Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad de Ingeniería Universidad de Antioquia
Print version ISSN 0120-6230
Rev.fac.ing.univ. Antioquia no.68 Medellín July/Sept. 2013
ARTÍCULO ORIGINAL
Passive sampling in the study of dynamic and environmental impact of pesticides in water
Muestreadores pasivos en el estudio de la dinámica de plaguicidas y el impacto ambiental en el agua
Jhon F. Narváez V. 1*,Carlos A. López2, Francisco J. Molina P.1
1Grupo de Investigación en Gestión y Modelación Ambiental-GAIA. Facultad de Ingeniería, Universidad de Antioquia A.A. 1226. Calle 67 N° 53-108. Medellín, Colombia.
2Laboratorio Análisis de Residuos. Instituto de Química. Universidad de Antioquia A.A. 1226. Calle 67 N° 53-108. Medellín, Colombia.
*Autor de correspondencia: teléfono: + 57 + 4 + 219 65 63, fax: + 57 + 4 + 219 65 68, correo electrónico: jhon.narvaez@udea.edu.co (J. Narvaez)
(Recibido el 9 de agosto de 2012. Aceptado el 5 de agosto de 2013)
Abstract
Pesticides are the most applied substances in agricultural activities which can contaminate water bodies by direct or indirect discharge, but large volumes and natural transformation processes can decrease the concentration of these substances and their degradates in watershed. Currently, conventional extraction methods such as: solid phase extraction (SPE) and solid phase micro extraction (SPME) among others do not permit low detection limits. However low levels of pesticides and degradates could produce chronic toxicity in different species. Nowadays, passive sampling is widespread used for monitoring pesticides and for ensuring the water quality and bioaccumulation studies due to this methodology allows the detection of pollutant from parts per quadrillion (ppq). The most popular membranes used in passive sampling are the semipermeable membrane devices (SPMD), which permit the concentration of lipophilic substances and the polar organic chemical integrative sampler (POCIS), which permits concentration of the hydrophilic ones. This review is about the application of passive samplers in pesticides analysis, the importance of these devices in the bioaccumulation studies and the evaluation of the ecotoxicological risks. Finally, passive sampling allows reducing costs, time and the amount of organic solvent used which classifies it within the environmental trends of ''green analytical chemistry''.
Keywords: Persistence, xenobiotics, metabolites, passive sampling, SPMD, POCIS and green analytical chemistry
Resumen
Los plaguicidas son las sustancias con mayor aplicación en la agricultura las cuales pueden contaminar los cuerpos de agua por descarga directa o indirecta, pero los grandes volúmenes y los procesos transformación pueden disminuir la concentración de estas sustancias y sus productos de degradación en las cuencas. Actualmente, los métodos convencionales de extracción tales como: la extracción en fase sólida (SPE) y la micro extracción en fase sólida (SPME) entre otras, no permiten bajos límites de detección, sin embargo bajos niveles de pesticidas y productos de degradación podrían producir toxicidad crónica en diferentes especies. Actualmente el muestreo pasivo es ampliamente usado en el monitoreo de plaguicidas y para el aseguramiento de la calidad del agua debido a que esta metodología es aplicada in estudios de bioacumulación y deteccion a partes por cuatrillón (ppq). Las membranas con mayor uso en el muestreo pasivo son las membranas tipo SPMD (Semi-permeable membrane devices), las cuales permiten la concentración de sustancias lipofílicas y las membranas tipo POCIS (Polar organic chemistry integrative sampler), las cuales permiten concentrar sustancias hidrofílicas. Esta revisión cubre la aplicación de los muestreadores pasivos en el análisis de plaguicidas, en los estudios de biacumulación y en la evaluación de los riesgos ecotoxicológicos. Finalmente los muestreadores pasivos permiten reducir costos, el tiempo de concentración y la cantidad de solventes orgánicos empleados en el tratamiento de muestra, lo que conduce a su clasificación dentro de las tendencias de la ''química analítica verde''.
Palabras clave: Persistencia, xenobióticos, metabolitos, muestreadores pasivos, SPMD, POCIS y química analítica verde
Introduction
Population growth and pesticides used in the agriculture have increased pollution of water bodies. Those substances can arrive to the water from crops or by direct discharge in water bodies where pesticides are transformed by biodegradation, photodegradation, chemical hydrolysis and other processes. However, metabolites may have higher toxicity than the parent compounds [1-2]. In Colombia, some pesticides such as chlorpyrifos, diazinon and mancozeb are widespread used in agricultural activities, but in many cases the presence in water of this substances and their degradates 3,5,6-trichloro-2-pyridinol (TCP), 4-hydroxy- 2-isopropyl-6-methylpyrimidine (IMHP) and ethylene thiourea (ETU) respectively is still unknown.
Recent studies have found that low levels of xenobiotics in the water could produce endocrine disruption, carcinogenicity and teratogenicity. Some pesticides such as organochlorines and some metabolites such as ETU are considered endocrine disruptors [3-4].
