Introduction
Autonomic neuropathy (AN) generally describes the affection of the small C nerve fibers, which impairs the autonomic nervous system. In diabetes, its symptoms include either extreme hot or cold sensation across the legs, acute pain, and low sweating capacity or even the absence of it. Furthermore, this set of symptoms usually leads to the so called "diabetic foot". Multiple diagnostic tools are available on the market, such as the monofilament 10g, the quantitative axon reflex test (QSART), the thermoregulatory sweat testing (TST) and the Neuropad (SánzCorbalán et al., 2011, Papanas et al., 2005). Caring strategies have also been exhaustively developed and promoted to improve the life quality and expectancy of those who suffer it. Still, 50% of diabetics will suffer from some type of neuropathy, responsible for 20% of ER visits. It has also been demonstrated that the risk of amputation triples when preceded by an ulceration in diabetics. In addition, foot ulcerations cause 80% of the non-traumatic amputations worldwide, leaving a life expectancy of 2 to 5 years (Boulton, Cavanagh and Rayman 2007, De Alcalá 2005). The electrical conductance of the skin and its behavior, normally referred to as electrodermal activity (EDA) or as galvanic skin response (GSR) (González-Correa 2018 a), is typically divided into low and high frequency components. The former is usually named skin conductance level (SCL) and the latter, skin conductance response (SCR). High frequency responses are also classified depending on whether they are produced by an intended stimulus (event-related SCR) or not (nonspecific SCR) (Braithwaite et al., 2013). EDA has been used to measure stress (Najstrom and Jansson 2007; Reinhardt et at., 2012; Sierra, et al., 2011), modulation during exercise (Posada Quintero et al., 2018) or to evaluate user experience (Rico-Olarte et al., 2018), just to name a few. For a review in Spanish on the topic, please refer to the study of Mojica-Londono (2017), or in English to Moncada and De la Cruz (2011). Earlier researches have studied the EDA as a method for monitoring diabetes or its correlation with AN in different regions of the body (Khalfallah et al., 2012; Deanfield et al., 1980; Colmenares Guillén et al., 2015; Li at al., 2004; Nazhel et al., 2002; Rivera Farina et al., 2009; Vlckova et al., 2016; Wang et al., 2008). From the aforementioned studies, only Vlckova, Srotova and Bednarik (2016) found no correlation. More recently, Mohanraj and Narayanan (2016) found a correlation between type 2 diabetes and low GSR in males. Finally, Ionescu-Tirgoviste et al. (2018) found significant differences in the electrical activity of type 2 diabetics in certain regions of the trunk. Colombian contributions relate to the usage of bio-impedance, including EDA, as a clinical diagnostic tool (C. A. González-Correa 2018, C. H. González-Correa 2018) and novel methods to analyse EDA based on frequency-domain features (Posada Quintero et al., 2016). The aims of this study were to independently study the correlation between EDA and AN among diabetic patients, addressing new variables, and to propose a novel low-cost method to diagnose the latter.
Material and Methods
Participants were patients of the diabetic foot care program conducted between September and December 2016 by the medical service of the University of Cauca, in Popayan, Colombia. The study was approved by the Ethics Committee of the same institution. Patients were classified based on the guidelines given by Boulton et al., (2007), De Alcalá (2005), and on the expertise and physical examination of their feet, performed by the head nurse of the program. Clinical data, including gender, age, and time suffering from diabetes, were collected. Therefore 3, groups were formed: the reference group, namely participants without diabetes, diabetics out of risk, and diabetics either at risk of diabetic foot or with already visible complications (ulcerations or toes amputation).
