Introduction
Turpentine is a well-known essential oil extracted by distillation from pine oleoresin1; It is widely used as a solvent in chemical industries, resins, and as an ingredient in paints2. Many perfumes contain products obtained from α-pinene and β-pinene3, the main constituents of turpentine. Through acid-catalyzed hydration, these compounds yield a complex mixture of monoterpenes, alcohols, and hydrocarbons with a wide industrial application. Some of the reaction products are α-terpineol, limonene, terpinolene, camphene, γ-terpinene, α- and β-fenchol4. Among these products, α-terpineol is the most important monoterpene alcohol used in the production of fragrances; α-terpineol also exhibits antimicrobial activity and is used for wound healing and the treatment of insect bites5. α-Terpineol can be obtained from the hydration of limonene, α- and β-pinene over an acid catalyst (Scheme 1) (6-8 that transfers a proton to the double bond of the alkene, forming a carbocation intermediate that reacts with water to form a protonated alcohol. The loss of H+ from the protonated alcohol generates the neutral product and the recovery of the catalyst9. α-Terpineol has been produced by the hydration of α-pinene with acid catalysts that leads to a complex mixture of monoterpenes (alcohols and hydrocarbons) (10.
The isomerization and hydration of α-pinene by homogeneous catalysts have been studied since 1947. The acid isomerization of α-pinene using 1-chloro-4-naftalen sulfonic acid was studied; α-pinene was dried over sodium and refluxed with sulfonic acid for 6 h, obtaining a yield of 30 % towards monocyclic terpenes such as β-pinene, limonene, terpinolene, and α-terpinene11. Oxalic and chloroacetic acid for the transformation of α-pinene produced a conversion of 80 % with a selectivity of 70 % to α-terpineol after 4 h at 70°C12. HPW12O40 as a catalyst and mixtures of acetic acid and water as a solvent were used for hydrating limonene, β-pinene, and α-pinene; all these substrates gave α-terpineol as the main product with a mixture of acetic acid and water (90/10 v/v) at 25 °C6; after 3 h of reaction, α-terpineol selectivity was near to 43 % and α-pinene conversion was 90 %; the conversion of β-pinene was 92 % with a selectivity of 42 % to α-terpineol at 2 h, while after 2.5 h of reaction, the limonene conversion and selectivity to α-terpineol were 7 % and 50 %, respectively. A yield of 67 % of α-terpineol using 15 % aqueous sulfuric acid and an excess of acetone (80-85 °C, 4 h) was reported3. α- Hydroxycarboxylic acid composite with boric acid produced α-pinene conversion of 96.1 % and selectivity to α-terpineol of 55.5 %13. Formic acid and sulfuric acid yielded 54 % of α-terpineol using turpentine14; a similar yield was obtained with phosphoric acid and acetic acid15. Furthermore, sulfuric acid in the presence of an ionic liquid was reported for the production of α-terpineol16. These homogeneous methods have some disadvantages, such as corrosion problems and the complexity of the final disposal of reaction residues.
α-Terpineol can also be obtained through the hydration of α-pinene over heterogeneous catalysts, which is a cleaner alternative because of the easier separation of solid catalysts from the reaction mixture. H-beta zeolite as a catalyst for the hydration and isomerization of α-pinene gave monocyclic terpenes and alcohols with α-terpineol as the main product with a selectivity of up to 48 %17. Zeolites dispersed on polymeric membranes for α-pinene hydration achieved 100 % conversion, and α-terpineol selectivity between 50 % to 70 %, but it required 150 h of reaction18,19. Zeolite catalysts for hydration reaction was reported with 100 % conversion and 57 % selectivity to alcohols, including α-terpineol20. Impregnated trichloroacetic acid (TCA) on different supports such as silica (TCA/SiO2), titania (TCA/TiO2), and zirconia (TCA/ZrO2·nH2O) as catalysts were reported; α-pinene conversion of 57 % and selectivity of 75 % of total alcohols, with 57 % selectivity to α-terpineol over TCA/ZrO2·nH2O were obtained21. The hydration reaction of α-pinene in the presence of natural clays (bentonite) treated with monochloroacetic acid as a catalyst produced oxygenated compounds at 80 °C, and achieved a conversion of around 80 % with selectivity toward oxygenated compounds of around 70 %, with α-terpineol as the main component 5. A sulfonated carbonaceous material was used with a 52.2 % yield to α-terpineol using α-pinene with almost complete conversion22.