Detection of metabolites and parent compounds are one of the major problems in the ecotoxicity and dynamic study of these substances. As long as conventional methods do not allow detection of these xenobiotics, their environment impact will be unknown. Techniques such as GC and HPLC represent only a small percentage of the analytical success compared with the larger part represented by separation methods and sampler treatments [5]. Although some conventional methods such as solid-phase micro extraction (SPME), solid phase extraction (SPE), Matrix solid-phase dispersion (MSPD), liquid-liquid extraction, soxhlet extraction, microwave/ sonication-assisted extraction, pressurized liquid extraction are widespread used to concentrate pesticides and their metabolites from water samples, in many cases the low levels of these substances are undetectable by those methods [6].
Passive sampling is a new methodology used in monitoring pharmaceutical and personal-care products, metabolites, pesticides and heavy metals in aquatic ecosystems, because it can concentrate different substances found in very low concentrations in water bodies. Some countries such as USA, Canada and European countries have used the passive sampling in pesticides' monitoring. Only Chile and Brazil in South America have used these methodologies. Passive sampling has been used for more than two decades; the first work about passive sampling for organic micro pollutants in water was published in 1987. This methodology allows a holistic understanding of the behavior of pesticides in aquatic ecosystems and allows obtaining values of the time-weighted average (TWA) for different substances [7].
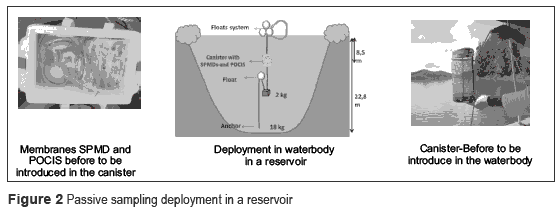
The SPMDs are membranes used in monitoring pesticides or other substances with log Kow between 4 and 8 (lipophilic organic compounds), as organochlorines and organophosphorus pesticides. On the other hand, the POCIS are membranes used widely in monitoring polar organic compounds which log Kow < 4 such as degradates, some antibiotics and other pharmaceutical compounds [8-9]. These devices can be located at key points in bodies of water (e.g. effluent treatment plants or tributary streams), thus substances present in water are transported across the membrane of the device by diffusion and finally the substances are retained by packed phase present on the membranes.
This paper discusses the importance of passive sampling in ecotoxicological analysis, and explains some basic topics, such as selecting and implementing passive samplers in water bodies. Furthermore, this paper shows the importance of passive sampling in pesticides' monitoring in Colombia. These substances are widely used in agricultural activities, for this reason it is a priority to evaluate the ecotoxicological impact and human risk of pesticides and metabolites in water bodies and especially in drinking water supplies [10].
Passive sampling: basic principles
Passive sampling includes any technique that enables the free transfer of substances in water, soil and air into a collector medium [11]. Therefore aquatic and terrestrial organisms are considered passive samplers because they concentrate different substances by free transfer.
The passive sampler has a packed phase that interacts with the analytes and permits their absorption in equilibrium with the concentration in the water body [12]. The passive samplers are membranes in which Fick's law is applied to estimate the diffusion of analytes. The analytes diffuse from higher concentration to lower concentration in a time (t) when the flux is linear and the membrane efficiency is 100%. Equation 1 describes the diffusion process:
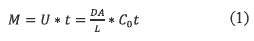
Where
• M: Mass of a substance transported
• U: Diffusive transport rate (mol/s).
• D: Molecular diffusion coefficient of the analyte (cm2/s).
• A: Cross section of the diffusion path (cm2).
• L: Total length of the diffusion path (cm).
• Co: Analyte concentration in the medium (mol/cm3).
• t: Diffusion time (s).
Therefore, DA/L is the flux (cm3/s). In some literature it is known as Rs and is related to the temperature and biofouling in the water body [13]. These conditions determine the device efficiency and the analyte losses by leaching. For this reason, often it is necessary to calibrate each sampler [14-16].
Through calibration, the analyst determines the field sampling time in order to determine how much time the passive sampler should remain in the water for detecting the possible analyte concentration. Furthermore, analyte losses by photodegradation or by leaching are controlled injecting performance reference compounds (PRC). These PRC allow finding the correction factor for the result (i.e. pesticides level in the water). Many substances monitored by SPMDs are susceptible to ultraviolet-A (UVA) and ultraviolet-B (UVB) due to the UV transparency of the SPMDs [10]. Some PRC such as the PAHs are used in studies of photodegradation processes. These are quantified at the beginning and at the end of the sampling time. The percentage loss of PAHs will be considered as the correction factor. On the other hand, PRCs like PBCs are commonly used in the detection of analyte losses during the fugacity process. For example, PCBs congeners 14, 29, and 50 are often used as PRCs since they do not occur in the environment under natural conditions [17].
The obtained correction factors are used to find the sampling rate (Rs). This value is fundamental to determine the concentration of pesticides monitored in the water. The models include the variables: log Kow , the PRC's release rate constant (Ke) and SPMD-water partition coefficient (Ksw) to find the Rs and, finally, the pesticide concentration [18]. Equation 2 shows the calculation of concentration:

Where:
• Cw: Chemical concentration in the water (ng/L).
