucrose agar was also used for this purpose. The correlation coefficient for the data was 0.99 and the relationship between these variables was expressed in terms of equation 3:
N: cell number CFU / mL and
ABS: absorbance at 600 nm.
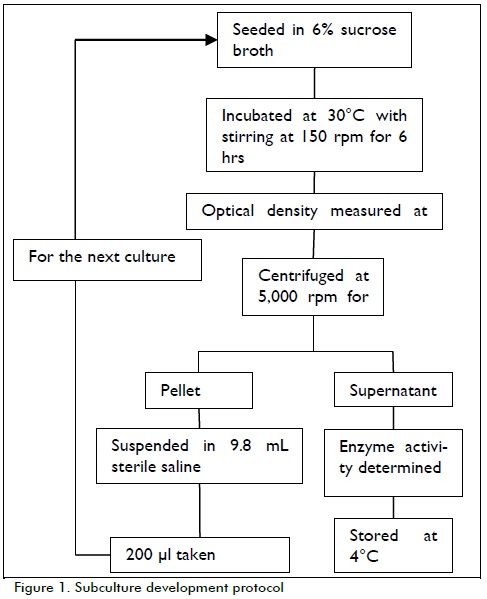
Subculture development
Subcultures were made as indicated in Figure 1. Culture time was established according to growth curve results.
Recovering enzyme extract
Final biomass concentration was determined at the end of culture exponential phase by measuring optical density at 600 nm in homogenised samples (Giraldo, 2002). The enzyme extract was also obtained at the end of the exponential phase and harvested and spun in 50 ml tubes at 5,500 rpm for 15 minutes. The supernatant contained the enzyme extract.
Determining enzyme activity
One unit of hydrolytic enzyme activity (HEA) was defined as the amount of enzyme required to produce 1μmol of fructose per minute at 30oC, 233 mM sucrose concentration, pH 5 and 50 mM phosphate buffer. One unit of transfer enzyme activity (TEA) was defined as the amount of enzyme required to transfer 1μmol of glucose per minute at 30oC, 233 mM sucrose concentration, pH 5 and 50 mM phosphate buffer.
Enzyme activity was determined in 1 mL volumes of 50 mM phosphate buffer, pH 5 and 233 mM sucrose and enzyme extract mixed in 1:1 ratio, thereby forming the reaction mixture. This was incubated at 30oC for 40 min. 0.1 mL aliquots were inactivated by being boiled for 5 min after 0, 5, 10, 15, 20 and 40 min of the reaction; enzyme activity was then determined. This activity was determined by quantifying the reducing sugar concentration using the colorimetric method from 3.5 dinitrosalicylic acid (DNS) at 540 nm (Miller, 1959); glucose production was measured by the enzymatic method (GOD-PAP kit, Boehringer ref. 166 391). The kit uses glucose oxidase, P- hydroxybenzene sulfonate as chromophore and iminoquinone as dye, developing great intensity with µ-glucose concentration. Absorbance was read at 510 nm, using a Spectronic spectrophotometer (Genesys 20) in both cases.
Using the values supplied with the kit, the following equation was used to quantify free glucose:
where GL was the glucose concentration in sample (mg / mL),
AB sample was sample absorbance at 510 nm and
AB standard was standard solution (100 mg glucose/100mL) absorbance at 510 nm.
Quantification of sugars was used to establish both hydrolytic and transfer enzyme activity. The free fructose obtained as the product of the enzyme reaction was determined as:
where FL was free fructose (mg / mL),
ART was total reducing sugars (mg / mL) determined by DNS and
GL was free glucose (mg/mL).
The unit of enzyme activity was defined per unit of time by plotting each value of free fructose regarding this variable; the slope of the graph represented hydrolysis or hydrolytic activity.
For determining transfer activity, it was assumed that total glucose (glucose + free glucose transferred) equalled the total fructose produced during hydrolysis. Glucose was then calculated as transferred, as the difference of total fructose and free glucose in the reaction:
after rearranging terms:
where FL was fructose produced during hydrolysis (mg/mL),
GL was free glucose (mg/mL) and
GT was transferred glucose (mg/mL).
Transfer activity was calculated from the slope of the graph: transferred glucose cf time elapsed.
Discussion and Results
Growth curve
The Leuconostoc mesenteroides IBUN 94.2.98 strain had 3 growth phases in the experimental conditions established for the cultures and time periods being studied. As shown in Figure 2, the time lag was 0 to 2 hours, the exponential growth phase lasted 3 to 5 hours and the beginning of the stationary phase occurred after 5 to 6 hours of incubation.
