Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.30 no.2 Bogotá Apr./June 2015
Malignant Hepatic Neoplasms: Part 1
Hepatocellular carcinoma: the roles of liver biopsies and immunohistochemical studies and other important issues
Rocío del Pilar López Panqueva MD. (1)
(1) Medical Pathologist in Anatomical Pathology Section at the Hospital Universitario Fundación Santa Fe de Bogotá and Professor in the Faculty of Medicine at Universidad de los Andes in Bogotá, Colombia.
Received: 08-05-15 Accepted: 22-05-15
Abstract
We continue with our review of liver tumors in which we will refer to the most common primary malignant liver tumors. As mentioned previously, hepatobiliary malignancies are a spectrum of invasive tumors whose names depend on their origins. According to the International Agency for Research on Cancer (IARC), malignant liver tumors are the second leading cause of death worldwide with approximately 745,000 deaths/year. (1)
As in the previous article, the aim here will be to identify the most relevant aspects of these neoplasms. In particular we will look at morphological findings that generate major diagnostic problems and at the usefulness of immunohistochemical studies and their differential diagnoses.
Hepatic carcinoma, the most frequent malignant epithelial tumor of the liver is the focus of the first part of this review. We will look at some of its variants and its precursor lesions, and at the controversial role of liver biopsies for diagnosis.
Keywords
Malignant liver tumors, hepatocellular carcinoma, dysplastic nodules, fibrolamellar hepatic carcinoma, percutaneous liver biopsy, aspiration biopsy, immunohistochemistry
HEPATOCELLULAR CARCINOMA OR HEPATOCELLULAR CARCINOMA (HCC), GENERAL ASPECTS
Worldwide, hepatocellular carcinoma (HCC) is the most common primary malignant liver tumor. Eighty-five percent of all cases occur in the context of chronic liver disease and cirrhosis of any etiology, but especially that associated with infection by Hepatitis B and C. Only 15% occur in non-cirrhotic livers. Patients with HCC have poor prognoses: it is the second leading cause of cancer-related death in men and the sixth in women (2).
HCC's varies according to age and geographical area. It is most frequent in people over the age of 65 years, and 80% of all HCC cases occur in developing countries. There is a high incidence in Asia and sub-Saharan Africa which reaches 80 per 100,000 inhabitants/year in women and more than 110 per 100,000 inhabitants/year in men. Half of the deaths occur in China. In low-incidence areas including developed countries and regions such as the USA, Europe, Australia and New Zealand, the incidence is 6.8 per 100,000 in men and 2.2 per 100,000 in women (3).
Colombia is a country with low HCC prevalence according to Globocan 2012 IARC data which shows that in Colombia the incidence of "liver cancer" without specifying the tumor subtype is 2.8 and 3.2 per 100,000 inhabitants. Female mortality adjusted for age is 3.5-4.0 per 100,000 inhabitants and male mortality is 4.1-4.5 per 100,000 inhabitants. The data also establish that the estimated number of new cases for 2015 will be 1,462 (1).
HCC develops especially in the context of chronic liver disease and cirrhosis. Many risk factors have been identified, but hepatitis B virus, hepatitis C and alcohol are the most prevalent in the world. HCC develops in a manner similar to other solid tumors. Especially in cirrhotic livers, hyperplastic nodules progress to low grade dysplastic nodules, then to early high grade HCC and then to HCC with even greater dedifferentiation (3). These changes depend on complex interactions among the host, the underlying disease and environmental factors. Modifying these is how the world can achieve lower mortality rates. Oxidative stress also plays an important role in carcinogenesis and tumor recurrence. It supports the finding of serum levels of derivatives of reactive oxygen metabolites (ROM-d) which are independent predictors of recurrence of HCC (p = 0.0185) (4).
These variations in the overall incidence reflect variations of risk factors. High-incidence Countries in which the prevalence of viral infections such as Hepatitis B and C is very high have high incidences of HCC and are responsible for 75% to 80% of total cases. It should be noted that hepatitis B causes 60% of cases in developing countries and only 23% in developed countries (3). Approximately 85% of cases occur in patients with cirrhosis of any etiology. In the USA hepatitis, C is one of its main causes, but the increased prevalence of metabolic syndrome, diabetes mellitus, obesity, alcoholic steatohepatitis and non-alcoholic steatohepatitis (NASH) are now contributing to increased incidence of other etiologies (5).
These variations seem to be related to various etiologies and different risk factors, mainly associated with parenchymal inflammation and infection with hepatitis B and C. The roles of genetic factors, epigenetic factors, activation and inactivation of oncogenes, and inactivation of tumor suppressor genes are being widely studied. An example of these is the deregulation of the Wnt/β-catenin pathway which is associated with the development of HCC in Europe and to a lesser extent in Asia. In Colombia, a study by the Gastro-hepatology Group at the University of Antioquia has shown that signaling Wnt/β-catenin is active in 42.6% of HCC samples, especially those associated with HBV infections. HBV is one of the most important risk factors for HCC in our country (6). Aflatoxins in the diet play an important role in carcinogenesis of HCC in some populations in sub-Saharan Africa, China and Asia, but have not been found to be important in Colombia. Here, the study of Navas and colleagues has found only 10.5% have mutations of codon 249 of gene p53 tumor suppressor gene (a common aflatoxin mutation hotspot) (7, 8).