Signal acquisition
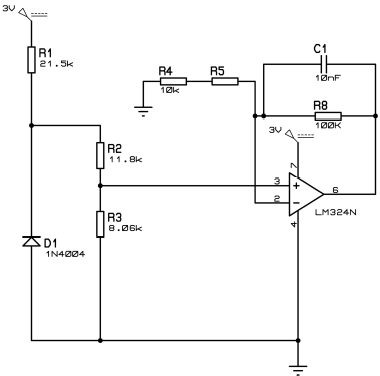
A hardware device and its software components, inspired by the circuits contained by the e-Health Arduino shield, CookingHacks (2019) and Zepeda and Mena (2014), were designed as part of the research. Apart from adding desired functionalities, the process of constructing a self-designed device was meant to remove some of the limitations observed when first exploring the e-Health shield, in which noise was the biggest obstacle. As observed, any given electronic device transmitting either wired or wirelessly, and any power outlet or cable nearby the circuit would produce enough noise to completely mask the EDA signal read by the hardware. Modifications to the designs included changing data transmission from Near Field Communication (NFC) to Bluetooth Low Energy (BLE), to allow longer distances between devices. Moreover, the CC2650 Texas Instruments micro controller was used for providing robustness and low energy consumption, in combination with the integrated circuit LM324N. The input was sampled at 4 Hz. The final hardware setup is observed in Figure 1. On the receiving side, a custom software, programmed to handle and plot the data, was written using C¥). The final prototype consisted on an 11 cm * 8 cm printed circuit board, which costed no more than USD 20. The prototype was capable of recording skin conductance for over 100 hours running on a coin battery; sending raw data wirelessly to a PC running Windows; and processing it to calculate mean values. Noise coming from near transmitting devices, power outlets or cables did no longer affect the signal in the self-designed device.
Experimental protocol
Lack of control concerning experimental variables was found in several projects (Deanfield, Daggett and Harrison 1980, Khalfallah, Ayoub, Calvet, Neveu, Brunswick, Griveau, Lair, Cassirand Bedioui2012, Rivera Farina, Perez Turiel, Gonzales, Gonzales Sarmiento, Herreros and Higuero 2009, Vlckova et al. 2016, Wang, Jia, Liang, Sun and Huang 2008). Hence, preventive measures were taken on this regard. Multiple protocols and stimuli were designed and put to test to assess them and obtain the best set of guidelines and the most reliable data. Specifically, body posture, relaxation prior to the test, resting between stimulus, placement of electrodes, and state of alertness were assessed.
First, the EDA on the sole of the foot was measured with the participant in three different postures: standing, sitting with the feet on the ground, and sitting with legs raised horizontally. The last posture reported the highest level of conductance, being eight-times the level measured while standing, and 60% higher than while sitting with no elevation of the legs. Both the relief of pressure from the measured surface and the free flow of blood (i.e. when laying horizontally instead of standing) are the reasons for the increase noticed in skin conductance. Furthermore, this applies to any specific region of measurement,thus it was analyzed and verified on the tip of the fingers, the palm of the hands and on the sole of the foot.
Change in posture also signified a reduction in the time needed to obtain a stable EDA. The first measure of 600 seconds was finally reduced to half by releasing weight on the sole of the foot and elevating the leg. With the defined basics, finding an adequate resting time between stimuli was relevant. After applying several stimuli, the time needed to recover the initial SCL was measured. As a result, a spacing of 60 seconds was defined. Concerning human error, a frame to place the electrodes always at the same distance was 3D printed. As the length of the conductor L is inversely proportional to the conductance, manually placing the electrodes would have introduced a source of human error and the recordings would not be comparable among them.
Finally, due to unexpected increases of SCL and appearances of SCRs while the patient intended to relax in a silent room with closed eyes, a method to capture the participant's attention to prevent such phenomenon was developed. After putting to test multiple techniques, such as reading an informative article and watching an emotionally neutral documentary, the most effective method for suppressing nonspecific SCRs was the task of following a white dot moving on a black screen. In this way, the researchers could recapture the participant's attention by changing the size and speed of the dot in a non-drastically way. In between the dot frames of the video, 7 stimuli covering different stimulation strategies were inserted to eliminate variance across participants due to susceptibility to one specific kind of stimulus. The final video lasted 7 minutes and 30 seconds and included 3 sudden changes of the screen color simultaneously with loud noises, 2 short 3-seconds-clips of physical injuries, and 2 messages displayed on screen asking to take a deep breath and to cough once. Thus, startle reflexes, and cognitive and physiological stimuli were assessed in this research. In summary, the final protocol started with data collection, followed by electrodes placement and 5 minutes of relaxation, while sitting comfortably on a gurney with the screen at approximately 80 cm. Next, the video was played. After it, the test concluded.
Kim et al. (2018) found that the EDA is affected by the circadian rhythm, showing variations of up to 15% from its mean value, which makes it an important factor to consider when performing multiple recordings. All participants of the present study took part in the test between 10 am and noon, that is, the period along which they were appointed for health controls. In consequence, data presented here should not contain spurious biases because of the circadian rhythm modulation.