• N: Amount of the chemical accumulated by the sampler (typically in ng).
• Rs: Sampling rate (L/d)
• t: Exposure time (d).
Currently, the U.S. Geological Survey (USGS) has two free versions in Microsoft Excel on their website to calculate the Rs [19].
Some factors, such as temperature, dissolved organic carbon and the flux, are related to the calculated Rs. Therefore Rs takes a specific value for each monitoring place [18].
Membranes used for pesticides monitoring and other pollutants
Passive sampler membranes can have ionic, lipophilic and hydrophilic packed phases which ensure the interaction between the membrane and the analytes present in the water. Some polyethylene membranes with organic internal solvent (TRIMPS) are adequate for capture of lipophilic pesticides such as endosulfan and chlorpyrifos [20]. However, polar pesticides and their degradates are not retained by this type of membrane. Therefore, packed cellulose and enzymatic inhibitors have been incorporated into polyethylene membranes for sampling water-soluble xenobiotics. Enzymatic inhibitors are added to increase resistance to biodegradation of cellulose which are known as passive sampling stained-cellulose or CIDS, which have shown stability for 21 days [21].
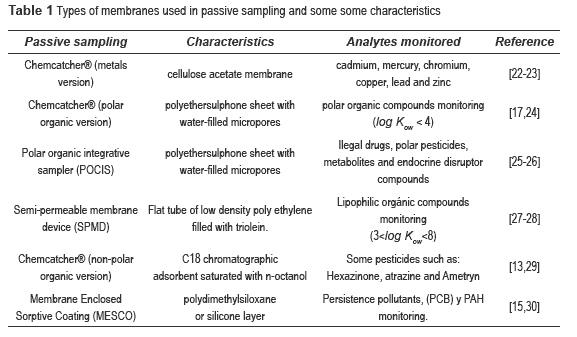
Some commonly used membranes are shown in the table 1.
SPMDs and POCIS are the most popular membranes used for monitoring of pesticides and their degradate due to their affinity for metabolites and parent compounds. For example, substances with log Kow between 1-8 can be easily monitored using SPMD s and POCIS [31-32].
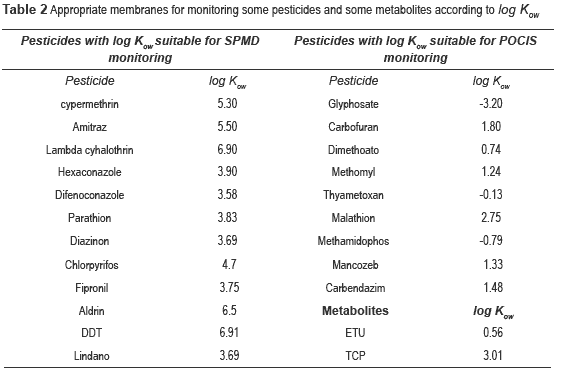
For the optimal membrane selection, log Kow is considered as an important physicochemical property due to necessary affinity between the membrane and the analytes. For example, triolein as packed phase in SPMDs absorbs lipophilic substances while the packed phase polyether sulfone used in POCIS absorbs polar pesticides. Table 2 shows the appropriate membrane (SPMDs or POCIS) for monitoring some pesticides and metabolites.
Selection of membranes number and field sampling time
An initial survey is fundamental in order to know what kinds of substances are used in a specific place. Also, log Kow, log Koc and solubility provide information about the dynamics of specific pesticides and allow selecting the appropriate membrane [33-34].
Additionally, the analyst needs to know what the frequency and quantity of pesticides applied for the study zone is and what the pesticides half-life is. These values allow estimating the pesticide concentration in the water and the amount of membranes required. Some equations allow to find the possible number of membranes necessary in the monitoring [18]. See equation 3.
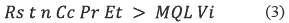
Where:
• Cc: Predicted environmental chemical concentration (ng/L).
• t: Deployment time in days.
• Rs: Sampling rate in liters of water extracted by the passive sampler per day (L/d).
• Pr: Overall method recovery for the analyte (expressed as a factor of one; therefore 0.9 is used for 90 percent recovery).
• n: Number of passive samplers combined into a single sample.
• Et: Fraction of the total sample extract that is injected into the instrument for quantification (0.001 if 1 microliter, μL, of a 1-milliliter, mL, sample is injected).
• MQL: Method quantification limit (ng/L).
• Vi: Volume of standard injection (commonly 1 μL).
As an example, for determining the amount of passive sampling for monitoring chlorpyrifos in a reservoir the analyst could apply the following steps:
1. The analyst needs to know:
• Possible quantity applied in the zone study.
• The possible quantity transformed in the time of study.
• The possible attenuation factor
• The volume of water body
2. The analyst could use the equation 4, which explains a first natural decay rates:
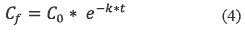
Where:
• Cf = Final concentration or final quantity (Kg).
• Co =Initial concentration or initial quantity (Kg).