In Western countries, it is interesting to see how changes in lifestyle, changes environmental factors, increasing rates of neonatal hepatitis B vaccinations, and decreasing exposure to aflatoxins in diets are all changing the evolution of the epidemiology of this tumor (2).
The guidelines suggested by the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL) have developed some strategies to follow since treatment, especially of high-risk patients, depends on early diagnosis of HCC. These guidelines are based on the size of the liver nodules and identification with imaging (9,10).
When should a liver biopsy be done?
Histological diagnosis is not necessary when the imaging diagnosis of HCC is clear. This is considered to be Evidence Level 1A and a Grade A Recommendation. Histological diagnosis by biopsy is indicated when imaging findings are atypical. This is a Grade C recommendation with Evidence Level 3b (9).
Indeterminate lesions should be imaged and have biopsies performed according to the level of suspicion for malignancy. Some studies have demonstrated the utility of combining radiology and biopsy which improves sensitivity without compromising specificity or positive or negative predictive value for the diagnosis of HCC in patients with cirrhosis who have nodules that are 20 mm or smaller. While the most common diagnosis is HCC intrahepatic cholangiocarcinoma, benign lesions, and metastasis (even when they are unusual in cirrhotic livers) can also be diagnosed. We have found and described metastases of neuroendocrine tumors, colon carcinomas and mammary gland cancers, among many others (11).
Given the clinical, pathological and molecular heterogeneity of HCC, there are several therapeutic modalities available which may be curative. These include surgical resection and liver transplantation, but they also include palliative treatments including local ablation, catheter directed therapies and systemic therapy.
When should histopathology not be done?
There is much controversy about when a biopsy should not be done, especially since diagnostic imaging has demonstrated great accuracy and excellent sensitivity and specificity for the diagnosis of hepatic masses (12). The overall complication rate of biopsies is 29%, but 90% of these are minor complications including pain and transient hypotension. There are also major complications such as intrahepatic or intra-peritoneal hemorrhaging, hemothorax, pneumothorax, injury to nearby organs and arteriovenous fistulas. The risk of seeding in the needle tract, intra-procedural hematogenous spread and recurrence after transplantation have a prevalence rate of 0.003% to 5%. The mortality rate after liver biopsies due to significant bleeding and peritonitis is thought to be between 0% and 0.18% (13).
We must ask. "What is the diagnostic challenge in a cirrhotic patient? When does a patient benefit from a histopathological study?"
At present, histopathological study of biopsies is done most often for patients with atypical images that do not allow achieving differentiation of HCC. Only judicious clinical judgment and individualization of each patient can determine the exact indication and benefit of a biopsy.
There are 4 major challenges in diagnosis of liver mass (14, 15):
- Achieving differentiation of whether a benign nodular hepatocellular lesion is a large regenerative nodule, a dysplastic nodule, focal nodular hyperplasia, a hepatocellular adenoma or simply reactive hepatocytes.
- Differentiating HCC from intrahepatic cholangiocarcinoma and metastatic tumors.
- Determining the histogenesis of malignant tumors.
- Establishing the primary site of origin of a metastatic liver tumor.
Which cytology method is better, fine needle aspiration (FNA) or percutaneous biopsy? Why?
Image guided FNA is useful for patients with significant comorbidities and advanced disease and is safe and minimally invasive. It has good sensitivity (91.5%) and overall diagnostic accuracy (92.4%), with excellent specificity (100%) and positive predictive value (100%) with acceptable negative predictive value (59.1%). It also correlates well with results from aspiration cytology for malignancy and levels of alpha fetoprotein of 400 micrograms/L (14). If used in liquid or block cytology base or in combination with biopsy, additional histochemical studies such as a reticle study or immunophenotype study which are useful for diagnosis of HCC can also be performed easily (16, 17).
The principal complication is bleeding which most frequently occurs in superficial locations of HCC, in large tumors and in severe cirrhosis. Implantation metastases form a subcutaneous nodule on the site or path of the previous biopsy, but have little effect on prognosis and can be treated with resection or radiation therapy.
The role of FNA has changed in the last decade. Although it usually produces only a small amount of material for cytology, and it focuses on cell morphology and can only identify subtle architectural changes with great difficult, this method has low risks and morbidity, is inexpensive, and gives quick results. Optimal results depend more on teamwork between radiologists and cell pathologists who are experts and have specialized training. Ideally, a cellular pathology service should exist in the same place where the procedure is done to allow the possibility of immunohistochemical studies and appropriate clinical-pathological correlation in a safe, fast, and economical system. These benefits make FNA most likely be done quickly as a starting point toward personalized medicine in the diagnosis of hepatic masses which is also recommended in the AASLD guidelines (12).