To carry out the hydration reaction of alkenes, the use of organic solvents was necessary; α-pinene, for example, is poorly soluble in water even at elevated temperatures (up to 100 °C) and forms a heterophase mixture23. Among the solvents that have been used, acetic acid6, acetone(3, 18), 1,4-dioxane20, and ethanol24 can be identified. Direct hydration of α-pinene in a pilot-scale jet reactor was reported25 using a mixture of isopropyl alcohol/ water as a solvent and Amberlyst-15 as a catalyst with 86 % conversion and 43 % selectivity toward α-terpineol (70 °C, 4 h). Amberlyst-15 is a commercially available catalyst that is easy to handle and that has been used in hydration26,27, esterification28, and hydrolyzation29 reactions. In this research, Ambelyst-15 commercial ion exchange was used to obtain α-terpineol under green conditions from α- and β-pinene, limonene, and turpentine. Results are significant since turpentine is a cheap raw material for hydration, and the final concentration of α-terpineol was the same as that using α-pinene as raw material.
Experimental section
2.1. Materials
Raw materials used in this research were (1S)-(-)-α-pinene (98 % w/w), (1S)-(-)-β-pinene (98 % w/w), S-(-)-limonene (96 % w/w), α-terpineol (90 % w/w, technical grade), and Amberlyst-15, supplied by Sigma Aldrich; 2-propanol (100 % w/w), acetonitrile (99.5 % w/w), and ethyl acetate (99.5 % w/w) supplied by J. T. Barker, and tert-Butanol (99.5 % w/w) which was purchased from Merck. Commercial turpentine oil with 50 % w/w of α-pinene, 40 % w/w of β-pinene, and 2.7 % w/w of limonene was obtained from a local market.
2.2. Hydration reactions
Initially, hydration reactions were performed with pure components (α-pinene, β-pinene, and limonene) in 2 mL closed vessel reactor consisting of a vial covered with inert silicon septum equipped with a magnetic stirrer, immersed in an oil bath, which temperature was controlled with a Corning® hot plate Model PC-420D equipped with a temperature and stirring speed controller. In a regular experiment, the temperature was set up at 70 °C with a catalyst loading of 15 % w/w of total reactants; 1.5 mmol of terpene, and a terpene:water:2-propanol mass ratio of 1:1:2. The samples were kept under constant stirring (1150 rpm) during the hydration process. The reaction products were analyzed by gas chromatography at regular intervals.
Reusing of the catalyst was carried out by washing the solid with ethanol and drying it at 50 °C before its reuse.
2.2. Analysis and quantification
Reaction products were analyzed using gas chromatography (7890 N, Agilent) with a FID detector. Pure α-terpineol was identified by comparison with the standard sample and the other substances by GC-MS. The chromatograph was equipped with a DB-1 capillary column (30 m, 320 μm, 0.25 μm), and the carrier gas was helium. The GC oven temperature was kept at 100 °C for 1 min, and then it was increased to 220 °C at a rate of 10 °C/min, where it remained stable for 1 minute. Both the injection port and the detector were set at 250 °C, and the split ratio was 1/25. The conversion and selectivity were calculated using the peak area normalization method with equations 1, 2. Yield was obtained with equation 3. The percentage corresponds to the percentage areas of the gas chromatographic analysis.
