Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957On-line version ISSN 2500-7440
Rev Col Gastroenterol vol.24 no.3 Bogotá July/Sept. 2009
The protective role of bilirubin in human beings
William Otero Regino MD(1), Héctor Velasco MD(2), Héctor Sandoval MD(2)
(1) Professor of Medicine, Unit of Gastroenterology, Universidad Nacional de Colombia, Gastroenterologist, Clínica Fundadores, Clínica Carlos Lleras Restrepo and Fundación Hospital San Carlos, Bogota, Colombia
(2) Internal Medicine Resident, Universidad Nacional de Colombia. Bogotá, Colombia.
Received: 21-07-09 Accepted: 18-08-09
Summary
Bilirubin is more than just the final product of heme catabolism. Today it is considered to be a fundamental substance which acts as an antioxidant and anti-inflammatory agent in the serum. It can neutralize free radicals and prevent peroxidation of lipids. In addition there is evidence that it protects the cardiovascular system, neuronal systems, the hepatobiliary system, the pulmonary system and the immune system. Recently the use of pharmacological agents which augment expression of Heme oxygenase 1 (HO-1) has been investigated. Consequently its metabolites such as carbon monoxide (CO), biliverdin (BV) and bilirubin (BR) could become parts of a therapeutic strategy for treatment of various inflammatory illnesses
Key words
Biliverdin, bilirubin, antioxidant, anti-inflammatory, cytoprotection.
Bilirubin (BR) has been commonly considered to be simply the "final product" of heme catabolism. Healthy newborns have high concentrations of it as a result of fetal erythrocytes breaking combined with its transitory inability to combine with glucuronic acid. In addition, high levels of it can end up accumulating inside the brain causing irreversible damage in areas such as the basal ganglia, producing kernicterus or bilirubin encephalopathy (1, 2). However, for the past 20 years bilirubin has increasingly become known for physiological functions in normal concentrations. It as a powerful antioxidant and anti-inflammatory that can help prevent lipid oxidation and other kinds of oxidation with better efficiency than vitamin E (3, 4). It has also been postulated as one of the principle defense mechanisms in the serum against oxidative stress (5, 6) and may have protective effects for the pulmonary, (7), cerebral (8), hepatobiliar (9), immunological and cardiovascular systems (1, 3).
The concept of non conjugated bilirubin as a powerful antioxidant could be the answer, from a teleological point of view, to mammals necessities for producing bilirubin and not stopping the catabolic chain from heme to biliverdin (BV) like amphibian, reptiles and birds do (10). Biliverdin is a hydrosoluble substance that is easily excreted into the bile without spending an extra amount of energy or using other enzyme systems such as biliverdin reductase to finally produce bilirubin, a substance that is insoluble in water. Moreover, it needs albumin for transportation and for its conjugate excretion with glucuronic acid through UDP glucuronyl transferase 1 which makes it hydrosoluble, so it can be excreted into the bile and the small intestine (11). This article will review evidence that supports the concept of bilirubin as a protective substance for human beings.
Every day human beings produce 4 mg of bilirubin per kilogram of weight. The process starts with molecules that contain heme which is present in the hemoglobin of red blood cells and in other heme proteins such as cytochromes, catalases, peroxidases and tryptophan pyrrolase (11, 12). 80% of the heme group comes from hemoglobin released by senescent erythrocytes and ineffective erythropoiesis. The other 20% is related to enzymatic non-erythroid sources (11, 12).
It has been proven that there are two bilirubin production peaks after an intravenous administration of porphyrin precursor radiation markers such as D aminolevulinic acid or glycine. One, due to ineffective erythropoiesis, occurs after 72 hours. The other, due to the destruction of senescent erythrocytes, occurs after 110 days (11-13).
Heme is a tetrapyrrole group, united by methane bridges. Each bridge is a different size due to the sizes of their chains their asymmetrical distributions (14).
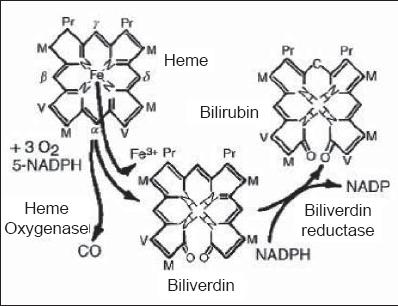
Free heme is dangerous in excessive quantities (15) which is why it is quickly removed from tissues through hydrolyzation by microsomal heme-oxygenase, specifically at the α-methane bridge. This results in production of biliverdin, a carbon monoxide molecule (CO), the release of iron, and consumption of oxygen. The process requires a reducing agent, NADPH (16). CO is a neurotransmitter and a powerful anti-inflammatory (17-19). There are three different kinds of heme-oxygenase isoforms. The first one is isoform HO-1, inducible by stress or by the aforementioned heme. Isoform HO-2 is a protein primarily made in the testicles and the brain. Isoform HO-3 has very low catalytic activity. Its main function is as a linking protein to heme (17-19). HO-1 has a high concentration in the spleen and is responsible for hemes quick elimination from circulation. It seems that HO-2 can protect neurons from oxidative damage (20). Heme oxigenase is part of the system which regulates the integrity of endothelial cells and which also regulates oxidative stress. The heme group of enzymes is very important for endothelial cells because it regulates activities of soluble guanylate cyclases (GCs), nitric oxide synthase (NOS), cytochrome P450 (CYP450), mono-oxigenase, cyclo-oxigenase (COX) and catalase (21, 22). As will be discussed later, its induction or overexpression plays an important role in cellular lesions mediated by oxidative stress. HO-1 can be expressed not only by stimulating its free heme substrate, but also by diverse pro-inflammatory stimuli. This expands its functions from degrading heme groups to eliminating inflammation (23) and to antioxidant and anti-inflammatory effects. This is mainly the result of biliverdin and bilirubin formation (24).
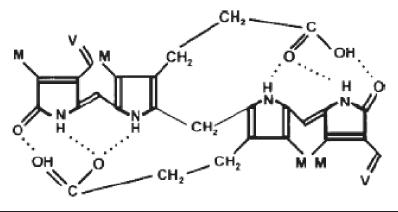
The next step is the reduction of biliverdin to bilirubin by the action of biliverdin reductase (BVR) (13, 21) (figure 1). Despite the presence of various polar groups like propionic acid and amines, bilirubin is water insoluble. This paradox is due to the internalization of its hydrogen ions (21) (figure 2).
Figure 1. From Heme to Bilirubin.
Figure 2. Internalization of Bilirubin’s Hydrogen Ions.