Pathologists prefer Trucut or percutaneous biopsy because it produces more tissue which can be analyzed not only for cell morphology but also for more evident architectural alterations. More histochemical and immunohistochemical techniques can be performed with precision including molecular studies which are necessary for diagnosis in some cases. This is especially important in light of the new targeted therapies which are showing great prognostic and predictive utility.
Generally, confirmation of a malignant tumor is required for initiation of any curative or palliative therapy. Several studies show false positives account for up to 20% of cases (7% to 31%) if only noninvasive studies are used for diagnoses. The recommendation calls for use of histopathology for cases with atypical radiological features in at least two consecutive studies. This evaluation can differentiate between premalignant lesions such as large cell and small cell dysplastic nodules and HCC. In addition, it can differentiate between early HCC and atypical HCC variants that must be recognized morphologically. This can confer significant prognostic value and lead to different therapeutic managements (18, 19, 20). Some groups have shown that a preoperative biopsy does not adversely affect whether a cancer patient will be able to receive a liver transplant. This is an important factor that supports preoperative biopsies rather than restricting their use in questionable situations (21).
IMPORTANT PATHOLOGICAL ISSUES
For more than one century many authors have described classification systems based on the macroscopic image. Generally it requires examination of the entire tumor and does not provide prognostic or predictive information by itself, nor does it give clues that can explain pathogenesis.
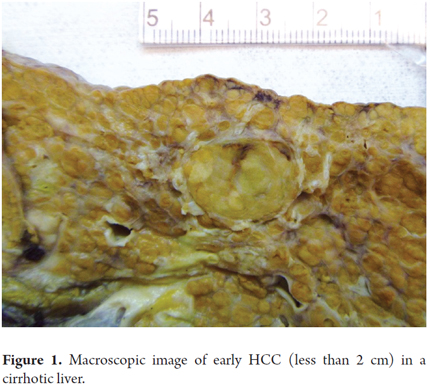
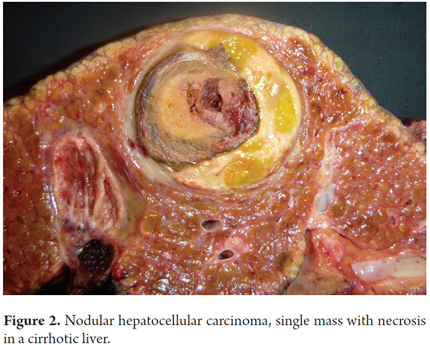
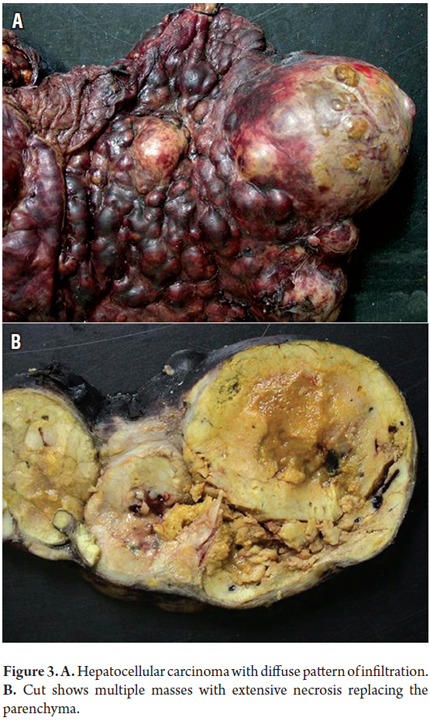
Table 1 summarizes existing macroscopic classifications. Small, pedunculated and encapsulated tumors without evidence of vascular invasion, and those not associated with cirrhosis have better prognoses. Large tumors, necrosis, vascular invasion, and biliary compromises have poorer prognoses. Macroscopic images of early HCC (less than 2 cm), and nodular masses with only diffuse and infiltrative patterns are shown in Figures 1, 2 and 3.
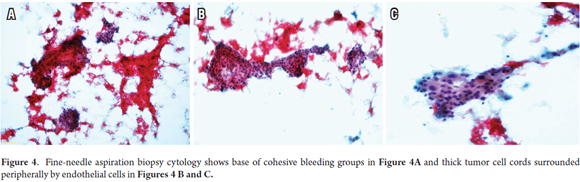
The main features of shown in the fine needle aspiration cytology study are the presence of polygonal cells with large eosinophilic cytoplasm, vesicular or hyperchromatic nuclei which vary according to the degree of differentiation, prominent nucleoli arranged in three-dimensional clusters, trabecular dispositions, pseudo-glandular or bile pigment, cohesive clusters with tree-like projections and thick strands of tumor cells which can be peripherally surrounded by endothelial cells (Figure 4).