Decomposition of the EDA
It has been shown that decomposing the EDA into SCL and SCR is not straightforward. Among the various reasons, one is related to the overlap of SCRs. If the inter stimulus interval is too short (i.e. less that 10-20 s) (Breska, Maoz and Ben-Shakhar 2011). SCRs may overlap making it difficult to discern between them.
Model-based decomposition methods with sound and physiologically inspired constraints have been proven to overcome this issue. The present study used the method proposed by (Greco et al., 2016) based on Bayesian statistics and mathematical convex optimization, while enforcing the sparsity and non-negativity of the reconstructed sudomotor nerve activity. Regarding the post analysis of the data, three values were calculated. First the mean SCL (M-SCL) was the average value between the average of the SCL of each foot. Second, the SCR (M-SCR) was the average value among the average of the SCR of the 7 stimuli of each foot. Finally, the difference between feet (DBF) was the absolute value of the difference between the M-SCL of both feet. Values of 0 represented a non-detectable change after the stimulus, or a skin conductance value below the sensitivity of the device at that moment.
Statistical Analysis
All the variables are presented as the mean ± standard deviations. Data was tested for Gaussianity with the Lilliefors test and failed to pass. Comparisons between groups were performed using the Kruskal-Wallis test followed by a post hoc test including the Bonferroni correction to reduce the family-wise error rate due to multiple comparisons. A value of p < 0,05 was used for statistical significance. Statistical analyses were performed using MATLAB R2019a.
Results
22 subjects participated in the final study: 5 of them were part of the control group (group 1), 6 were classified as out of risk diabetics (group 2) and 11 as at risk (group 3). 3 subjects were excluded from the final analysis due to erroneous data caused by a device failure at the moment of the test. Age correlated well with classification between groups 2 and 3 (4,4 ± 4,43 years vs 11,64 ± 7,66 years, p < 0,05).
Differences in reaction to stimuli among subjects were found. In Figure 2, a higher response towards loud unexpected noises is evident (stimuli correspondent to vertical dashed lines 1, 2 and 3), followed by lower responses to the other 4 stimuli. In contrast, Figure 3 shows a participant with particularly high sensitivity when deep breathing (sixth dashed line). This heterogeneity was present among all groups.
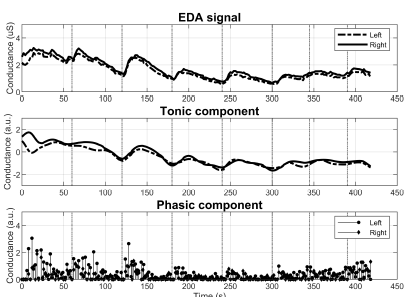
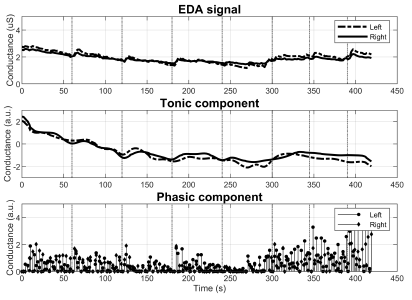
Patients belonging to group 2 frequently presented an enlarged DBF. In the top graph of Figure 4, notice the gap between the baseline of the feet. This difference is not present in the tonic component (middle graph) as the algorithm receives as input the Z-transform of the EDA signal.
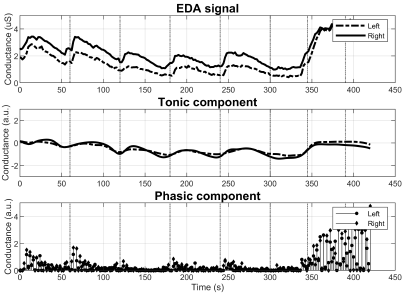
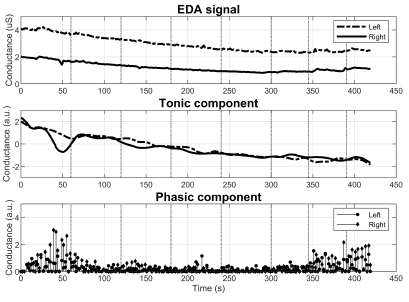
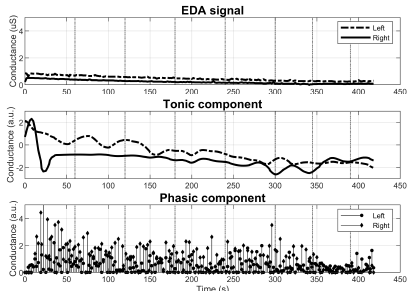
With respect to group 3, the differences in the EDA behavior are noticeable at plain sight. Figure 5 reports a subject with diminished M-SCR, but preserved M-SCL. Figure 6 illustrates abnormality in both metrics. More specifically, the signal starting at 0,7 µS contains no visible SCRs regardless of the 7 stimuli (top graph). Notice as well the estimated phasic component losing its sparsity as a result of the abnormality.