Once bilirubin has been formed it can interact with free oxygen radicals, producing its own oxidation which transforms it once again into biliverdin (1, 13).This reconversion is catalyzed by BVR which, by virtue of the same, detoxifies up to 10,000 times the oxidant excesses (1) (figure 3). When bilirubin acts as an antioxidant it is converted into biliverdin. Since bilirubin is soluble in lipids it can protect cells against lipid peroxidation. All soluble oxidants are neutralized or detoxified by glutathione using a cycle that requires two enzymes: glutathione peroxidase and glutathione reductase (1, 25). This protects hydrosoluble proteins from oxidation by glutathione while lipids are protected by bilirubin. This explains the physiologically complementary antioxidant and cytoprotective functions of these two substances (1). They may have other superimposed activities due to the fact that glutathione can also protect against lipid oxidation (1, 2, 13). Other known antioxidant defense mechanisms are superoxide dismutase enzymes and catalase that turn superoxide anions into water (1, 2).
In nature hydrogen bridges are hydrolyzed by the glucuronidation of the carboxyls of propionic acid. As a result bilirubin becomes hydrosoluble (conjugated bilirubin) and is excreted into the bile. Conjugated bilirubin reacts quickly through the Van den Bergh reaction (VdB) due to the accessibility of its central methane group while non-conjugated bilirubin reacts very slowly to Diazo reactive substances because of its internalized hydrogen and the methane bridge which is inaccessible to the reactive substance (13, 21). Since total bilirubin can be measured by accelerators that can break hydrogen bridges (VdB), the difference between total bilirubin measured by VdB and conjugated bilirubin is considered to be non conjugated bilirubin. Less than 4% of normal total bilirubin is conjugated, but methods based on the Diazo reaction such as VdB give false results as high as 10 to 15% of total bilirubin. This is why it has classically been considered that this kind of reaction overestimates conjugated bilirubin (13). Exposure to the sun breaks the hydrogen bridges and changes the non conjugated bilirubin configuration allowing it to be excreted into the bile (26).
CARDIOVASCULAR SYSTEM
Today there is evidence of the protective effect of mild to moderately elevated levels of bilirubin against diseases related to oxidative stress. Among these are ischemic cardiovascular disease, Alzheimers disease and ischemia-reperfusion, suggesting that increased production of bilirubin is an adaptive response against oxidation (27). Several studies have found that elevated levels of bilirubin are associated with a decreased risk of coronary disease (28, 29). The inverse association between bilirubin and risk of coronary disease was analyzed in the European study "Prospective Epidemiological Study of Myocardial Infarction (PRIME)" (30). In this study bilirubin was measured in 216 coronary patients over 5 years in which 434 examinations were conducted. Bilirubin levels were significantly lower among the coronary patients than within the control group. The mean bilirubin level of the patient group was 0.46 mg/dL, while the range was from 0.31 to 0.72 mg/dL. The mean bilirubin level of the control group was 0.53 mg/dL, while the range was from 0.36 to 0.75 mg/dL. Based on these findings it has been suggested that bilirubin is a new indicator of coronary risk in middle-aged men (30). In people with Gilberts syndrome, the frequency of ischemic heart disease is lower than the general population (2% vs. 12%) (31). Apparently the protective effect of bilirubin is higher than the HDL level, classically considered to be the protective cholesterol fraction (31).
It has also been found that high levels of bilirubin preserve coronary flow and microvascular coronary functions. Hakan and colleagues (32) found that the diastolic peak was directly related to total bilirubin levels. In contract to the behavior of the ultrasensitive reactive protein C (PCR) which is inversely proportional, thus confirming that elevated levels of bilirubin can prevent atherosclerosis. 12% of the population has elevated levels of bilirubin (33) which seem to be genetically determined.
In a recent study of 55 families (33), 188 male and 144 female patients were randomized for several cardiovascular risk factors to determine if low bilirubin levels are related to early cardiovascular events (Early defined for men as before the age of 55, and for women before the age of 65 years). High albumen levels and low levels of high density lipoproteins (HDL) were related to high bilirubin levels in women but not in men. Low bilirubin levels were related to small increases in cardiovascular events in men, but not in women. Bilirubin gene secretion was found in 23% of the population. It was concluded that high bilirubin levels have a small effect on decreasing cardiovascular risk in men with no differences in women, possibly due to low HDL-C levels (33). In the Framingham Heart Study, the homozygous allele UGT1A1*28 was related to high bilirubin levels and had a B association with low cardiovascular risk (34). Low bilirubin levels are also independent of, and inversely related to, the deterioration of carotid flow. This is mediated through vasodilation and increased carotid artery intima-media thickness in both men and women, making these two factors predictors of atherosclerosis (35).
A correlation was also found between the highest bilirubin levels and lower risk of peripheral arterial disease. The National Health and Nutrition Examination Survey (NHANES), undertaken from 1999 to 2004 (36), analyzed 7075 patients levels of total bilirubin and risk factors for peripheral arterial disease (EAP). It found, that a 0.1mg/dl bilirubin increment was associated with a 6% decrease in EAP. The odds ratio (OR) was 0.94. The 95% confidence interval was 0.9 to 0.98. Hyperbilirubin results were not due to chronic hepatic disease or alcohol consumption. An inverse relation was found between EAP and bilirubin levels in men with an OR of 0.90 and a 95% confidence interval between 0.85 and 0.96 while in women the OR was 0.97 and the 95% confidence interval was between 0.91 and 1.04. There was a B association between smokers whose OR was 0.81 with a 95% confidence interval between 0.73 and 0.90) and non-smokers whose OR was 0.97 and whose 95% confidence interval was between 0.93 and 1.02. The study results led to the conclusion that high bilirubin levels are associated with lower EAP prevalence. In a metaanalysis a negative association was also found between high bilirubin levels and severity of atherosclerosis (37).