FNA poses problem for differential diagnosis between well-differentiated hepatocellular lesions and metastatic tumors with poorly differentiated HCC. The presence of necrosis may suggest an abscess or atypical reactive hepatocytes near metastatic lesions that can be confused with HCC. The help of immunohistochemical methods is useful and necessary (22, 23). Nevertheless, their use is usually limited by the absence of sufficient aspirated cytological material.
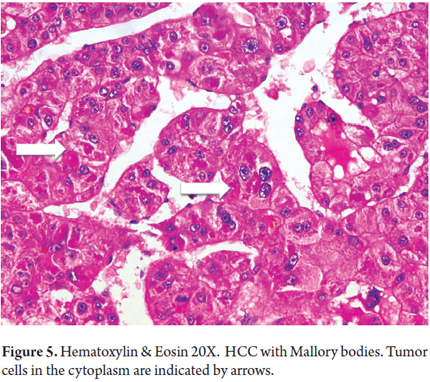
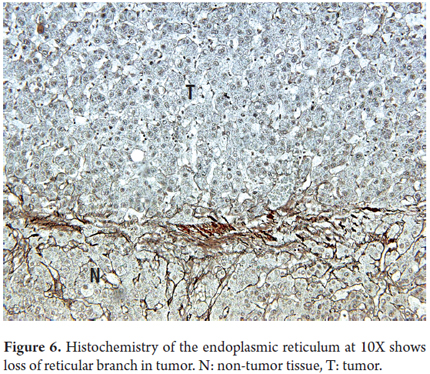
Histopathological biopsies or resected HCC tumor cells resemble normal liver cells, the presence of atypical cells depends on the degree of tumor differentiation. Tumor hepatocytes are arranged in trabeculae of rows of more than two cells. Their cores vary in size and shape, evidence hyperchromasia and irregularity of the membrane, have increased nucleus/cytoplasm ratios and have prominent nucleoli with cytoplasmic eosinophilia. HCC can contain normal cellular products such as liver or bile glycoproteins, fat, Mallory bodies, alpha1 antitrypsin cells, fibrinogen and other cellular proteins (Figure 5). The bile canaliculi are frequently observed surrounding the tumor or plugs in dilated bile canaliculi cells. This condition is very useful for differential diagnosis of poorly differentiated or metastatic tumors. The reticular pattern is lost in HCC (Figure 6) (24, 25).
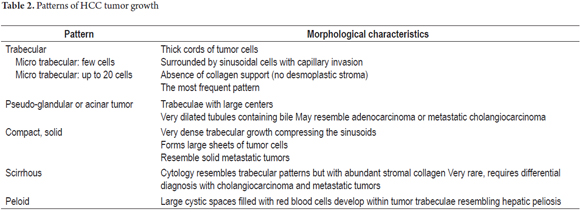
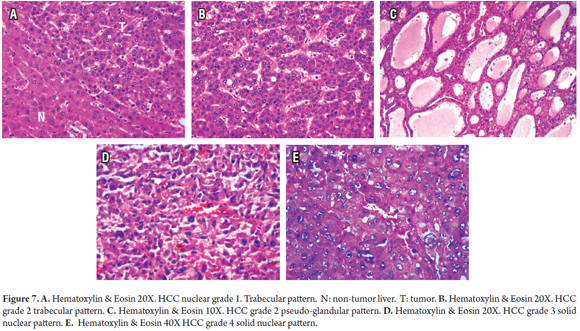
Several architectural patterns can be observed in hepatocellular carcinoma. Table 2 summarizes the main characteristics of the most commonly observed patterns. Microscopic graduation is based on nuclear alterations. In 1954, Edmondson and Steiner proposed a scale from I to IV which takes into account the size, nuclear irregularity, hyperchromasia, and nuclear/cytoplasmic ratios associated with tumor differentiation (Figure 7). Since it is impossible to cytologically differentiate hepatocellular adenoma in Grade I, the diagnosis must be made on the basis of architectural changes, growth patterns, vascular invasion and/or metastasis.
Necrosis and vascular invasion are common. The latter is observed in resection and transplant explants, but is unusual to find in biopsies. Intrahepatic metastases occur in 60% of tumors that are smaller than 5 cm and in more than 95% of larger tumors. Invasion of bile ducts is rare and occurs in less than 5% of cases.