• K = Degradation constant (1/d)
• t = Time (d)
Therefore, the half time in the nature is given by equation 5:

Finally the possible pesticides in water are given by equation 6:
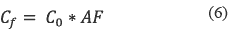
Where:
• AF = Is the attenuation factor
Equations 4, 5 and 6 are applied in order to determine the level of pesticides in water after transformation and leaching processes. In this paper, the following values were used in order to find the possible concentration of chlorpyrifos in a drinking water reservoir in Colombia:
• Quantity of application of chlorpyrifos in the zone of influence: 9.42 Kg (This value was calculated according to application frequency)
• Half time in soil: 50 days [35]
• Degradation constant: -0.0139/d(Obtained by Equation 5)
• The possible attenuation factor: 0.001
• Reservoir volume in liters: 2.5 x 107 L Time of study: 15 days
To find the quantity of chlorpyrifos in soil, It was applied the equation 4.
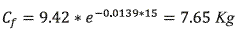
According to equation 4, in the zone of influence there are 76.49 Kg of chlorpyrifos after 15 days.
To calculate the possible quantity of chlorpyrifos in the reservoir, It was applied the equation 6.
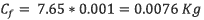
According to equation 6, 0.0076 kg ofchlorpyrifos arrives at the reservoir.
Using the reservoir volume, it was found that the possible concentration of chlorpyrifos in the water is 0.031 ng/L. This concentration is undetectable by conventional separation methods, but passive sampling methodology does detect it.
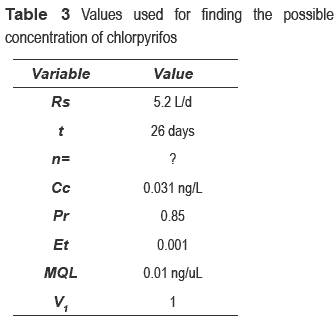
Equation 1 and the estimated chlorpyrifos concentration are used in order to find the number of passive samplings through the following values in table 3.
Results indicate that three passive samplers are necessary to detect 0.031 ng/L of chlorpyrifos in the reservoir. Also, according to log Kow of chlorpyrifos (see table 2.), SPMDs membranes could be appropriate for monitoring this pesticide.
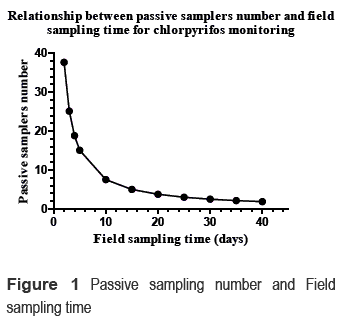
The analyst has the possibility of modifying either the number of passive samplers or the field sampling time. For example, when the analyst uses less time, he should increase the amount of passive samplers, and if the analyst uses more time, he could decrease the amount of passive samplers. Figure 1 shows the relationship between the number of passive samplers and the field sampling time.
Figure 1 shows the exponential relationship between the number of passive samplers and the field sampling time for the chlorpyrifos monitoring. According to figure 1, 25 days of monitoring are necessary using 3 passive samplers in order to determine a chlorpyrifos concentration of 0.031 ng/L in the reservoir. Similarly, according to the figure, it is possible shorten the time by using more passive samplers. As comparative methods, some conventional separation methods, such as SPE, require about 1000-1200 liters of water in order to detect this chlorpyrifos concentration with 0.01 ng/uL limit of detection (LOD).
The deployment of passive sampling is presented in the figure 2.
Processing spmds and pocis: analyte desorption
To recover the trapped analytes in the packed phase ofthe passive samplers, desorption methods are used. Some desorption methods for pesticides trapped in SPMDs and POCIS are described below [36].
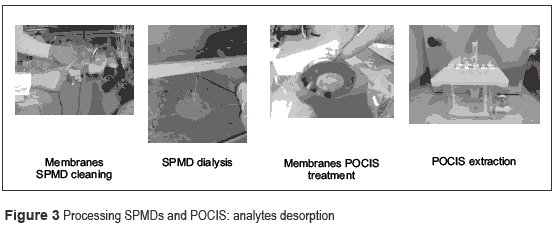
SPMDs dialysis
After elapse of the field sampling time, the passive samplers are recovered and transported to a laboratory. Each SPMD is removed from the canister and immediately cleaned with a soft toothbrush to remove biofouling and particles.
Then the SPMD membrane is immersed in dilute hydrochloric acid to remove salts and rinsed with deionized water. The cleanliness is improved with acetone and hexane [37].
The SPMD membrane needs a dialysis time to desorb the analytes trapped in the triolein packed phase. Membranes are placed in a glass container with sufficient volume of hexane at 18° C for 18 to 24 hours. Repeating this step using hexane as solvent and a minimum dialysis time of 6 hours improves the desorption process. The dialysis times may vary depending on the properties of the analytes that are extracted. Usually, a period of 18 hours followed by another 6 hours is sufficient for most analytes, though, some pesticides, such as pyrethroids, may require up to three periods of 24 hours to achieve an adequate recovery [18]. However, long dialysis times increase the number of interferences in the assay.