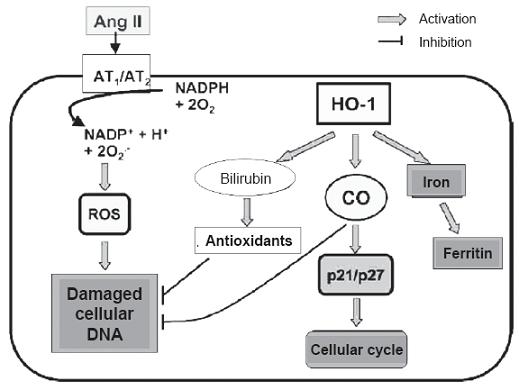
In addition to neutralizing oxygen radicals, unconjugated bilirubin also acts as a reducing agent of some peroxides including prostaglandin H synthase (PGH) when in the presence of hydrogen peroxide or organic hydroperoxides (38). Recently, Mazza and colleagues demonstrated bilirubins antioxidant and cytoprotective effects in relation to damage to endothelial cells mediated by angiotensin II (38). Other authors have found that angiotensin II significantly stimulates peroxide formation in monocytes. Exogenous application of bilirubin not only suppresses peroxide formation, but also suppresses the chemotactic activity of angiotensin II in these cells (39). Consequently bilirubins action can be particularly relevant to preventing vasoconstriction mediated by oxidation mechanisms. Within the mechanisms of action along this pathway it has been demonstrated that inhibiting NADPH oxidase and protein kinase C (PKC) activity prevents vascular damage mediated angiotensin II (40, 41). Long ago has it been known that angiotensin II is frequently elevated in hypertense people and associated to high levels of free radicals in oxygen which increase renal damage (42) and therefore, natural antioxidants like bilirubin have protector effects (figure 3).
Figure 3. Hypothesis on OH-1 protective mechanisms to the angiotensin II induced lesion. Ang II: Angiotensin II. AT1/2: Receptor 1 and 2 of Angiotensin II. HO-1: Heme Oxigenase 1; CO carbon monoxide. ROS: Reactive Oxygen Species. Angiotensin II. ATI/ 2: Angiotensin II receptors 1 and 2. HO-1: Heme oxigenase 1; CO Carbon monoxide. ROS: Reactive Oxygen Species.
It has recently been found that in diabetic mice both biliverdin and bilirubin can preserve endothelial cell integrity, preventing cellular death and augmenting vascular reactivity and vascular restenosis (43, 44). (45, 46). In experimental diabetes bilirubin is implicated in the reduction of oxidative stress which augments the bioavailability of nitric oxide (NO) needed for the integrity of endothelial cells (46). HO-1 attenuates the generation of oxidants by diabetes and uncouples nitric oxide synthase (NOS) to mediate the inhibition of PKC and NADPH oxidase in the endothelial cell (43, 46). Glucose increases the production of peroxynitrites which are NO/O2- reaction products. In turn, the peroxynitrites inactivate the precursor of nitric oxide synthase (NOS) by increasing the uncoupling of the enzyme creating a greater quantity of O2- (peroxide) than of NO (47-49). Functional NOS can increase with HO-1. The peroxynitrites stimulate the genetic expression of HO-1 in non-diabetic people, but in diabetics glucose suppresses the effects on the expression HO-1. The HO-1 increases the expression of super oxide dismutase which can protect against uncoupling of NOS (47-49).
In the renal blood vessels of rodents treated with biliverdin, the expression of E selectins and P selectins, by lipopolysaccharide (LPS) was significantly reduced. This confirmed that biliverdin and bilirubin have anti-inflammatory properties (50). In experimental models of endotoxemia in rodents, Want and colleagues (51) demonstrated that bilirubin is a key mediator of cytoprotection. It improved cellular lesions in response to the infusion of LPS when levels of NO and TNF-α had already been reduced and the inducible synthase of hepatic NO was significantly less.
Arteriosclerosis is an inflammatory disease of the walls of large and medium size arteries which is precipitated by elevated levels of low density lipoproteins in the blood (52). Oxidative modifications in the plasma of low density lipoproteins (LDL), mainly at the level of arterial walls, markedly increases the atherogenicity of these (53) and, with the deposit of oxidized LDL, form part of the crucial lesions of arteriosclerosis (54). Minimally modified LDL induces chemotactic protein-1 monocytes and colony-stimulating factor which produce recruitment and differentiation of macrophages in the arterial wall (54). The endothelial cell dysfunction induced by oxidized LDL is one of the first steps in the development of arteriosclerosis, so the adaptive vascular responses and /or oxidative stress protectors are important in the prevention of arteriogenesis (55). Inhibition of LDL oxidation is one of the anti-arteriogenic properties of bilirubin. This includes neutralizing free oxygen radicals generated from phospholipids, triglycerides and cholesterol esters (56, 57). Even at low concentrations it has the capacity to inactivate oxygen radicals in Vitro, decreasing oxidative cell damage and attenuation of oxidative stress in vivo (58-60). The mechanisms by which bilirubin reacts with oxygen radicals are not completely understood, however its hydrophobic tetrapyrrole structure has been reported to be an inhibitor of NADPH oxidase and protein kinase C (PKC) among other mediators of pro-arteriogenic factors (58-60).
THE IMMUNE SYSTEM
Experimentally, bilirubin has immunosuppresor effects which act on lymphocytes and granulocytes (61). In vitro, bilirubin in concentrations from 100 to 200 micromoles inhibits cytotoxic T lymphocyte activity (62). Similarly, it alters the proliferation of T cells induced by phytohemagglutinin (63). HO 1 has important immunosuppresor effects and its induction decreases episodes of acute and chronic rejection in solid organ transplants (64, 65). These effects seem to be mediated by biliverdin and bilirubin (BV/BR) (62, 65). BVs anti-inflammatory effects in organ transplants is expressed by less proliferation of T cells, less leukocyte infiltration and longer survival of cardiac transplants (66). BR inhibits the activation of endothelial cells in the suppression of E-selectin adhesion molecules (67). An important effect of the BV/BR system is inhibition of nuclear factor-kB which is necessary for transcription of pro-inflammatory genes (66, 67). Both substances are antiapoptotic and can suppress the responses involved in chronic dysfunctions of transplants (24).
HEPATOBILIARY EFFECTS
Experimentally, it has been found that the BV/BR system prevents acetaminophen toxicity (68) by neutralizing toxic free radicals produced by the metabolism of this medication (69). Biliverdins cytoprotective effects against lesions produced by post-ischemia hepatic reperfusion have been demonstrated in rats (70). CO has been demonstrated to modulate the formation and flow of bile (71).