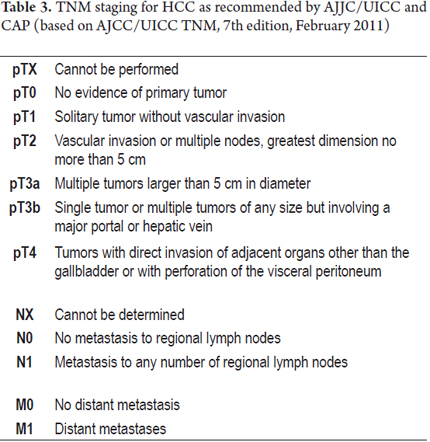
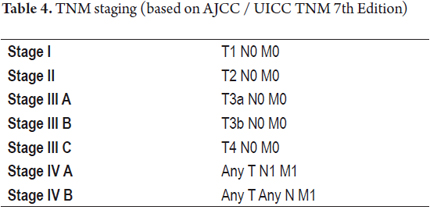
The TNM Classification of Malignant Tumors cancer staging notation system is coded according to the recommendations of the AJCC/UICC (Joint Committee for the Study of Cancer and the International Union for Cancer Control) and CAP (College of American Pathologists) (Tables 3 and 4).
HCC VARIANTS
Recognized morphological variants of HCC include scirrhous carcinoma, sarcomatoid carcinoma, clear cell carcinoma, inflammatory carcinoma, lymphoepithelioma-like carcinoma (LELC) and medullary carcinoma. Differentiation of biliary types usually accompanies these classic forms. Mixed tumors such as hepatocholangiocarcinoma that contain foci of these variants or combinations of several of them can also occur.
Some, like clear cell carcinoma in which large tumor cells have clear, transparent cytoplasm, are identified by abundant glycogen content, but this can cause problems for diagnosis since they resemble adrenal cortical carcinoma, renal carcinoma and metastatic clear cell neuroendocrine carcinoma.
Scirrhous HCC has abundant fibrous stroma which resemble sarcomas, cholangiocarcinoma and metastatic adenocarcinoma. In these cases it is useful to use immunohistochemical studies.
FIBROLAMELLAR HCC
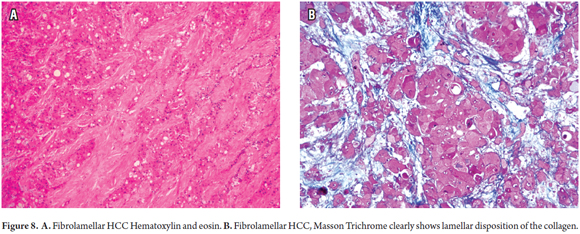
Fibrolamellar HCC is a distinct subset of HCC, different from those described above. It has very different clinical and prognostic features. It usually occurs in young women, men and adolescents who are on average 23 years old. It has no association with chronic liver disease, cirrhosis, or other known associated risk factors. In addition, it grows more slowly than other types and has better prognoses with 50% to 70% 5 year survival rates. In this variant, cells have abundant eosinophilic and granular cytoplasm, round nuclei, often with inclusions, and prominent nucleoli. Most characteristic is the arrangement of collagen which is abundant, forms parallel plates, "lamellar" between trabeculae or nests of hepatocyte cell tumors (Figure 8).
The main differential diagnosis of masses in young people with non-cirrhotic livers were described in our previous article. They include adenoma, focal nodular hyperplasia and nodular regenerative hyperplasia. Sclerosing variants of scirrhous carcinoma and those associated with hypercalcemia should also be considered in the differential diagnosis of fibrolamellar HCC (24). We recently described an unusual variant of HCC mixed with classic and fibrolamellar components (26).
It is in these cases where histopathological examination is essential for determining the histogenesis of nodular liver lesions. A biopsy or complete resection of the lesion is preferred because the fibrolamellar component is not very cellular or readily apparent in FNA samples so a definitive diagnosis is difficult or impossible (24).
PRECURSOR LESIONS: DYSPLASTIC NODULES
Dysplastic nodule (DN) is the term used to identify a benign lesion that may be confused with HCC including the lesions described above such as adenomatous hyperplasia and macro regenerative nodules, or any nodule in a cirrhotic liver that is grossly different in form and color from the surrounding cirrhotic nodules which are detected by imaging studies. Histological examination is necessary to classify whether the nodules are low grade or high grade based on morphological characteristics.
Low grade nodules are portal nodules containing dysplastic areas within them that consist of liver cells that are very similar to normal cells with normal or slightly increased nucleus/cytoplasm ratios, minimal atypical nuclei, but without mitosis.
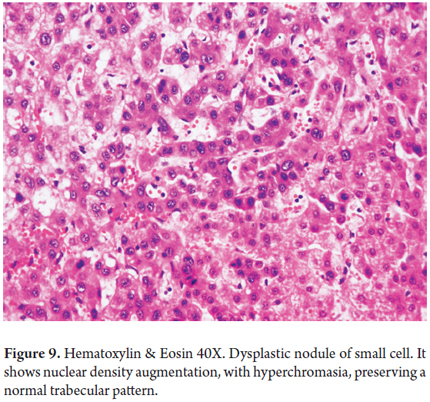
High grade nodules are dysplastic and characterized by altered small cells. When the hepatocytes are considerably smaller than normal cells and appears as an area of nuclear agglomeration (or they may be arranged separately) with increased nuclear density, it is considered to be a pre-neoplastic lesion. There is more basophilic cytoplasm than in normal hepatocytes, but no significant number of atypical nuclei, nor invasion of stroma (Figure 9). They may have pseudo-glandular dispositions and may have the appearance of nodules within nodules. In some cases it may be extremely difficult or impossible to differentiate between a high-grade DN and HCC especially in fine needle biopsies (21, 22).