3. Results and discussion
3.1. Hydration of α-pinene
α-Pinene hydration was carried out following the best reaction conditions reported25. The temperature was set up at 70 °C over 4 h with a catalyst loading of 15 % w/w of total reactants with a pinene:water:2-propanol mass ratio equal to 1:1:2. Table 1 shows the distribution of the largest number of products identified by GC-MS of the hydration process of α-pinene over Amberlyst-15, using a mixture of 2-propanol/water as a solvent, achieving a conversion of 93 %. Selectivity of the main reaction products, Table 1, decreased in the following order: α-terpineol > terpinolene > limonene > camphene. These results agreed with previous reports25,30 where the formation of camphene, limonene, and terpinolene also took place during the hydration of α-pinene to α-terpineol.
As α-pinene is poorly soluble in water even at high temperatures (up to 100 °C) and forms a heterophase mixture, it is necessary to ensure an adequate amount of organic solvents for getting one liquid phase with water to perform a hydration reaction23. Table 2 shows α-pinene hydration over Amberlyst-15 in the presence of four different solvents classified according to their polarity31. It is possible to conclude that the conversion is strongly affected by the type of solvent, but there is no trend regarding their polarity. When methyl ethyl ketone or toluene were used as solvents for the hydration process, Amberlyst-15 did not show activity. At the same reaction conditions (70 °C, 4 h with a catalyst loading of 15 % w/w of the total weight of the mixture with a pinene:water:2-propanol mass ratio of 1:1:2), the same conversion (84 %) was obtained with 2-propanol and acetonitrile, with a slightly higher selectivity with acetonitrile; these two solvents were selected for further experiments.
Table 1 Products of α-pinene hydration at 93 % of conversion*
| Name | % w/w | S (%) |
|---|---|---|
| α-Pinene | 6.3 | - |
| β-Pinene | 0.1 | 0 |
| Canfene | 9.6 | 10 |
| α-Terpinene | 2.6 | 3 |
| Limonene | 10.8 | 11 |
| γ-Terpinene | 1.2 | 1 |
| Terpinolene | 11.0 | 12 |
| Fenchol | 3.2 | 3 |
| 1-Methyl-4-(1 methylethenyl) cyclohexanol beta-Terpineol | 1,1 | 1 |
| Borneol | 4.7 | 3 |
| α-Terpineol | 36.9 | 39 |
| Terpin hydrate | 2.4 | 3 |
| Others not identified | 10.1 | - |
* Reaction conditions: α-pinene (1.5 mmol), Amberlyst-15 (15 % w/w), mass ratio α-pinene: H2O:2-propanol =1:1:2, 1150 rpm, 70 °C, 4 h. S: selectivity. % w/w was calculated as a percent area of the compound.
Table 2 Effect of different solvents on the hydration of α-pinene
| Solvent | Type | C (%) | S (%) | Y (%) |
|---|---|---|---|---|
| 2-Propanol | Protic | 84 | 43 | 36 |
| Acetonitrile | Dipolar aprotic | 84 | 48 | 40 |
| t-Butanol | Dipolar aprotic | 20 | 33 | 7 |
| Ethyl acetate | Polar aprotic | 35 | 45 | 16 |
Reaction conditions: α-pinene (1.5 mmol), Amberlyst-15 (15 % w/w);
mass ratio of α-pinene:H2O: solvent was 1:1:2, 1150 rpm,
70 °C, 4 h. C: conversion, S: selectivity, Y: yield.
In the literature, there are no reports about the use of acetonitrile as a solvent in the hydration of α-pinene. The effect of five different solvents (normal butanol, isobutyl alcohol, normal propyl alcohol, dioxane, 2-propanol) on the conversion of turpentine and the selectivity of terpineol was investigated25; there was not a trend, but 2-propanol had a good affinity to both H2O and turpentine, and the mutual solubility of H2O and turpentine was better under the reaction conditions.