The hexane extract is passed through the SPE cartridge to increase the purity of the sample. This process protects the equipment and decreases the interference in GC and HPLC chromatograms. Some investigations suggest using gel permeation chromatography to remove the triolein residues in the sample, although, with a better dialysis, the last step is not needed. The extracts are finally concentrated and injected into the analysis equipment. The GC is more common in pesticide analysis after SPMD dialysis [38-41].
POCIS dialysis
After field recovery, the POCIS membranes are removed from each canister. The analyte extraction methods may vary according to available resources in the laboratory. Due to high polar characteristics of the chemicals absorbed, the extraction methods include the use of chromatographic columns or SPE cartridges. Contrary to SPMD dialysis, the POCIS membranes are opened gently on the top of chromatography column or directly on the top of the SPE cartridge. Afterward, the absorbent is washed with a suitable solvent in order to ensure the complete transfer of analytes. Methanol and water are commonly used as solvents in this procedure [36].
The combination of three solvents: methanol, toluene and dichloromethane are the most common solvent mix used for analyte extraction. The solvent proportions in this combination depend primarily on the POCIS configuration (type of absorbent) and specific substances studied. A mixture of methanol: toluene: dichloromethane can be used for pesticide analysis, while for drug analysis only methanol (40 ml) is used [18].
When analytes are volatile, for example tetrachloroethylene, methanolis notrecommended as a solvent, because it requires high temperatures of evaporation (Rota-evaporation), resulting in the loss of some volatile analytes. An alternative method includes the use of 25 mL of a mixture of dichloromethane:methyl-tert-butyl ether 8:2 (v:v) [36].
Regardless of the method of extraction of analytes from the membranes, analysts should consider evaluation of recoveries by the method applied. The passive sampling treatments in the laboratory are showed in figure 3.
Passive samplers and assessment of eco-toxicological risks
Some environmental organic pollutants have high toxicological risk on biota. Lipophilic substances can bioaccumulate in fish tissue or other aquatic organisms and trigger environmental problems related to the bio-magnification process [42-43].
Pesticide and metabolite monitoring ensure the control of exposure of aquatic organisms to these substances. Organochlorine pesticides such as DDT, PCBs and PAHs have high affinity to fish tissue because their non-polar structure. The passive sampler is an adequate monitoring device for this purpose, because the packed phase mimics the fish tissue and allows paralleling the bioaccumulation process [44]. Also, some passive samplers, such as POCIS, mimic the intake of contaminants in aquatic organisms by respiratory exposure [45].
Therefore, after the SPMD cleanup, the desorbed analytes or extract obtained can be used in toxicity bioassays such as Daphnia spp, Vibrio fischeri or Pseudokirchneriella subcapitata [46-48]. These kinds of bioassays describe the toxicity behavior for many compounds in the environment. Toxicity results can be compared with determinations by instrumental techniques. Both represent the possible bioaccumulation during the field sampling time. For example, passive sampling applied in wastewater treatment plants allows analyzing the efficiency of the removal process for the substances of interest [49]. Some substances such as PAHs and PCBs were analyzed with GC/MS in sewage plants in Asia through SPMD monitoring [50]. The results indicate a low concentration of these persistent substances in wastewater. Also, the toxicity analysis demonstrated the low reduction of toxic impact and therefore the low reduction of persistent organic compounds by conventional processes, such as pretreatment (screens, grit traps, pre-aeration), primary clarifiers, aeration tanks, and secondary clarifiers [50].
Monitoring by passive sampling is appropriate in ecotoxicity analysis for different organic substances present at low levels in water [51-52]. The passive sampling technique combined with chemical analyses and bioassays are a valuable tool for monitoring priority organic pollutants in aquatic ecosystems [53-54].
Conclusions
Some environmental problems such as endocrine disruption, carcinogenicity and teratogenicity have a major impact on global public health. Substances such as pesticides and metabolites induce chronic and acute toxicity at low concentration levels. Therefore, passive sampling is a new methodology used in some countries for monitoring pesticides, their degradates and other organic pollutants at low concentrations in the water. These methodologies provide a better assessment of environmental risk and help to improve the understanding what is the possible effect of substances to low level. Passive sampling methods such as SPMDs and POCIS have a wide range for monitoring organic pollutants in water. These membranes provide a dynamic approach and can estimate the ecotoxicity and risk factor for major substances of anthropogenic origin. Analysts should consider the physicochemical properties of specific analytes to select adequate membranes, the number and the field sampling time. Passive sampling permits evaluation of the exposure of humans and aquatic organisms to low levels of pollutants where the impact of these substances at low levels remains unknown.
Acknowledgements
The authors thank Jaime Alberto Palacio Baena (PhD) for his valuable scientific suggestions and kind help in ecotoxicological knowledge. The authors thank Santiago Alvarez and James Smith for their suggestions in English writing. Aditionally the authors thank Environmental Sampling Technologies (Est-Lab) for advice and recommendations about SPMDs and POCIS membranes. Finally, this work was supported of Sustainability program 2011-2012 at University of Antioquia.