CONCLUSIONS
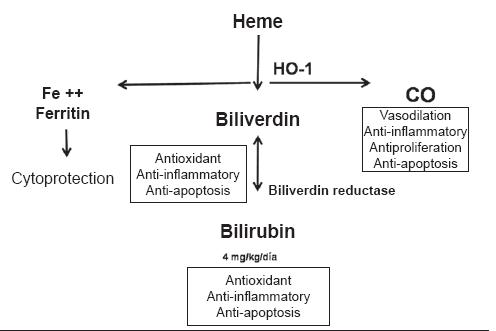
In spite of the evidence of the antioxidant effect of bilirubin it is important to highlight that this effect basically occurs in the serum while the most important protective mechanisms are within the tissues. Since bilirubins concentration is 100 to 1000 times greater in the serum than within cells, we still lack an elucidation of the interrelation of bilirubins activities within the serum and its activities which occur inside the cells (1). The great challenge involved in understanding these physiological events is to apply what we learn to therapeutic applications. Recently, the results of biliverdin and bilirubins therapeutical application in solid organ transplants have been checked (1, 72). The use of pharmacological agents that increase HO-1 expression, and therefore its metabolites (carbon monoxide, biliverdin and bilirubin) can become a therapeutic strategy for handling different inflammatory diseases (24). This last possibility acquires greater relevance if we take into account the fact that the only currently available strategy is the use of immunosuppresors which increase the incidence of tumors and infectious bacterial, fungal and viral diseases in patients with transplants (73). Similarly, HO-1 is being considered as a potential therapeutic target for hepatoprotection (9). In figure 4, the beneficial effects of the metabolites involved in the metabolic chain of heme metabolism (CO, BV/BR) are shown.
Figure 4. Benefits of heme metabolism.
REFERENCES
1. Sedlack TW, Zinder SH. Bilirrubin benefits: cellular protection by a biliverdin reductase antioxisant cycle. Pediatrics 2004; 113: 1776-82.
2. kapitulnik J. Bilirrubin: an endogenous product of heme degradation with both cytotoxic and cytoprotective properties. Mol, Pharmacol 2004; 66: 773-9.
3. Stocker R, Glazer AN, Ames BN. Antioxidant activity of albumin-bound bilirrubin. Proc Natl Acad Sci USA 1987; 84: 5918-22.
4. Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirrubin is an antioxidant of possible physiological importance. Science 1987; 235: 1043-6.
5. Belanger S, Lavoie JC, Chessex P. Influence of bilirrubin on the antioxidant capacity of plasma in newborn infants. Biol Neonat 1997; 71: 233-8.
6. Copinhatan V, Miller NJ, Milner AD, Rice-Evans CA. Bilirrubin and ascorbate antioxidant activity in neonatal plasma. FEBS Lett 1994; 349: 197-200.
7. Sarady-Andrews JK, Liu F, Gallo D, Nakao A, Overhaus M, Ollinger AM, et al. Biliverdin administration protects against endotoxin-induced acute lung injury in rats. Am J Physiol Lung Mol Physiol 2005; 289: L1131-L1137.
8. Syapin PJ. Regulation of heme oxygenase-1 for treatment of neuroinflammation and brain disorders. Br J Pharmacol 2008; 11: 623-40.
9. Farombi EO, Surh YJ. Heme Oxygenase-1 as a potential therapeutic target for hepatoprotection. J Bioch Mol Biol 2006; 39: 479-91.
10. McDonagh AF. Bile pigments: bilatrienes and 5, 15 biladiens. En: Dolphin D (Editor). The porphyrins. New Cork, NY: Academic Press 1979. p. 293-491.
11. Roy Chowdhury J, Wolkoff AW, Roy Chowdhury N, Arias I. Hereditary jaundice and disorders of bilirubin metabolism. The metabolic and molecular bases of inherited disease. 8th ed. McGraw-Hill 2001. p. 3063-101.
12. Roy-Chowdhury, N., Wang, X., and Roy-Chowdhury, J. Bile pigment metabolism and its disorders. En Rimoin DL, Connor JM, Pyeritz RE, Korf BR, (edits). Principles and practice of medical genetics, Fifth Edition 2006. p. 28-35.
13. Choudhry N, Arias IM, Wolkoff AW, Chowdhry JR, Disroders of bilirubin metabolism. En: Arias IM, Boyer JL, Chisari FV (edit). The liver: Biology and Pathobiology 4th,. Philadelphia. 2001. p. 291-309.
14. Roy Chowdhury N, Arias IM, Wolkoff AW. Disorders of bilirubin metabolism. In Zakim D, Boyer TD: Hepatolog. A textbook of liver disease. 3 ed. 1996, p 791-200.
15. Kumar S, Bandyopadhyay U. free heme toxicity and its detoxofication systems in human. Toxicol Lett 2005; 157: 175-88.
16. Maines MD. Heme oxigenase: function, multiplicity, regulatory mechanisms and clinical applications. FASEB J 1988; 2: 2557-68.
17. Zakhary R, Poss KD, Jaffrey SR, Ferris CD, Tonegawa S, Snyder SH. Targeted gene deletion of heme oxigenase 2 reveals neural role for carbon monoxide. Proc Natl Acad Sci USA 1997; 94: 1848-53.
18. Baranano DE, Ferris CD, Snyder SH. Atypical neural messengers. Trend Neurosci 2001; 24: 99-106.
19. Ryter SW, Alam J, Choi AM. Heme oxigenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev 2006; 86: 583-650.
20. Dore S, Goto S, Sampei K, et al. Heme-oxigenase 2 acts to prevent neuronal death in brain cultures and following transient cerebral ischemia. Neoroscience 2000; 99587-92.
21. Roy Chowdhry J, Roy Chowdhury N, Jansen PLM. Bilirubin metabolism and its disorders. In: Zakim D, Boyer T, editors. Hepatology: a textbook of liver disease. 4th ed. 2003. p. 233-69.
22. Xia Wang, Jayanta Roy Chowdhury, Namita Roy. Bilirubin metabolism: Applied physiology. Current Paediatrics 2006; 16: 70-74.
23. Willoughby DA, Moore AR, Colville-Nash PR, Gilroy D. Resolution of inflammation. Int J Immunopharmacol 2000; 22: 1131-5.
24. Ollinger R, Wang H, Yamashita K, Wegiel B, Thomas M, Margreiter R, et al. Therapeutics applications of bilirrubin and biliverdin in transplantation. Antioxid Redox signal 2007; 9: 2175-85.
25. Sedlak TW, Sqaleh M, Higgison DS, Paul BD, Juluri KR, Snyder SH. Bilirubin and gluthatione have complementary antioxidant and cytoprotective roles. PNAS 2009; 106: 5171-6.
26. Bonnett R, Davis E, Hursthouse MB. Structure of bilirubin. Nature 1976; 262: 327-8.
27. Morita, T. Heme Oxygenase and Atherosclerosis. Arterioscler Thromb Vasc Biol 2005; 25: 1786-795.
28. Clark JE, Foresti R, Sarathchandra P, Kaur H, Green CJ, Motterlini R. Postischemic myocardial dysfunction. Am J Physiol Heart Circ Physiol 2000; 278: H643-H651.