As shown in Table 1, diabetic participants reported both a diminished M-SCL and M-SCR when compared to the reference group. Numerically, group 2 had in average a 351% lower M-SCL and 55% lower M-SCR to stimuli. Additionally, group 3 presented 236% and 79% lower numeric values for these same two groups of variables.
Table 1 Comparison of the metrics between groups
| Characteristics | Reference group (n = 5) | Diabetics out of risk ( n = 6) | Diabetics at risk (n = 11) |
| Age | 42,80 ± 18,34 | 54,33 ± 18,37 | 63,45 ± 8,72 |
| TWD | 0 ± 0 | 4,40 ± 4,43 | 11,64 ± 7,66 |
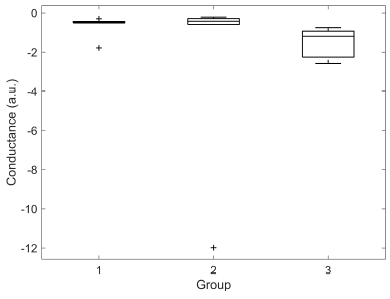
| M-SCL | -0,66 ± 0,55 | -2,32 ± 4,74 | -1,56 ± 0,74 |
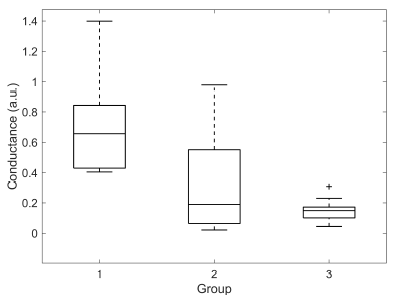
| M-SCR | 0,73 ± 0,36 | 0,33 ± 0,37 | 0,15 ± 0,08 |
| DBF | 0,23 ± 0,32 | 1,50 ± 3,10 | 1,66 ± 1,63 |
Abbreviations: TWD, time with diabetes; MSCL, mean skin conductance level; MSCR, mean skin conductance response; DBF, difference between feet. Age and TWD are in years, the rest in arbitrary units.
Source: Authors
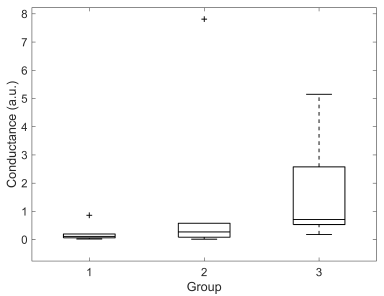
Significant difference was found for M-SCR between groups 1 and 3 (0,73 ± 0,36 vs 1,66 ± 1,63, p < 0,01). M-SCLs of groups 2 and 3 were significantly different (-2,32 ± 4,74 vs -1,56 ± 0,74, p < 0,05). DBF was significantly different between groups 1 and 3 (0,23 ± 0,32 vs 1,66 ± 1,63, p < 0,05). No other significant differences were found. Box plots illustrating the distributions for each group of M-SCL, M-SCR and DBF are presented in Figures 7, 8 and 9, respectively.