References
1. I. Cavoski, P. Caboni, G. Sarais, T. Miano. ''Degradation and Persistence of Rotenone in Soils and Influence of Temperature Variations''. Journal of agricultural and food chemistry. Vol. 56. 2008. pp. 8066-8073. [ Links ]
2. A. Belfroid, M. Van Drunen, M. Beek, S. Schrap, C. Van Gestel, B. Van Hattum. ''Relative risks of transformation products of pesticides for aquatic ecosystems''. The Science of the total environment. Vol. 222. 1998. pp.167-183. [ Links ]
3. C. Colosio, S. Fustinoni, S. Birindelli, I. Bonomi, G. De Paschale, T. Mammone, et al. ''Ethylenethiourea in urine as an indicator of exposure to mancozeb in vineyard workers''. Toxicology letters. Vol. 134. 2002. pp. 133-140. [ Links ]
4. G. Darko, O. Akoto, C. Oppong. ''Persistent organochlorine pesticide residues in fish, sediments and water from Lake Bosomtwi, Ghana''. Chemosphere. Vol. 72. 2008. pp. 21-24. [ Links ]
5. Y. Chen, Z. Guo, X. Wang, C. Qiu. ''Sample preparation''. Journal of Chromatography A. Vol. 1184. 2008. pp. 191-219. [ Links ]
6. Nollet L. ''Handbook of Water Analysis''. Tribaldo EB. Analysis of Pesticides in Water. 2nd ed. Ed. CRC Press Taylor and Francis Group. Boca Raton, FL, USA. 2007. pp. 449-481. [ Links ]
7. B. Vrana, I. Allan, R. Greenwood, G. Mills, E. Dominiak, K. Svensson, et al. Passive sampling techniques for monitoring pollutants in water. TrAC Trends in Analytical Chemistry. Vol. 24. 2005. pp. 845-868. [ Links ]
8. C. Harman, O. Beyum, K. Tollefsen, K. Thomas, M. Grung. ''Uptake of some selected aquatic pollutants in semipermeable membrane devices (SPMDs) and the polar organic chemical integrative sampler (POCIS)''. J. Environ. Monit. Vol. 10. 2008. pp. 239-247. [ Links ]
9. C. Harman, K. Tollefsen, O. Beyum, K. Thomas, M. Grung. ''Uptake rates of alkylphenols, PAHs and carbazoles in semipermeable membrane devices (SPMDs) and polar organic chemical integrative samplers (POCIS)''. Chemosphere. Vol. 72. 2008. pp. 1510-1516. [ Links ]
10. Van der WerfHMG. ''Assessing the impact ofpesticides on the environment''. Agriculture, Ecosystems & Environment. Vol. 60. 1996. pp. 81-96. [ Links ]
11. F. Stuer. ''Review of passive accumulation devices for monitoring organic micropollutants in the aquatic environment''. Environmental Pollution. Vol.136. 2005. pp. 503-524. [ Links ]
12. T. Gorecki, J. Namiesnik. ''Passive sampling''. TrAC Trends in Analytical Chemistry. Vol. 21. 2002. pp. 276-291. [ Links ]
13. R. Greenwood, G. Mills, B. Vrana. ''Potential applications of passive sampling for monitoring non-polar industrial pollutants in the aqueous environment in support of REACH''. Journal of Chromatography A. Vol. 1216. 2009. pp. 631-639. [ Links ]
14. B. Vrana, G. Mills, E. Dominiak, R. Greenwood. ''Calibration of the Chemcatcher passive sampler for the monitoring of priority organic pollutants in water''. Environmental Pollution. Vol. 142. 2006. pp. 333-343. [ Links ]
15. G. Ouyang, J. Pawliszyn. ''Configurations and calibration methods for passive sampling techniques''. Journal of Chromatography A. Vol. 1168. 2007. pp. 226-235. [ Links ]
16. S. MacLeod, E. McClure, C. Wong. ''Laboratory calibration and field deployment of the polar organic chemical integrative sampler for pharmaceuticals and personal care products in wastewater and surface water''. Environmental Toxicology and Chemistry. Vol. 26. 2007. pp. 2517-2529. [ Links ]
17. S. Seethapathy, T. Górecki, X. Li. ''Passive sampling in environmental analysis''. Journal of Chromatography A. Vol. 1184. 2008. pp. 234-253. [ Links ]
18. D. Alvarez. Guidelines for the use of the semipermeable membrane device (SPMD) and the polar organic chemical integrative sampler (POCIS) in environmental monitoring studies. US Geological Survey, Techniques and Methods. 2010:1-D4. Available on: http://pubs.usgs.gov/tm/tm1d4/pdf/tm1d4.pdf. Accessed: April 2012 [ Links ]
19. U.S. Geological Survey. Columbia Environmental Research Center. Available on: http://www.cerc.usgs.gov/Branches.aspx?BranchId=8. Accessed: April 2012 [ Links ]
20. F. Esteve, V. Yusá, A. Pastor, M. De La Guardia. ''New perspectives in the use of semipermeable membrane devices as passive samplers''. Talanta. Vol. 74. 2008. pp. 443-457. [ Links ]