29. Hill-Kapturczak N, Chang SH, Agarwal A. Heme oxygenase and the kidney. DNA Cell Biol 2002; 21: 307-321.
30. Troughton J, Woodside J, Young S, Arveiler D, Amouyel P, Ferrieres J, et al. Bilirubin and coronary heart disease risk in the Prospective Epidemiological Study of Myocardial Infarction (PRIME). European Journal of Cardiovascular Prevention and Rehabilitation 2007; 14: 79-84.
31. Vítek L, Jirsa M, Brodanová M, Kalab M, Marecek Z, Danzig V, Novotný L, Kotal P. Gilbert syndrome and ischemic heart disease: a protective effect of elevated bilirubin levels. Atherosclerosis 2002; 160: 449-56.
32. Hakan G, Erdogan D. High serum bilirubin concentrations preserve coronary flow reserve and coronary microvascular functions. Arterioscler Thromb Vasc Biol 2005; 25: 2289-2294.
33. Lingenhela A, Kolleritsa B, Schwaigera J, Huntb S, Gressb R, Hopkins P, et al. Serum bilirubin levels, UGT1A1 polymorphisms and risk for coronary artery disease. Atherosclerosis 2006; 184: 431-437.
34. Jing-Ping L, ODonnell C, Schwaiger J, Cupples A, Lingenhel A, Hunt S, et al. Association between the UGT1A1*28 allele, bilirubin levels and coronary heart Disease in the Framingham heart study. Circulation 2006; 114: 1476-1481.
35. Jing-Ping L, Cupples A, Wilson P, Heard-Costa N, ODonnell C. Early carotid atherosclerosis and family history of vascular disease. Atherosclerosis 2001; 154: 747-754.
36. Perlstein T, Pande R, Beckman J, Creager M. Serum total bilirubin level and prevalent lower-extremity peripheral arterial disease: National Health and Nutrition Examination Survey (NHANES) 1999 to 2004. Arterioscler Thromb Vasc Biol 2008; 28: 166-172.
37. Novotni L, Vitek L. Inverse relationship between serum bilirubin and atherosclerosis in men: A meta-analysis of published studies. Experimental Biology and Medicine 2003; 228: 568-571.
38. Mazza F, Goodman AI, Lombardo G, Vanella A, Abraham NG. Heme oxygenase I gene expression attenuates angiotensin II mediated DNA damage in endothelial cells. Exp Biol Med 2003; 228: 576-583.
39. Morita T, Imai T, Yamaguchi T, Sugiyama T, Katayama S, Yoshino G. Induction of heme oxygenase-1 in monocytes suppresses angiotensin II-elicited chemotactic activity through inhibition of CCR2: role of bilirubin and carbon monoxide generated by the enzyme. Antioxid Redox Signal 2003; 5: 439-447.
40. Kwak JY, Takeshige K, Cheung BS, Minakami S. Bilirubin inhibits the activation of superoxide-producing NADPH oxidase in a neutrophil cell-free system. Biochim Biophys 1991; 1076: 369-373.
41. Sano K, Nakamura H, Matsuo T. Mode of inhibitory action of bilirubin on protein kinase C. Pediatric Research 1985; 19: 587-590.
42. Kiemer AK, Bildner N, Weber NC, Vollmar AM. Characterization of heme oxygenase 1 (heat shock protein 32) induction by atrial natriuretic peptide in human endothelial cells. Endocrinology 2003; 144: 802-812.
43. Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest 1996; 97: 1916-1923.
44. Ishizaka N, Aizawa T, Mori I, Taguchi J, Yazaki Y, Nagai R, Ohno M. Heme oxygenase-1 is upregulated in the rat heart in response to chronic administration of angiotensin II. Am J Physiol Heart Circ Physiol 2000; 279: H672-H678.
45. McClung JA, Morita T, Rodella L, Rezzani R, Weiss MB, Abraham NG. Heme oxygenase-1 prevents superoxide anion associated vascular smooth muscle growth and decreases circulating endothelial cells in a rat model of balloon injury and restenosis in diabetes mellitus. Circulation 2004; 110: III-282.
46. Milstien S, Katusic Z. Oxidation of tetrahydrobiopterin by peroxynitrite: implications for vascular endothelial function. Biochem. Biophys Res Commun 1999; 263: 681-4.
47. Foresti R, Sarathchandra P, Clark JE, Green CJ, Motterlini R. Peroxynitrite induces haem oxygenase-1 in vascular endothelia to apoptosis. Biochem J 1999; 339: 729-36.
48. Rezzani R, Quan S, Rodella L, Bianchi R, Goodman A, Abraham NG. Heme oxygenase-1 upregulation attenuates glucose-mediated oxidative stress renal injury in HO-2 knockout mice. Hypertension 2003; 42: 421 (abstract).
49. Chang SH, Barbosa-Tessmann I, Chen C, Kilberg MS, Agarwal A. Glucose deprivation induces heme oxygenase-1 gene expression by a pathway independent of the unfolded protein response. J Biol Chem 2002; 277: 1933-40.
50. Vachharajani TJ, Work J, Issekutz AC, Granger DN. Heme oxygenase modulates selectin expression in different regional vascular beds. Am J Physiol Heart Circ Physiol 2000; 278: H1613-H1617.
51. Wang WW, Smith DL, Zucker SD. Bilirubin inhibits iNOS expression and NO production in response to endotoxin in rats. Hepatology 2004; 40: 424-33.
52. Galkina E, Ley K. Immune and inflammatory mechanisms of aterosclerosis. Ann Rev Immunol 2009; 27: 165-97.
53. Wiztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest 1991; 88: 1785-92.
54. Berliner JA, Heinecke JW. The role of oxidized lipoproteins in atherosclerosis. Free Radic Biol Med 1996; 20: 707-27.
55. Morita, T. Heme Oxygenase and Atherosclerosis. Arterioscler Thromb Vasc Biol 2005; 25: 1786-795.
56. Xia Wang, Jayanta Roy Chowdhury, Namita Roy. Bilirubin metabolism: Applied physiology. Current Paediatrics 2006; 16: 70-74.
57. Hill-Kapturczak N, Chang SH, Agarwal A. Heme oxygenase and the kidney. DNA Cell Biol 2002; 21: 307-21.
58. Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science 1987; 235: 1043-1046.