A study of the endoplasmic reticulum may help is the lattice. Its structure is preserved or only focally absent. CK7 staining shows reactivity of ducts which have proliferated in the periphery of the nodule in more than 95% of cases of high-grade DN. In HCC this ductal reaction is absent or is seen as focal with CK7 staining.
IMMUNOHISTOCHEMISTRY STUDIES USEFUL IN THE DIAGNOSIS OF HCC
There are many difficult-to-interpret immunohistochemical markers that may be useful in for confirming a diagnosis of HCC in selected cases. In Table 5 we list only the most commonly used and the key points to keep in mind (27-32).
- Polyclonal carcinoembryonic antigen (pCEA) is useful for demonstrating bile canaliculi of both normal liver tissue and HCC.
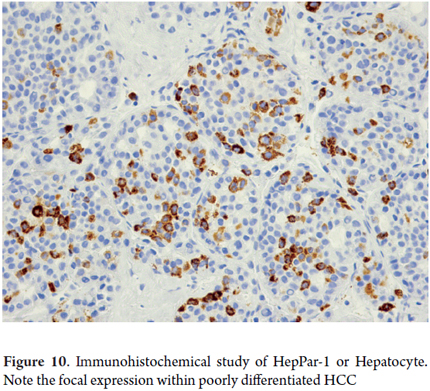
- Hep par 1 antibody stain is a monoclonal antibody that reacts with an epitope of liver mitochondria. It produces positive staining in about 90% of HCC cases, even though it is not exclusive to hepatocellular tumors and should be interpreted in an appropriate clinical and morphological context (Figure 10).
- Alpha-Fetoprotein (AFP) is abundant in the serum of patients with HCC, even when immune-staining is negative for the tumor. Patchy staining is found in more than 50% of HCC cases.
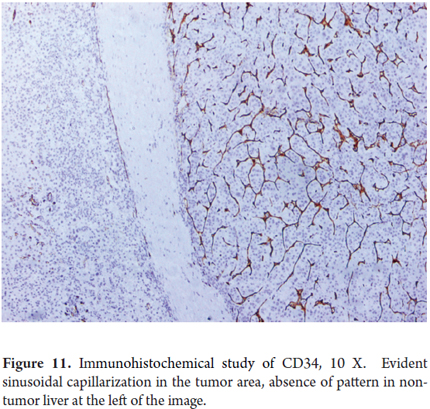
- CD34 is a marker of endothelial cells but specialized liver sinusoidal endothelium does not normally express this marker. A phenomenon in HCC known as sinusoidal capitalization. Cells surrounding the trabeculae of HCC are generally positive for CD34 while benign hepatocellular lesions are typically CD34 positive only in peripheral areas or near the fibrous septa. Diffuse sinusoidal reactivity to CD34 may be useful for distinguishing a cirrhotic nodule from well differentiated HCC (Figure 11).
- HSP70 is a protein that plays an important role in cell regulation, cycle progression and apoptosis. It is expressed in more than 80% of early HCC cases.
- Glutamine Synthetase (GS) is the result of nuclear translocation of β-catenin and correlates with activation of HCC. Diffuse expression can be found in up to 70% of early HCC cases. It can also be found early in cases of high grade dysplastic nodules, but is focal and occurs in less than 15% of these cases.
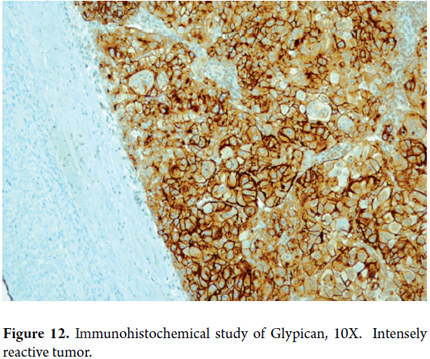
- Glypican-3 (GPC-3) is an oncoprotein that is identified in most cases of HCC. It is a good marker of hepatocellular differentiation in primary liver neoplasias. It is not expressed in normal tissue, nor in pseudotumors. Immunostaining exists only focally in some dysplastic nodules and tissue compromised by hepatitis with marked inflammatory activity (Figure 12).
- Arginase-1 (Arg-1) is a very sensitive marker for benign and malignant hepatocytes which can be a useful diagnostic tool for liver tumor pathology. It is detected in 96% of HCC cases, but never occurs in metastatic tumors.