21. R. Hyne, M. Aistrope. Continuous sampling of pesticides in waterways. Centre for Ecotoxicology, Department of Environment & Conservation, NSW. Available on: http://www.irec.org.au/farmer_f/pdf_171/Continuous%20sampling%20of%20pesticides%20in%20waterways.pdf. Accessed: April 2012. [ Links ]
22. R. Aguilar, M. Gómez, R, Greenwood, G. Mills, B. Vrana, M. Palacios. ''Application of Chemcatcher passive sampler for monitoring levels of mercury in contaminated river water''. Talanta. Vol. 77. 2009. pp. 1483-1489. [ Links ]
23. R. Aguilar, R. Greenwood, G. Mills, B. Vrana, M. Palacios, M. Gomez. ''Assessment of Chemcatcher passive sampler for the monitoring of inorganic mercury and organotin compounds in water''. International Journal of Environmental and Analytical Chemistry. Vol. 88. 2008. pp. 75-90. [ Links ]
24. R. Schäfer, A. Paschke, B. Vrana, R. Mueller, M. Liess. ''Performance of the Chemcatcher passive sampler when used to monitor 10 polar and semi-polar pesticides in 16 Central European streams, and comparison with two other sampling methods''. Water research. Vol. 42. 2008. pp. 2707-2717. [ Links ]
25. E. Bailly, Y. Levi, S. Karolak. ''Calibration and field evaluation of polar organic chemical integrative sampler (POCIS) for monitoring pharmaceuticals in hospital wastewater''. Environmental Pollution. Vol. 174. 2013. pp. 100-105. [ Links ]
26. A. Arditsoglou, D. Voutsa. ''Passive sampling of selected endocrine disrupting compounds using polar organic chemical integrative samplers''. Environmental Pollution. Vol 156. 2008. pp. 316-324. [ Links ]
27. F. Esteve, A. Pastor, V. Yusa, M. De La Guardia. ''Using semi-permeable membrane devices as passive samplers''. TrAC Trends in Analytical Chemistry. Vol. 26. 2008. pp. 703-712. [ Links ]
28. Y. Wang, Z. Wang, J. Liu, M. Ma, N. Belzile.''Monitoring priority pollutants in the Yanghe River by dichloromethane extraction and semipermeable membrane device (SPMD)''. Chemosphere. Vol. 39. 1999. pp. 113-131. [ Links ]
29. M. Shaw, M. Furnas, K. Fabricius, D. Haynes, S. Carter, G. Eaglesham, et al. ''Monitoring pesticides in the Great Barrier Reef''. Marine Pollution Bulletin. Vol. 60. 2010. pp. 113-122. [ Links ]
30. J. Namiesnik, B. Zabiegala, A. Kot, M. Partyka, A. Wasik. ''Passive sampling and/or extraction techniques in environmental analysis: a review''. Analytical and bioanalytical chemistry. Vol. 381. 2005. pp. 279-301. [ Links ]
31. J. Huckins, D. Alvarez. Semipermeable membrane devices (SPMD). Columbia Environmental Research Center. Available on: http://www.cerc.usgs.gov/pubs/center/pdfDocs/SPMD.pdf. Accessed: April 2012. [ Links ]
32. J. Huckins, D. Alvarez. Polar Organic Chemical Integrative Sampler. Columbia Environmental Research Center. Available on: http://www.cerc.usgs.gov/Content/UploadedFiles/ExternalDocs/POCIS.pdf. Accessed: April 2012. [ Links ]
33. T. Reemtsma, M. Jekel. Organic Pollutants in the Water Cycle: Properties, Occurrence, Analysis & Environmental Relevance of Polar Compounds. Ed. Thorsten Reemtsma and Martin Jekel. Berlin, Germany. 2006. pp. 2-59. [ Links ]
34. C. Harman, S. Brooks, R. Sundt, S. Meier, M. Grung. ''Field comparison of passive sampling and biological approaches for measuring exposure to PAH and alkylphenols from offshore produced water discharges''. Marine Pollution Bulletin. Vol. 63. 2011. pp. 141-148. [ Links ]
35. IUPAC. Global availability of information on agrochemicals. Available on: http://sitem.herts.ac.uk/aeru/iupac/154.htm. Accessed: April 2012. [ Links ]
36. N. Fork. Investigation of Organic Chemicals Potentially Responsible for Mortality and Intersex in Fish of the North Fork of the Shenandoah River, Virginia, during Spring of 2007. Available on: http://pubs.usgs.gov/of/2008/1093/pdf/0FR2008-1093.pdf. Accessed: April 2012. [ Links ]
37. G. Liu, G. Zhang, J. Li, X. Li, X. Peng, S. Qi. ''Spatial distribution and seasonal variations of polycyclic aromatic hydrocarbons (PAHs) using semi-permeable membrane devices (SPMD) and pine needles in the Pearl River Delta, South China''. Atmospheric Environment. Vol. 40. pp. 3134-3143. [ Links ]