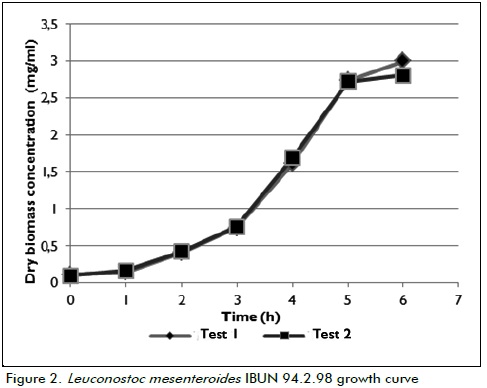
The end of the exponential phase was established as the point for stopping growth and harvest according to previous studies which considered enzyme extract characterisation (Soler and Buitrago, 2010); 6 hours was thus established as the time to harvest and quantify enzyme activity.
Subcultures
Biomass behaviour
Successive cultures were made once the time required for producing the enzymatic extract had been set. Biomass was determined indirectly as described above, both at the beginning and end of each subculture. The results presented in Figure 3 indicate that the strain achieved stable behaviour after 6 hours' culture at an average 3.1 ± 0.3 mg / mL dry biomass concentration through 40 subcultures.
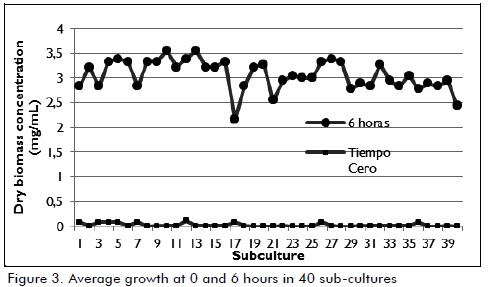
Equivalent number of generations
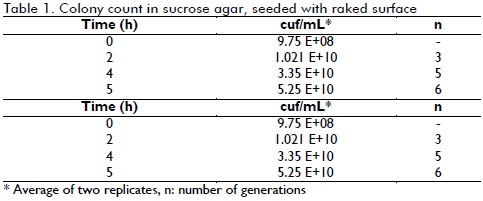
Table 1 shows the cfu/mL concentration regarding time spent in culture (data was obtained using equation 3). The final concentration after 5 hours indicated that each subculture produced 6 generations on average.
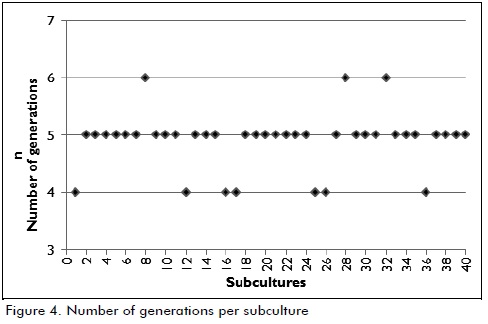
The number of generations in each subculture was calculated following the same protocol (values are presented in Figure 4). It was observed that 4 to 6 generations were obtained from each subculture after 6 hours' incubation, as reported in the pertinent literature. It was found that a bacterial cell took about forty minutes to produce a new generation (Kobayashi, 1975).
Enzymatic activity
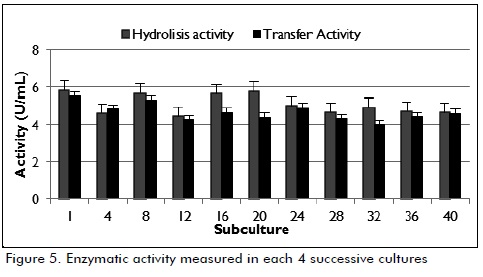
Enzymatic activity values were calculated from the results obtained from sugar quantification. Regarding hydrolysis activity, the results shown in Figure 5 show that this activity was greater than or equal to the transfer activity since the enzyme split sucrose into fructose and glucose and glucose was then transferred to dextran.
The relationship of the number of generations to scale-up
The information given in Figure 4 (number of generations - subculture) showed that around 196 generations of the Leuconostoc mesenteroides IBUN 91.2.98 strain had been produced by the end of the 40 subcultures. No significant changes regarding biomass production and glucosyltransferase activity were observed in test conditions. An industrial enzyme production stage would presuppose useful fermenter volume of 8,000 litres, 400 litres of inoculum and 20 litres pre-inoculum (obtained with 1L culture from bench level); 18 generations would thus be obtained according to the information presented in Table 2.
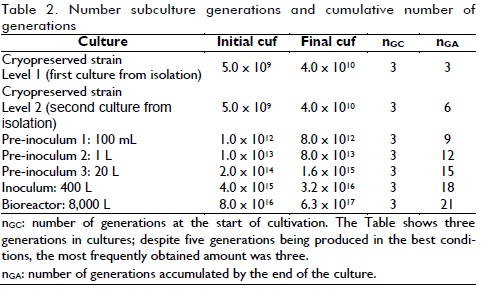
The data used for the calculations presented in Table 2 was inferred from laboratory culture results using 1 mL, 100 mL, 1 L, 20 L and 400 L. Such data corresponded to batch cultures and verified that the strain used had high stability regarding enzyme activity production for the hypothetical production conditions. It could be inferred that this strain could be used in continuous culture for extended periods of time without presenting problems associated with the number of generations and enzyme activity production measured in glucosyltransferase units.