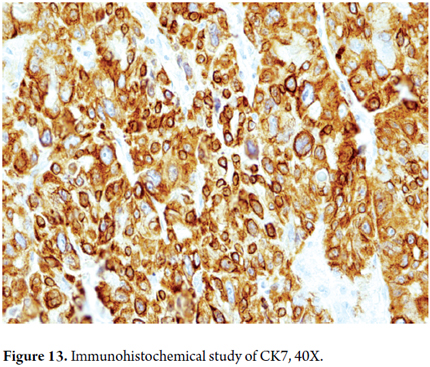
- Cytokeratins are also useful. Those with low molecular weights such as CAM5.2, cytokeratin 8 and cytokeratin 18 react to HCC while cytokeratins 7, 20 and AE1/AE3 are negative to classic HCC. The fibrolamellar variant shows only expression for CK7 (Figure 13).
- Epidermal growth factor receptor (EGFR) has shown utility as a predictive biomarker of response to EGFR antagonists in fibrolamellar carcinoma.
Differential diagnosis is usually required between well-differentiated hepatocellular carcinoma and adenoma, between hepatocellular carcinoma and cholangiocarcinoma, and between hepatocellular carcinoma and metastatic tumors. It is precisely in these cases where immunohistochemical studies are useful. Obviously the interpretation must be done in conjunction with the clinical history, images, morphological pattern, and the combination of histochemistry of the reticulum and two or more of the immunohistochemical studies.
CONCLUSION
The most frequent malignant liver tumors are metastatic or secondary and second primary tumors such as hepatocellular carcinoma. This is especially true for patients with cirrhosis of any etiology. In light of modern medicine, more and better imaging techniques, and the rise of therapies targeted to the patient's need, the decision to do a biopsy may be an ethical necessity that benefits and helps to optimize the interpretation of results and definition of subgroups of patients who may benefit from one or another therapy, thus avoiding the possibility of side effects while lowering costs for the health care system. This scenario can be major clinical challenge both for the simple pathologist and for surgical pathology by the most highly specialized pathologists. It requires a detailed and careful study of cytology and tumor architecture with routine hematoxylin and eosin staining often accompanied by additional histochemistry studies of the reticulum and a panel of immunohistochemical studies. Obviously, it requires knowledge of the patient's medical history, imaging studies, and epidemiology.
REFERENCES
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-86. [ Links ]
2. Wallace MC, Preen D, Jeffrey GP, Adams LA. The Evolving epidemiology of hepatocellular carcinoma: a global perspective. Expert Rev Gastroenterol Hepatol. 2015;1:1-15. [ Links ]
3. Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69. [ Links ]
4. Suzuki Y, Imai K, Takai K, et al. Hepatocellular carcinoma patients with increased oxidative stress levels are prone to recurrence after curative treatment: a prospective case series study using the d-ROM test. J Cancer Res Clin Oncol. 2013;139(5):845-52. [ Links ]
5. Yazici C, Niemeyer DJ, Iannitti DA, Russo MW. Hepatocellular carcinoma and cholangiocarcinoma: an update. Expert Review of Gastroenterology & Hepatology. 2014;8(1):63-82. [ Links ]
6. Suarez I, Uribe D, Jaramillo CM, Osorio G, Pérez JC, López R, et al. Wnt/β-catenin signaling pathway in hepatocellular carcinomas cases from Colombia. Ann Hepathol. 2015;14(1):54-64. [ Links ]
7. Kew MC. Aflatoxins as a cause of hepatocellular carcinoma. J Gastrointestin Liver Dis. 2013;22(3):305-10. [ Links ]
8. Navas MC, Suárez I, Carreño A, Uribe D, Ríos WA, Cortés-Mancera F, et al. Hepatitis B and hepatitis C infection biomarkers and TP53 mutations in hepatocellular carcinomas from Colombia. Hepat Res Treat. 2011;2011:582945. [ Links ]
9. Vilarinho S, Taddei T. Therapeutic strategies for hepatocellular carcinoma: new advances and challenges. Curr Treat Options Gastroenterol. 2015;13(2):219-34. [ Links ]
10. Deepali Jain. Tissue diagnosis of hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4:S67-73. [ Links ]
11. Darnell A, Forner A, Rimola J, Reig M, García-Criado Á, Ayuso C, et al. Liver imaging reporting and data system with MR imaging: evaluation in nodules 20 mm or smaller detected in cirrhosis at screening US. Radiology. 2015;141132. [ Links ]
12. Wee A. Fine needle aspiration biopsy of hepatocellular carcinoma and hepatocellular nodular lesions: role, controversies and approach to diagnosis. Cytopathology. 2011;22(5):287-305. [ Links ]