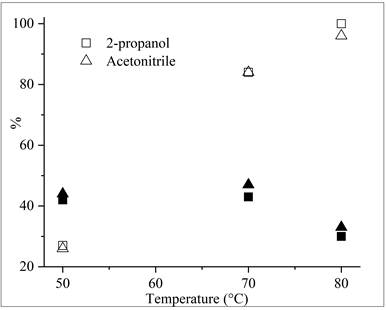
The effect of temperature on the conversion of α-pinene and selectivity to α-terpineol is shown in Figure 1. The maximum conversion and selectivity were reached at 70 °C in both solvents, acetonitrile, and 2-propanol. The conversion of α-pinene increased from 27 % to 99 % when the temperature changed from 50 °C to 80 °C in the presence of 2-propanol. The selectivity to α-terpineol remained constant in around 43 % in the range of temperature between 50 °C and 70 °C. Still, it started to decrease when the temperature reached 80 °C, possibly due to a displacement of the thermodynamic equilibrium of the reaction that favors the formation of other compounds from α-terpineol. It was suggested that in this case, the main products are limonene and other p-menthadienes as a result of acid-catalyzed α-pinene isomerization and reverse dehydration reaction of p-menthenols 23. At low temperatures, the hydration reaction is very slow (sometimes taking hundreds of hours), whereas at high temperatures, its thermodynamic equilibrium reverses toward dehydration22. Acetonitrile has a similar behavior to 2-propanol, but the hydration of α-pinene in the presence of acetonitrile is in two phases (Table 3). One of the phases is rich in unreacted α-pinene and its isomers, while the other phase has a high content of α-terpineol. This result indicates that two liquid phases can afford the α-terpineol and quite similar yields. Acetonitrile is a polar solvent used in pharmaceutical industry32, for example in the manufacture of deoxyribonucleic acid (DNA) oligonucleotide and two-carbon building block inorganic molecules to construct many useful chemicals, including acetamidine hydrochloride, thiamine, and α-naphthalene acetic33, and in acetamide synthesis for applications in the manufacture of plastics, fibers, and resins 34. Although acetonitrile is a highly flammable substance and ignites in the presence of flames, under the developed reaction conditions, it can be safely used.
3.2. Hydration of α-pinene, β-pinene and limonene
Table 4 shows that α-pinene conversion increased rapidly from 2 to 6 h, then the conversion kept rising very slowly until achieving 99 % at 8 h. The selectivity to α-terpineol reached the maximum value at 4 h (43%). An α-terpineol selectivity of 42 % and a substrate conversion of 91% were obtained with β-pinene (70°C, Amberlyst-15 (15 % w/w), terpene: water: 2-propanol mass ratio of 1: 1: 2, 1150 rpm), but at shorter time in the case of β-pinene (2 h). The hydration of limonene also produces α-terpineol with a selectivity of 58 % and a conversion of 27 % after 8 h of reaction.
Table 3 Hydration of α-pinene (purity 98 %) using acetonitrile as solvent
| Substance | % Mass composition Aqueous phase | % Mass composition Organic phase |
|---|---|---|
| α-Pinene | 41.1 | 10.0 |
| Camphene | 13.1 | 4.8 |
| Limonene | 14.6 | 0 |
| α-Terpinolene | 1.3 | 5.9 |
| γ-Terpinene | 1.4 | 0 |
| Terpinolene | 14.6 | 5.0 |
| Fenchol, exo | 0 | 3.8 |
| Borneol | 0 | 3.2 |
| α-Terpineol | 9.6 | 54.9 |
| 1,8-Terpin | 0 | 4.7 |
| Others | 4.3 | 7.5 |
Reaction conditions: α-pinene (1.5 mmol), Amberlyst-15 (15 % w/w); α-pinene:
H2O: acetonitrile mass ratio of 1:1:2, 1150 rpm, 70 °C, 4 h.