Discussion
Several stimuli techniques have been assessed in scientific literature (Deanfield et al. 1980, Khalfallah et al. 2012, Nazhel, Yetkin, Irkec and Kocer 2002, Rivera Farina et al. 2009, Vlckova et al. 2016, Wang et al. 2008). Most of them have come to the same conclusion: skin conductance does differ regarding autonomic neuropathy diagnose. For instance, a correlation was found between ulcerations in the feet and the voltage amplitude of the response to startle reflexes (Deanfield et al. 1980). In another research, current through the skin was induced and measured by means of electrochemical reactions (Khalfallah et al. 2012). Their investigation lead to a positive correlation between EDA and AN. In a study by Wang et al. (2008), diabetic patients were associated with higher latency between initiation of the stimulus and waves N and P. However, other study by Vlckova et al. (2016) did not associate lower voltage amplitude nor longer latency with patients diagnosed either with autonomic neuropathy or polyneuropathy.
Although EDA has been extensively studied due to its non-invasive qualities, it only informs about the direct current (DC) response of the body. Its alternating current (AC) counterpart is called bio-impedance analysis (BIA). Phase angles measured at different frequencies using the BIA proved to be useful features when classifying diabetes mellitus in age and sex-matched groups (Jun et al., 2018). For the same group, however, EDA showed marginal results (S. Kim et al., 2019). Compared to this study, measurement protocol of the EDA included both feet and hands, lasted 5 minutes and was repeated 30, 60, 90 and 120 minutes after meal intake. While taking into account glucose variation, subjects were asked to stand still and no intended stimuli were presented during each 5-minutes recording session. As such, the recorded data may be the combination of SCL information and spurious non-specific SCRs, making them highly variable. Mohanraj and Narayanan (2016) studied males with type 2 diabetes during the change from a supine to a standing position. In this study, significantly different EDA was found between diabetics and age and sex-matched controls. In line with our results, studies show that M-SCL by itself is not enough to discriminate between the control group and either of the two other groups (p > 0,05) here described. Statistical difference exists only when event-related SCRs are included.
To the best of our knowledge, researches fail to evaluate at once all kinds of stimulation and to assess external variables, since EDA is shaped by multiple other factors such as emotions, alertness and distress. There are several potential reasons for researchers failing to agree on the correlation between EDA, diabetes and autonomic neuropathy. Studies do not take into account the state of alertness, fail to capture the attention of the participant during the whole experiment or do not stimulate the subject at all. Therefore, emotional factors concerning or not the experiment might get involved (e.g. stress due to a possible bad outcome or boredom). Moreover, reasons have been given for one or another type of stimulation. Those in favor of intrinsic ones (e.g. Valsava Maneuver or deep breathing) argue that secondary health issues appear if the stimulus comes from outside, such as deficiencies in the autonomous efferent pathways (Rivera Farina et al. 2009). Thus, it was of paramount priority to conduct a test capable of gathering data regarding all the aforementioned stimuli under a single experiment.
One possible explanation for the variation in the 7 stimuli is the natural consequences of aging. It is well known that diabetes prevails among the elderly, and even though patients were asked to use glasses, and the volume of the computer was adjusted for those with reported hearing loss, decay in reflexes may affect the intended outcome of the stimulus. Similarly, elderly participants might be less prone to be stimulated by videos or crude scenes. While younger participants may become less agitated when asked to take a deep breath or to cough, and more aroused when disturbed by an unexpected loud noise.
Limitations
The study presented some limitations on its scope. First, even though the average age was similar among groups, the population sample was not big enough to stratify it based on it. Hence, decay in SCL and SCR inherent to aging could not be studied. Second, data regarding blood sugar level, weight, and other factors associated with diabetic foot were not gathered for all the participants. Thus, a more exhaustive correlation analysis could not be made.
Further research should be conducted applying the here exposed diversity of stimuli, in order to study in depth the characteristics and responses provoked by each one, while increasing at the same time the study population to conduct a deeper analysis based on age, sex and diabetes duration. Future clinical tests will serve as well to study the necessary modifications in order to comply with FDA standards.
Conclusion
A portable low-cost custom device for the acquisition of EDA was developed. It was then used to study the behavior of EDA and an experimental protocol accounting for a variety of stimuli was designed. This may allow health centers and clinics to apply an early screening where known but costly tests cannot be afforded, either because they are disposable and thus become costly for large populations, or because they are too expensive for the available budget.
A total of 22 subjects participated in a preliminary study to test the device. Lower M-SCR and higher DBF were associated with patients classified as at risk of AN. Lower M-SCL may not be directly associated with AN, although it could be taken into account when analyzed by a doctor. Finally, the abnormal state of only one of the above variables might indicate the beginning of AN.