59. Stocker R, Glazer AN, Ames BN. Antioxidant activity of albumin-bound bilirubin. Proc Natl Acad 1987; 84: 5918-5922.
60. Clark JE, Foresti R, Sarathchandra P, Kaur H, Green CJ, Motterlini R. Heme oxygenase-1-derived bilirubin ameliorates. Free Radical Biology & Medicine 2005; 19: 1-25.
61. Sima P, Mala J, Miller I, Hodr R, Truxova E. The suppresisve effect of continuous infusion of bilirubin on the immune response in mice. Folia Microbiol 1980; 25: 483-90.
62. Haga Y, Tempero MA, Zetterman RK. Unconjugated bilirubin inhibits in vitro cytotoxic T lynphocyte activity of human lymphocytes. Biochim Biophys Acta 1996; 1317: 65-70.
63. Haga Y, Tempero MA, kay D, Zetterman RK. Intracellular accumulation of unconjugated bilirubin inhibits phytohemagglutin induced proliferation and interleukin-2 production of human lymphocytes. Dig Dis Sci 1996; 41: 1468-74.
64. Camara NO, Soares MP. Heme oxigenase-1 (HO-1), a protective gene that prevents chronic graft dysfunction. Free Radic Biol Med 2005; 38: 426-35.
65. Tsui TY, Wu X, Lau CK, Ho DW, Xu T, Siu YT, et al. Prevention of chronic deterioration of heart allograft by recombinant adeno-associated virus-mediated heme oxygenase-1 gene transfer. Circulation 2003; 103: 2623-9.
66. Yamashita K, McDaid J, Ollinger R, Tsui TY, Berberat PO, Usheva A, et al. Biliverdin, a natural product of heme catabolism induces tolerance to cardiac allografts. FASEB J 2004; 18: 765-7.
67. Soares MP, Seldon MP, Gregoire IP, Vassilevskaia T, Berberat PO, et al. Heme-oxygenase 1 modultes the expression of adhesion molecules associated with endotelial cell activation. J Immunol 2004; 172: 553-63.
68. Chiu H, Brittingham JA, Lakim DL. Differential induction of heme oxigenase-1 in macrophages and hepatocytes during acetaminophen-induced hepatotoxicity in the rat: effects oh hemin and biliverdin. Toxicol Appl Pharmacol 2002; 181: 106-15.
69. Nelson SD. Molecular mechanisms of the hepatotocixity caused by acetaminophen. Sem liver Dis 1990; 10: 267-78.
70. Fondevila C, Shen XD, Tsuchiyashi S, Yamshita K, Csizmadia E, Lassman C, et al. Bilivedin therapy protects rat livers from ischemia and reperfusion injury. Hepatology 2004; 40: 1333-41.
71. Wunder C, Potter RF. The heme oxygenase system: its role in liver inflammation. Curr Drug Targets Cardiovasc Haematol Disord 2003; 3: 199-208.
72. Pae HO, Chung HT. Heme Oxygenase-1: its therapeutic roles in inflammatory disease. Immune Network 2009; 9: 12-19.
73. Buell JF, Gross TG, Woodle ES. Malignancy after transplantation. Transplantation 2005; 80: S254-S264.
74. McCarty MF. "Iatrogenic Gilbert syndrome"--a strategy for reducing vascular and cancer risk by increasing plasma unconjugated bilirubin. Med Hypotheses 2007; 69: 974-94.
1. Sedlack TW, Zinder SH. Bilirrubin benefits: cellular protection by a biliverdin reductase antioxisant cycle. Pediatrics 2004; 113: 1776-82. [ Links ]
2. Kapitulnik J. Bilirrubin: an endogenous product of heme degradation with both cytotoxic and cytoprotective properties. Mol, Pharmacol 2004; 66: 773-9. [ Links ]
3. Stocker R, Glazer AN, Ames BN. Antioxidant activity of albumin-bound bilirrubin. Proc Natl Acad Sci USA 1987; 84: 5918-22. [ Links ]
4. Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirrubin is an antioxidant of possible physiological importance. Science 1987; 235: 1043-6. [ Links ]
5. Belanger S, Lavoie JC, Chessex P. Influence of bilirrubin on the antioxidant capacity of plasma in newborn infants. Biol Neonat 1997; 71: 233-8. [ Links ]
6. Copinhatan V, Miller NJ, Milner AD, Rice-Evans CA. Bilirrubin and ascorbate antioxidant activity in neonatal plasma. FEBS Lett 1994; 349: 197-200. [ Links ]
7. Sarady-Andrews JK, Liu F, Gallo D, Nakao A, Overhaus M, Ollinger AM, et al. Biliverdin administration protects against endotoxin-induced acute lung injury in rats. Am J Physiol Lung Mol Physiol 2005; 289: L1131-L1137. [ Links ]
8. Syapin PJ. Regulation of heme oxygenase-1 for treatment of neuroinflammation and brain disorders. Br J Pharmacol 2008; 11: 623-40. [ Links ]
9. Farombi EO, Surh YJ. Heme Oxygenase-1 as a potential therapeutic target for hepatoprotection. J Bioch Mol Biol 2006; 39: 479-91. [ Links ]
10. McDonagh AF. Bile pigments: bilatrienes and 5, 15 biladiens. En: Dolphin D (Editor). The porphyrins. New Cork, NY: Academic Press 1979. p. 293-491. [ Links ]
11. Roy Chowdhury J, Wolkoff AW, Roy Chowdhury N, Arias I. Hereditary jaundice and disorders of bilirubin metabolism. The metabolic and molecular bases of inherited disease. 8th ed. McGraw-Hill 2001. p. 3063-101. [ Links ]
12. Roy-Chowdhury, N., Wang, X., and Roy-Chowdhury, J. Bile pigment metabolism and its disorders. En Rimoin DL, Connor JM, Pyeritz RE, Korf BR, (edits). Principles and practice of medical genetics, Fifth Edition 2006. p. 28-35. [ Links ]
13. Choudhry N, Arias IM, Wolkoff AW, Chowdhry JR, Disroders of bilirubin metabolism. En: Arias IM, Boyer JL, Chisari FV (edit). The liver: Biology and Pathobiology 4th,. Philadelphia. 2001. p. 291-309. [ Links ]
14. Roy Chowdhury N, Arias IM, Wolkoff AW. Disorders of bilirubin metabolism. In Zakim D, Boyer TD: Hepatolog. A textbook of liver disease. 3 ed. 1996, p 791-200. [ Links ]
15. Kumar S, Bandyopadhyay U. free heme toxicity and its detoxofication systems in human. Toxicol Lett 2005; 157: 175-88. [ Links ]
16. Maines MD. Heme oxigenase: function, multiplicity, regulatory mechanisms and clinical applications. FASEB J 1988; 2: 2557-68. [ Links ]
17. Zakhary R, Poss KD, Jaffrey SR, Ferris CD, Tonegawa S, Snyder SH. Targeted gene deletion of heme oxigenase 2 reveals neural role for carbon monoxide. Proc Natl Acad Sci USA 1997; 94: 1848-53. [ Links ]