Conclusions
Leuconostoc mesenteroides IBUN 91.2.98 had high reproducibility and growth regarding enzyme complex production in the conditions evaluated here.
After 40 successive cultures (activity being measured every 4 cultures), it was observed that hydrolytic and transfer enzymatic activity values became stable at a value close to 5 U/mL during the final stage of the exponential phase, indicating that this strain was stable.
There were no restrictions concerning scale-up regarding the number of generations obtained and the proposed hypothetical conditions because no significant changes were observed in the cultures during enzyme activity production.
References
Breton, C., Snajdrová, L. S., Jeanneau, C., Koca, J., Imberty, A., Structures and mechanisms of glycosyltransferases., Glycobiology, Vol. 16, No. 2, 2006, pp. 29 - 37. [ Links ]
Cardozo, A., Gómez, M., Aislamiento e identificación de cepas nativas con actividad levansacarasa., BSc Bacteriology thesis, Universidad Colegio Mayor de Cundinamarca, 1998. [ Links ]
Cuesta, B., Aseguramiento de Calidad. Cultivos de referencia., Norma ISO 17025, pp. 1-44. [ Links ]
Dols, S., Willemot, R. M., Vignon, M., Monsan, P., Characterization of the different dextransucrase activities excreted in glucose, fructose, or sucrose medium by Leuconostoc mesenteroides NRRL B-1299., Applied and Environmental Microbiology, Vol. 64, No. 4, Abril, 1998, pp. 1298-302. [ Links ]
Dols, M., Remaud-Simeon, M., Monsan, P. F., Dextransucrase production by Leuconostoc mesenteroides NRRL B-1299, comparison with L. mesenteroides NRRL B-512F., Enzyme Microbiology Technology, Vol. 20, 1997, pp. 523-530. [ Links ]
García, M., Villamizar, L., Torres, L., Cotes, A., Efecto de subcultivos sucesivos de Beauveria bassiana sobre sus características y actividad contra Premnotrypes voraz., Manejo Integrado de Plagas y Agroecología, Vol. 77, 2006, pp. 50-56. [ Links ]
Giraldo, S. E., Mora, C., Ospina, S., Caracterización de las propiedades de hidrofilidad de levana de origen biotecnológico., 1er Congreso Colombiano de Biotecnología, Bogotá, Universidad Nacional de Colombia, 2002, pp. 160-160 v. [ Links ]
Kaneko, Y., Sato, R., Aoyagi, H., Evaluation of Chinese hamster ovary cell stability during repeated culture for large scale antibody production., Journal of Bioscience and Bioengineering, Vol. 109, No. 3, 2010, pp. 274-280. [ Links ]
Kobayashi, M., Matsuda, K., Purification and characterization of two activities of the intracellular dextransucrase from Leuconostoc mesenteroides NRRL B-1299., Biochim Biophys Acta, Vol. 397, 1975, pp. 69-79. [ Links ]
Miller, G. L., Use of DinitrosaIicyIic acid reagent for determination of reducing sugar,. Analytical Chemistry, Vo.l 31, 1959, pp. 426-428. [ Links ]
Moulis, C., Joucla, G., Harrison, D., Fabre, E., Monsan, P., Understanding the polymerization mechanism of glycoside-hydrolase family 70 glucansucrases., Journal of Biological Chemistry, Vol. 281, No. 42, Oct., 2006, pp. 31254-67. [ Links ]
Purama, R. K., Goyal, A., Identification, effective purification and functional characterization of dextransucrase from Leuconostoc mesenteroides NRRL B-640., Bioresource Technology, 2008, pp. 3635-3642. [ Links ]
Saenz, A. C., Prado, M., Aislamiento de cepas nativas con actividad levansacarasa., BSc Bacteriology thesis, Universidad Colegio Mayor de Cundinamarca, 1998. [ Links ]
Soler, A., Buitrago, H., Evaluación de la transferencia de oxígeno en cultivos con Lactococcus lactis empleando un sistema de fermentación con aireación externa., Revista Colombiana de Biotecnología, Vol. 12, No. 2, 2010, pp. 124-138. [ Links ]
Thiry, M., Cingolani, D., Optimizing scale-up fermentation process., Trends in Biotechnology, Vol. 3, 2002, pp. 103-105. [ Links ]