13. Molla N, AlMenieir N, Simoneau E, Aljiffry M, Valenti D, Metrakos P, et al. The role of interventional radiology in the management of hepatocellular carcinoma. Current Oncology. 2014;21(3):480-92. [ Links ]
14. Wang P, Meng ZQ, Chen Z, et al. Diagnostic value and complications of fine needle aspiration for primary liver cancer and its influence on the treatment outcome. A study based on 3011 patients in China. Eur J Surg Oncol. 2008;34:541-6. [ Links ]
15. Stigliano R, Marelli L, Yu D, et al. Seeding following percutaneous diagnostic and therapeutic approaches for hepatocellular carcinoma. What is the risk and the outcome? Seeding risk for percutaneous approach of HCC. Cancer Treat Rev. 2007;33:437-47. [ Links ]
16. Kuo FY, Chen WJ, Lu SN, Wang JH, Eng HL. Fine needle aspiration cytodiagnosis of liver tumors. Acta Cytol. 2004;48(2):142-8. [ Links ]
17. Castro-Villabón D, Avello Y, Ruiz N, Rodríguez-Urrego PA. Implementation of routine thromboplastin-plasma cell block technique in the evaluation of non-gynecologic specimens: A methodologic comparison with conventional cytology. J Microscopy Ultrastructure. 2014;2(3):177-81. [ Links ]
18. Jain D. Tissue diagnosis of hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4(Suppl 3):S67-73. [ Links ]
19. Compagnon P, Grandadam S, Lorho R, et al. Liver transplantation for hepatocellular carcinoma without preoperative tumor biopsy. Transplantation. 2008;86:1068-76. [ Links ]
20. International Consensus Group for Hepatocellular Neoplasia. International consensus on the pathologic diagnosis of early hepatocellular carcinoma: a report of the International Consensus Group for Hepatocellular Neoplasia. Hepatology. 2009;49:658-64. [ Links ]
21. Fuks D, Cauchy F, Fusco G, Paradis V, Durand F, Belghiti J. Preoperative tumour biopsy does not affect the oncologic course of patients with transplantable HCC. J Hepatol. 2014;61(3):589-93. [ Links ]
22. Barbhuiya M, Bhunia S, Kakkar M, Shrivastava B, Tiwari PK, Gupta S. Fine needle aspiration cytology of lesions of liver and gallbladder: An analysis of 400 consecutive aspirations. J Cytol. 2014;31(1):20-4. [ Links ]
23. Saleh HA, Aulicino M, Zaidi SY, Khan AZ, Masood S. Discriminating hepatocellular carcinoma from metastatic carcinoma on fine-needle aspiration biopsy of the liver: the utility of immunocytochemical panel. Diagn Cytopathol. 2009;37(3):184-90. [ Links ]
24. Goodman ZD. Neoplasms of the liver. Modern Pathology. 2007;20:S49-60. [ Links ]
25. Ferrell L. Liver pathology: cirrhosis, hepatitis, and primary liver tumors. Update and diagnostic problems. Mod Pathol. 2000;13(6):679-704. [ Links ]
26. Castro-Villabón D, Barrera-Herrera LE, Rodríguez-Urrego PA, Hudacko R, Vera A, Álvarez J, et al. Hepatocellular carcinoma with both fibrolamellar and classical components: an unusual morphological pattern. Case Rep Pathol. 2015;2015;609780. [ Links ]
27. Shafizadeh N, Kakar S. Diagnosis of well-differentiated hepatocellular lesions: role of immunohistochemistry and other ancillary techniques. Adv Anat Pathol. 2011;18:438-45. [ Links ]
28. Geller SA, Dhall D, Alsabeh R. Application of immunohistochemistry to liver and gastrointestinal neoplasms liver, stomach, colon, and pancreas. Arch Pathol Lab Med. 2008;132:490-9. [ Links ]
29. Krings G, Ramanchandran R, Jain D, Wu TT, Yeh MM, Torbenson M, et al. Immunohistochemical pitfalls and the importance of glypican 3 and arginase in the diagnosis of scirrhous hepatocellular carcinoma. Modern Pathology. 2013;26:782-91. [ Links ]
30. Kandil SK, Cooper K. Glypican-3: a novel diagnostic marker for hepatocellular carcinoma and more. Adv Anat Pathol. 2009;16:125-9. [ Links ]
31. Yan BC, Gong C, Song J, Krausz T, Tretiakova M, et al. Arginase-1: a new immunohistochemical marker of hepatocytes and hepatocellular neoplasms. Am J Surg Pathol. 2010;34(8):1147-54. [ Links ]
32. Lagana SM, Salomao M, Bao F, Moreira RK, Lefkowitch JH, Remotti HE. Utility of an immunohistochemical panel consisting of glypican-3, heat-shock protein-70, and glutamine synthetase in the distinction of low-grade hepatocellular carcinoma from hepatocellular adenoma. Appl Immunohistochem Mol Morphol. 2013;21(2):170-6. [ Links ]











 text in
text in