18. Baranano DE, Ferris CD, Snyder SH. Atypical neural messengers. Trend Neurosci 2001; 24: 99-106. [ Links ]
19. Ryter SW, Alam J, Choi AM. Heme oxigenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev 2006; 86: 583-650.
20. Dore S, Goto S, Sampei K, et al. Heme-oxigenase 2 acts to prevent neuronal death in brain cultures and following transient cerebral ischemia. Neoroscience 2000; 99587-92. [ Links ]
21. Roy Chowdhry J, Roy Chowdhury N, Jansen PLM. Bilirubin metabolism and its disorders. In: Zakim D, Boyer T, editors. Hepatology: a textbook of liver disease. 4th ed. 2003. p. 233-69. [ Links ]
22. Xia Wang, Jayanta Roy Chowdhury, Namita Roy. Bilirubin metabolism: Applied physiology. Current Paediatrics 2006; 16: 70-74. [ Links ]
23. Willoughby DA, Moore AR, Colville-Nash PR, Gilroy D. Resolution of inflammation. Int J Immunopharmacol 2000; 22: 1131-5. [ Links ]
24. Ollinger R, Wang H, Yamashita K, Wegiel B, Thomas M, Margreiter R, et al. Therapeutics applications of bilirrubin and biliverdin in transplantation. Antioxid Redox signal 2007; 9: 2175-85. [ Links ]
25. Sedlak TW, Sqaleh M, Higgison DS, Paul BD, Juluri KR, Snyder SH. Bilirubin and gluthatione have complementary antioxidant and cytoprotective roles. PNAS 2009; 106: 5171-6. [ Links ]
26. Bonnett R, Davis E, Hursthouse MB. Structure of bilirubin. Nature 1976; 262: 327-8. [ Links ]
27. Morita, T. Heme Oxygenase and Atherosclerosis. Arterioscler Thromb Vasc Biol 2005; 25: 1786-795. [ Links ]
28. Clark JE, Foresti R, Sarathchandra P, Kaur H, Green CJ, Motterlini R. Postischemic myocardial dysfunction. Am J Physiol Heart Circ Physiol 2000; 278: H643-H651. [ Links ]
29. Hill-Kapturczak N, Chang SH, Agarwal A. Heme oxygenase and the kidney. DNA Cell Biol 2002; 21: 307-321. [ Links ]
30. Troughton J, Woodside J, Young S, Arveiler D, Amouyel P, Ferrieres J, et al. Bilirubin and coronary heart disease risk in the Prospective Epidemiological Study of Myocardial Infarction (PRIME). European Journal of Cardiovascular Prevention and Rehabilitation 2007; 14: 79-84. [ Links ]
31. Vítek L, Jirsa M, Brodanová M, Kalab M, Marecek Z, Danzig V, Novotný L, Kotal P. Gilbert syndrome and ischemic heart disease: a protective effect of elevated bilirubin levels. Atherosclerosis 2002; 160: 449-56. [ Links ]
32. Hakan G, Erdogan D. High serum bilirubin concentrations preserve coronary flow reserve and coronary microvascular functions. Arterioscler Thromb Vasc Biol 2005; 25: 2289-2294. [ Links ]
33. Lingenhela A, Kolleritsa B, Schwaigera J, Huntb S, Gressb R, Hopkins P, et al. Serum bilirubin levels, UGT1A1 polymorphisms and risk for coronary artery disease. Atherosclerosis 2006; 184: 431-437. [ Links ]
34. Jing-Ping L, ODonnell C, Schwaiger J, Cupples A, Lingenhel A, Hunt S, et al. Association between the UGT1A1*28 allele, bilirubin levels and coronary heart Disease in the Framingham heart study. Circulation 2006; 114: 1476-1481. [ Links ]
35. Jing-Ping L, Cupples A, Wilson P, Heard-Costa N, ODonnell C. Early carotid atherosclerosis and family history of vascular disease. Atherosclerosis 2001; 154: 747-754. [ Links ]
36. Perlstein T, Pande R, Beckman J, Creager M. Serum total bilirubin level and prevalent lower-extremity peripheral arterial disease: National Health and Nutrition Examination Survey (NHANES) 1999 to 2004. Arterioscler Thromb Vasc Biol 2008; 28: 166-172. [ Links ]
37. Novotni L, Vitek L. Inverse relationship between serum bilirubin and atherosclerosis in men: A meta-analysis of published studies. Experimental Biology and Medicine 2003; 228: 568-571. [ Links ]
38. Mazza F, Goodman AI, Lombardo G, Vanella A, Abraham NG. Heme oxygenase I gene expression attenuates angiotensin II mediated DNA damage in endothelial cells. Exp Biol Med 2003; 228: 576-583. [ Links ]
39. Morita T, Imai T, Yamaguchi T, Sugiyama T, Katayama S, Yoshino G. Induction of heme oxygenase-1 in monocytes suppresses angiotensin II-elicited chemotactic activity through inhibition of CCR2: role of bilirubin and carbon monoxide generated by the enzyme. Antioxid Redox Signal 2003; 5: 439-447. [ Links ]
40. Kwak JY, Takeshige K, Cheung BS, Minakami S. Bilirubin inhibits the activation of superoxide-producing NADPH oxidase in a neutrophil cell-free system. Biochim Biophys 1991; 1076: 369-373. [ Links ]
41. Sano K, Nakamura H, Matsuo T. Mode of inhibitory action of bilirubin on protein kinase C. Pediatric Research 1985; 19: 587-590. [ Links ]
42. Kiemer AK, Bildner N, Weber NC, Vollmar AM. Characterization of heme oxygenase 1 (heat shock protein 32) induction by atrial natriuretic peptide in human endothelial cells. Endocrinology 2003; 144: 802-812. [ Links ]
43. Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest 1996; 97: 1916-1923. [ Links ]
44. Ishizaka N, Aizawa T, Mori I, Taguchi J, Yazaki Y, Nagai R, Ohno M. Heme oxygenase-1 is upregulated in the rat heart in response to chronic administration of angiotensin II. Am J Physiol Heart Circ Physiol 2000; 279: H672-H678. [ Links ]