Table 4 Conversion of α, β-pinene, and limonene and selectivity to α-terpineol over Amberlyst-15
| Component | t (h) | C (%) | S (%) | Y (%) |
|---|---|---|---|---|
| α-Pinene | 2 | 65 | 45 | 29 |
| 4 | 84 | 43 | 36 | |
| 6 | 95 | 38 | 36 | |
| 8 | 99 | 36 | 35 | |
| β-Pinene | 2 | 91 | 42 | 38 |
| 4 | 99 | 39 | 38 | |
| 6 | 100 | 37 | 37 | |
| 8 | 100 | 35 | 35 | |
| Limonene | 2 | 9 | 52 | 4 |
| 4 | 14 | 60 | 8 | |
| 6 | 20 | 60 | 12 | |
| 8 | 27 | 58 | 15 |
Reaction conditions: terpene (1.5 mmol), Amberlyst-15 (15 % w/w), terpene: water: 2-propanol
mass ratio of 1: 1: 2, 1150 rpm, 70°C. C: conversion, S: selectivity, Y: yield
Liquid-phase hydration and acetoxylation of limonene, β-pinene, and α-pinene with acetic acid/water mixture of 90/10 v/v at 25 °C, catalyzed by dissolved heteropoly acid H3PW12O40 or supported on silica were studied6. They found that the three substrates give α-terpineol as the main product, obtaining a selectivity of 43 % with α-pinene, 42 % with β-pinene and a selectivity of 50 % with limonene; the authors also reported that α-terpenyl acetate formation and the reaction rate increased depending on the substrate, in the following order: limonene < α -pinene < β-pinene.
3.3. Hydration of commercial turpentine oil
Table 5 shows the effect of different initial mass ratio of reactants on the hydration of commercial turpentine oil over Amberlyst-15 as a catalyst at 70 °C. Varying the mass ratio of water from 0.5 to 0.25 with respect to turpentine and keeping constant the mass ratio between turpentine and 2-propanol, the composition of α-terpineol tends to remain constant between 32 to 35 %. When the water-to-turpentine ratio changed from 1 to 0.5, almost total consumption of β-pinene was observed; however, the terpineol production did not change (entries 1 and 4). When the mass ratio between turpentine and solvent changed from 1:1 to 1:2 there was an increase in the α-terpineol content from 22 to 35 % (entries 7 and 4), probably due to an improvement in the solubility of the substances. But in the case that the amount of solvent exceeded three times the amount of turpentine (entry 6), a lower production of α-terpineol was observed with a high consumption of the reactants. Therefore, the best mass ratio of turpentine: H2O:2-propanol was 1:0.5:2 (entry 4), achieving a decrease in the amount of solvent reported25 where the mass ratio of turpentine: H2O:2-propanol was equal to 1:1:2; achieving a conversion of 86% and 43% selectivity toward α-terpineol at 70 °C and 4 h using α-pinene. Reutilization of the catalyst (entries 2 and 3) showed minor changes in the composition of α-terpineol, in agreement with the previous results25.
When the catalyst amount was varied (entries 2, 7, and 8), the highest α-terpineol content (35 % w/w) was obtained with 15 % w/w of total reactants. This behavior was related to more acidic active sites, which resulted in a higher reaction rate for the conversion of turpentine25. The effect of reaction time on α-terpineol production is shown in Table 5. When the reaction time ranged between 2 (entry 9) and 4 h (entry 2), the content of α-terpineol increased from 30 to 35 % w/w, while the content of α-pinene decreased from 25 to 8 % w/w; the content of α-terpineol remained almost constant until 8 h (entry 11). In all cases, there was almost total consumption of β-pinene in the mixture.
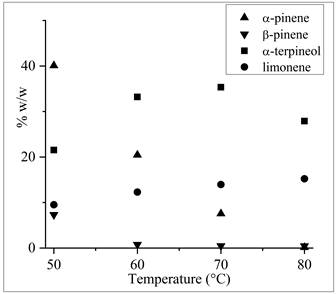
The hydration of commercial turpentine also depended on reaction temperature as the pure α-pinene did. Figure 2 shows the effect of temperatures ranging from 50 °C to 80 °C on the main components of turpentine oil. While the content of α-pinene and β-pinene decreased, the limonene content of the turpentine oil increased after 4 h of reaction, possibly due to collateral isomerization reactions. The hydration reaction at different temperatures was also studied6; they used heteropoly acid H3PW12O40 as catalyst and mixture of acetic acid and water for limonene and α-pinene hydration at three temperatures (15, 25, and 40 °C), obtaining a conversion of 89 % from α-pinene, selectivity of 36 % to α-terpineol at 25 °C for 180 min with an acetic acid / water ratio of 95/5 v/v, under homogeneous conditions.