38. N. Felsvik, E. Brevik, J. Berge. ''Monitoring of organotin compounds in seawaterusing semipermeable membrane devices (SPMDs)—tentative results''. J Environ Monit. Vol. 2. 2000. pp. 281-284. [ Links ]
39. S. Goodbred, W. Bryant, M. Rosen, D. Alvarez, T. Spencer. ''How useful are the ''other'' semipermeable membrane devices (SPMDs); the mini-unit (15.2cm long)?''. Science of the Total Environment. Vol. 407. 2009. pp. 4149-4156. [ Links ]
40. L. Setková, J. Hajslová, P. Bergqvist, V. Kocourek, R. Kazda, P. Suchan. ''Fast isolation of hydrophobic organic environmental contaminants from exposed semipermeable membrane devices (SPMDs) prior to GC analysis''. Journal of Chromatography A. Vol. 1092. 2005. pp. 170-181. [ Links ]
41. D. Sabaliunas, J. Ellington, I. Sabaliuniene. ''Screening bioavailable hydrophobic toxicants in surface waters with semipermeable membrane devices: role of inherent oleic acid in toxicity evaluations''. Ecotoxicology and Environmental Safety. Vol. 44. 1999. pp. 160-167. [ Links ]
42. F. Cid, R. Antón, E. Caviedes. ''Organochlorine pesticide contamination in three bird species of the Embalse La Florida water reservoir in the semiarid midwest of Argentina''. Science of the Total Environment. Vol. 385. 2007. pp. 86-96. [ Links ]
43. L. Kalyoncu, I. Agca, A. Aktumsek. ''Some organochlorine pesticide residues in fish species in Konya, Turkey''. Chemosphere. Vol. 74. 2009. pp. 885-889. [ Links ]
44. V. Yusá, A. Pastor, M. Guardia. ''Microwave-assisted extraction of OCPs, PCBs and PAHs concentrated by semi-permeable membrane devices (SPMDs)''. Analytica chimica acta. Vol. 540. 2005. pp. 355-366. [ Links ]
45. D. Alvarez, P. Stackelberg, J. Petty, J. Huckins, E. Furlong, S. Zaugg, et al. ''Comparison of a novel passive sampler to standard water-column sampling for organic contaminants associated with wastewater effluents entering a New Jersey stream''. Chemosphere. Vol. 61. 2005. pp. 610-622. [ Links ]
46. M. Isidori, M. Ferrara, M. Lavorgna, A. Nardelli, A. Parrella. ''In situ monitoring of urban air in Southern Italy with the Tradescantia micronucleus bioassay and semipermeable membrane devices (SPMDs)''. Chemosphere. Vol. 52. 2003. pp. 121-126. [ Links ]
47. D. Sabaliunas, A. Sodergren. ''Use of semi-permeable membrane devices to monitor pollutants in water and assess their effects: A laboratory test and field verification''. Environmental Pollution. Vol. 96. 1997. pp. 195-205. [ Links ]
48. C. Gourlay, C. Miége, A. Noir, C. Ravelet, J. Garric, J. Mouchel. ''How accurately do semi-permeable membrane devices measure the bioavailability of polycyclic aromatic hydrocarbons to Daphnia magna?''. Chemosphere. Vol. 61. 2005. pp. 1734-1739. [ Links ]
49. B. Tan, D Hawker, J. Müller, F. Leusch, L. Tremblay, H. Chapman. ''Comprehensive study of endocrine disrupting compounds using grab and passive sampling at selected wastewater treatment plants in South East Queensland, Australia''. Environment international. Vol. 33. 2007. pp. 654-669. [ Links ]
50. C. Wang, Y. Wang, F. Kiefer, A. Yediler, Z. Wang, A. Kettrup. ''Ecotoxicological and chemical characterization of selected treatment process effluents of municipal sewage treatment plant''. Ecotoxicology and Environmental Safety. Vol. 56. 2003. pp. 211-217. [ Links ]
51. D. Sabaliunas, J. Lazutka, I. Sabaliuniene. ''Acute toxicity and genotoxicity of aquatic hydrophobic pollutants sampled with semipermeable membrane devices''. Environmental Pollution. Vol. 109. 2000. pp. 251-265. [ Links ]
52. R. Muller, J. Tang, R. Thier, J. Mueller. ''Combining passive sampling and toxicity testing for evaluation of mixtures of polar organic chemicals in sewage treatment plant effluent''. J Environ Monit. Vol. 9. 2007. pp. 105-110. [ Links ]
53. S. Bonetta, E. Carraro, C. Pignata, I. Pavan, C. Romano, G. Gilli. ''Application of semipermeable membrane device (SPMD) to assess air genotoxicity in an occupational environment''. Chemosphere. Vol. 75. 2009. pp. 1446-1452. [ Links ]
54. E. Vermeirssen, N. Bramaz, J. Hollender, H. Singer, B. Escher. ''Passive sampling combined with ecotoxicological and chemical analysis of pharmaceuticals and biocides-evaluation of three Chemcatcher (TM) configurations''. Water research. Vol. 43. 2009. pp. 903-914. [ Links ]