45. McClung JA, Morita T, Rodella L, Rezzani R, Weiss MB, Abraham NG. Heme oxygenase-1 prevents superoxide anion associated vascular smooth muscle growth and decreases circulating endothelial cells in a rat model of balloon injury and restenosis in diabetes mellitus. Circulation 2004; 110: III-282. [ Links ]
46. Milstien S, Katusic Z. Oxidation of tetrahydrobiopterin by peroxynitrite: implications for vascular endothelial function. Biochem. Biophys Res Commun 1999; 263: 681-4. [ Links ]
47. Foresti R, Sarathchandra P, Clark JE, Green CJ, Motterlini R. Peroxynitrite induces haem oxygenase-1 in vascular endothelia to apoptosis. Biochem J 1999; 339: 729-36. [ Links ]
48. Rezzani R, Quan S, Rodella L, Bianchi R, Goodman A, Abraham NG. Heme oxygenase-1 upregulation attenuates glucose-mediated oxidative stress renal injury in HO-2 knockout mice. Hypertension 2003; 42: 421 (abstract). [ Links ]
49. Chang SH, Barbosa-Tessmann I, Chen C, Kilberg MS, Agarwal A. Glucose deprivation induces heme oxygenase-1 gene expression by a pathway independent of the unfolded protein response. J Biol Chem 2002; 277: 1933-40. [ Links ]
50. Vachharajani TJ, Work J, Issekutz AC, Granger DN. Heme oxygenase modulates selectin expression in different regional vascular beds. Am J Physiol Heart Circ Physiol 2000; 278: H1613-H1617. [ Links ]
51. Wang WW, Smith DL, Zucker SD. Bilirubin inhibits iNOS expression and NO production in response to endotoxin in rats. Hepatology 2004; 40: 424-33. [ Links ]
52. Galkina E, Ley K. Immune and inflammatory mechanisms of aterosclerosis. Ann Rev Immunol 2009; 27: 165-97. [ Links ]
53. Wiztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest 1991; 88: 1785-92. [ Links ]
54. Berliner JA, Heinecke JW. The role of oxidized lipoproteins in atherosclerosis. Free Radic Biol Med 1996; 20: 707-27. [ Links ]
55. Morita, T. Heme Oxygenase and Atherosclerosis. Arterioscler Thromb Vasc Biol 2005; 25: 1786-795. [ Links ]
56. Xia Wang, Jayanta Roy Chowdhury, Namita Roy. Bilirubin metabolism: Applied physiology. Current Paediatrics 2006; 16: 70-74. [ Links ]
57. Hill-Kapturczak N, Chang SH, Agarwal A. Heme oxygenase and the kidney. DNA Cell Biol 2002; 21: 307-21. [ Links ]
58. Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science 1987; 235: 1043-1046. [ Links ]
59. Stocker R, Glazer AN, Ames BN. Antioxidant activity of albumin-bound bilirubin. Proc Natl Acad 1987; 84: 5918-5922. [ Links ]
60. Clark JE, Foresti R, Sarathchandra P, Kaur H, Green CJ, Motterlini R. Heme oxygenase-1-derived bilirubin ameliorates. Free Radical Biology & Medicine 2005; 19: 1-25. [ Links ]
61. Sima P, Mala J, Miller I, Hodr R, Truxova E. The suppresisve effect of continuous infusion of bilirubin on the immune response in mice. Folia Microbiol 1980; 25: 483-90.
62. Haga Y, Tempero MA, Zetterman RK. Unconjugated bilirubin inhibits in vitro cytotoxic T lynphocyte activity of human lymphocytes. Biochim Biophys Acta 1996; 1317: 65-70. [ Links ]
63. Haga Y, Tempero MA, kay D, Zetterman RK. Intracellular accumulation of unconjugated bilirubin inhibits phytohemagglutin induced proliferation and interleukin-2 production of human lymphocytes. Dig Dis Sci 1996; 41: 1468-74. [ Links ]
64. Camara NO, Soares MP. Heme oxigenase-1 (HO-1), a protective gene that prevents chronic graft dysfunction. Free Radic Biol Med 2005; 38: 426-35. [ Links ]
65. Tsui TY, Wu X, Lau CK, Ho DW, Xu T, Siu YT, et al. Prevention of chronic deterioration of heart allograft by recombinant adeno-associated virus-mediated heme oxygenase-1 gene transfer. Circulation 2003; 103: 2623-9. [ Links ]
66. Yamashita K, McDaid J, Ollinger R, Tsui TY, Berberat PO, Usheva A, et al. Biliverdin, a natural product of heme catabolism induces tolerance to cardiac allografts. FASEB J 2004; 18: 765-7. [ Links ]
67. Soares MP, Seldon MP, Gregoire IP, Vassilevskaia T, Berberat PO, et al. Heme-oxygenase 1 modultes the expression of adhesion molecules associated with endotelial cell activation. J Immunol 2004; 172: 553-63.
68. Chiu H, Brittingham JA, Lakim DL. Differential induction of heme oxigenase-1 in macrophages and hepatocytes during acetaminophen-induced hepatotoxicity in the rat: effects oh hemin and biliverdin. Toxicol Appl Pharmacol 2002; 181: 106-15. [ Links ]
69. Nelson SD. Molecular mechanisms of the hepatotocixity caused by acetaminophen. Sem liver Dis 1990; 10: 267-78. [ Links ]
70. Fondevila C, Shen XD, Tsuchiyashi S, Yamshita K, Csizmadia E, Lassman C, et al. Bilivedin therapy protects rat livers from ischemia and reperfusion injury. Hepatology 2004; 40: 1333-41. [ Links ]
71. Wunder C, Potter RF. The heme oxygenase system: its role in liver inflammation. Curr Drug Targets Cardiovasc Haematol Disord 2003; 3: 199-208. [ Links ]
72. Pae HO, Chung HT. Heme Oxygenase-1: its therapeutic roles in inflammatory disease. Immune Network 2009; 9: 12-19. [ Links ]
73. Buell JF, Gross TG, Woodle ES. Malignancy after transplantation. Transplantation 2005; 80: S254-S264. [ Links ]
74. McCarty MF. "Iatrogenic Gilbert syndrome"--a strategy for reducing vascular and cancer risk by increasing plasma unconjugated bilirubin. Med Hypotheses 2007; 69: 974-94. [ Links ]











 text in
text in