Table 5 Results of the hydration of commercial turpentine oil
| Entry | Turpentine:water:2-propanol mass ratio | Catalyst loading (% w/w) | α-Pinene (% w/w) | β-Pinene (% w/w) | α-Terpineol (% w/w) |
|---|---|---|---|---|---|
| 1 | 1:01:02 | 15 | 8 | 0.5 | 35 |
| 2ᵃ | 1:01:02 | 15 | 6 | 0.2 | 35 |
| 3ᵇ | 1:01:02 | 15 | 4 | 0.2 | 34 |
| 4 | 1:0.5:2 | 15 | 8 | 0.5 | 35 |
| 5 | 1:0.25:2 | 15 | 3 | 0.5 | 32 |
| 6 | 1:0.5:3 | 15 | 3 | 0.5 | 34 |
| 7 | 1:0.5:1 | 15 | 32 | 9 | 17 |
| 8 | 1:0.5:0.5 | 15 | 40 | 20 | 14 |
| 9 | 1:0.5:2 | 20 | 2 | 0.5 | 35 |
| 10 | 1:0.5:2 | 10 | 11 | 1 | 32 |
| 11ᶜ | 1:0.5:2 | 15 | 25 | 2 | 30 |
| 12ᵈ | 1:0.5:2 | 15 | 2 | 0.5 | 35 |
| 13ᵉ | 1:0.5:2 | 15 | 0.5 | 0.4 | 33 |
Reaction conditions: Amberlyst-15 as catalyst, 1150 rpm, 70 °C, 4 h.
aFirst reuse. aSecond reuse. c2 h of reaction. d6 h of reaction. e8 h of reaction.
The conversion and selectivity to α-terpineol increased significantly when temperature increased in the acid catalyzed reaction of α-pinene over Y-zeolite; the maximum conversion was obtained at 65 °C35.
Conclusions
The synthesis of α-terpineol was successfully carried out from several substrates. β-Pinene showed yields to α-terpineol over Amberlyst-15 catalyst up to 38 % in just 2 h of reaction, while with α-pinene and limonene yields of 36 and 8 %, respectively, were reached at 4 h. Proving that the selected catalytic system is even more active to obtain the desired product starting from β-pinene. The hydration of α-pinene was successfully carried out in the presence of different solvents. Despite the conversion being strongly affected by the type of solvent, there was no trend regarding their polarity. In the presence of acetonitrile, it was possible to obtain a conversion of 84 % for α-pinene and a selectivity of 48 % over Amberlyst-15 (4 h, 70 °C), providing an opportunity to improve the performance of the reaction from the study of the effect of different solvents to 2-propanol. Besides, with our methodology, acetonitrile emerges as an alternative solvent since the product of the reaction has two liquid phases that could be separated by liquid-liquid extraction followed by distillation.
A turpentine mixture containing α-pinene, β-pinene, and limonene yielded α-terpineol (35 % w/w) in the presence of Amberlyst-15. By increasing the amount of solvent with respect to the amount of turpentine, the terpineol yield improved. However, an excess of solvent (3 times the amount of turpentine) did not affect the final content of α-terpineol. The increase in temperature above 70 °C produced a decrease in some of the components of turpentine oil, but not in the final content of α-terpineol. The results of the turpentine hydration process showed a composition of α-terpineol (35 % w/w) similar to that obtained with α-pinene (36.9 % w/w). Despite this, the limonene content in turpentine oil increased rather than being transformed to α-terpineol.