Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.30 no.4 Bogotá Oct./Dec. 2015
Evidence Based Review of the Impact of Treatments of Gastroesophageal Reflux Disease
Roger Castillo MD. (1), William Otero MD. (2), Alba Trespalacios. MSc. PhD. (3)
(1) Internal Medicine Resident (final year) at the National University of Colombia in Bogotá, Colombia.
(2) Professor of Medicine in the Gastroenterology Unit of the National University of Colombia and Gastroenterologist at Clínica Fundadores in Bogotá, Colombia.
(3) Medical Microbiology Specialization Director and Director of the Microorganism Collection in the Microbiology Department at Pontificia Universidad Javeriana in Bogotá, Colombia.
Received: 16-01-15 Accepted: 20-10-15
Abstract
Background: Gastroesophageal reflux disease (GERD) is a very prevalent disease among adults that alters the quality of life. Many treatments have been investigated, some of which require changes in lifestyle related to associated risk factors. Although changes in lifestyle are recommended, the evidence that supports these recommendations is controversial and scarce.
Objective: The objective of this study is to estimate the impact of lifestyle changes on patients with GERD.
Methodology: A systematic search of the literature in PubMed, Science Direct and Embase was conducted using the following keywords: gastroesophagueal (sic) reflux, heartburn, bed head elevation, Carbonated Beverages, mint, cocoa, citrus, Drinking Alcohol, caffeine, coffee, late-evening meal, spicy food, fatty foods, obesity, weight loss, exercise and Smoking Cessation. Controlled clinical trials and prospective cohort studies that studied lifestyle changes and their effects on GERD were included in the study.
Results: Of the 2,731 articles found, fifteen were included in our analysis. There is little evidence that suspending consumption of food or drink items such as peppermint, chocolate, citrus, carbonated beverages, fatty foods and spicy foods clinically improves GERD. Decaffeinated coffee may decrease the amount of reflux, and quitting smoking is associated with improvement in symptoms. Meal times at night can change some parameters of pH monitoring but did not alter symptoms. Some exercises, mainly respiratory, may improve symptoms. There is evidence that elevating the head while in the bed and weight loss (in cases of overweight or obese patients) improve symptoms, but there is also evidence against these methods and studies of them have methodological limitations.
Conclusion: Adequate evidence does not exist that changes in lifestyle improve GERD symptoms. Most studies are uncontrolled clinical trials or observational studies. Additional clinical trials with better quality are needed to define the impact of these measures on GERD.
Keywords
Gastroesophageal reflux, heartburn, beverages.
Gastroesophageal reflux disease (GERD) is a condition in which the reflux of stomach's contents causes troublesome symptoms and/or complications (1). The symptoms are considered to be bothersome and affect the quality of life when they occur two or more times per week (1). The prevalence of GERD is high in the general population: in Japan it affects 6.5% to 9.5% of the population (2), in North America it affects 10% to 20%, in Europe its prevalence is between 10% and 20% (3), and in Latin America its prevalence ranges from 11.9% to 31.3% (4). It is estimated that its incidence is 4.5 to 5.4 for every 1,000 patients per year (3). The most common manifestations include regurgitation and heartburn. Both negatively impact quality of life (5, 6), and both are considered to be typical symptoms of GERD. Besides these esophageal symptoms, GERD also produces structural changes in the esophagus including esophagitis in 35% of patients and strictures. Barrett's esophagus occurs in 10% of cases and can even lead to esophageal adenocarcinoma (7, 8). GERD is also associated with various extraesophageal entities such as asthma, lung disease, laryngitis, and coughing (9, 10, 11). While these associations are consistent, causality has been difficult to prove except in the cases of a few ear and throat conditions (1). Some experts believe that it is often not possible to determine whether an abnormality is the cause or consequence of GERD (7). The incidence of esophageal cancer in patients with GERD ranges from 1.0/100,000 to 60.8/100,000 person-years depending on age. It is more common among those over 70 years of age (8), men, smokers, and regular consumers of alcohol (12). Despite GERD's negative impact on the quality of life, it does not decrease survival (13).
The most important mechanisms leading to GERD are related to transitory relaxation of the lower esophageal sphincter (LES) which is mediated by the vagovagal reflex (14). These abnormal relaxations are independent of swallowing and last about 20 seconds. This is longer than typical relaxation during swallowing (15). Although after food consumption acidity decreases, it is known that the large amount of acid in reflux after meals is produced by the formation of an "acid pocket" consisting of a pocket of acid within the food in the proximal stomach (15). An acid pocket that remains above the diaphragm, especially in a person with a hiatal hernia, is a major risk factor for development of gastroesophageal reflux (16). Treatment of GERD has traditionally been based on pharmacological measures alone without surgery. The gold standard has been proton pump inhibitors (17), but additional measures are often recommended, especially changes in lifestyle. These include losing weight; elevating the head of the bed; and avoiding tobacco, alcohol, caffeine, spicy food, acidic food, high-fat food and eating late at night (17). Other measures that have been evaluated include consumption of chocolate and carbonated drinks and sleeping on the right side. The usefulness of these measures is controversial. A systematic review in 2006 found no evidence that, with the exception of weight reduction, none of these measures confers any additional benefits in relation to the symptoms of GERD (17). However, these changes continue to be recommended despite the absence of sufficient evidence in the literature. Given the controversy about the real utility of lifestyle change, we decided to conduct this systematic review with the aim of estimating the impact of changes in lifestyle on patients with GERD.
METHODOLOGY
A systematic literature search of PubMed, Science Direct and Embase was conducted in August 2014. The search was limited to the past 20 years and to articles in English and Spanish. The keywords used in the search were gastroesophagueal reflux, heartburn, bed head elevation, Carbonated Beverages, mint, cocoa, citrus, Drinking Alcohol, caffeine, coffee, late-evening meal, spicy food, fatty foods, obesity, weight loss, exercise and Smoking Cessation (spelling and capitalization of search terms from the Spanish original). Studies eligible for inclusion were controlled trials and prospective cohort studies done in the past 20 years. Studies were included if the study only included people over 18 years of age who had been diagnosed with GERD and only if the reports studied interventions in subjects lifestyles. The results were supported by symptoms, impedance and esophageal pH monitoring.
Study selection, data extraction and synthesis
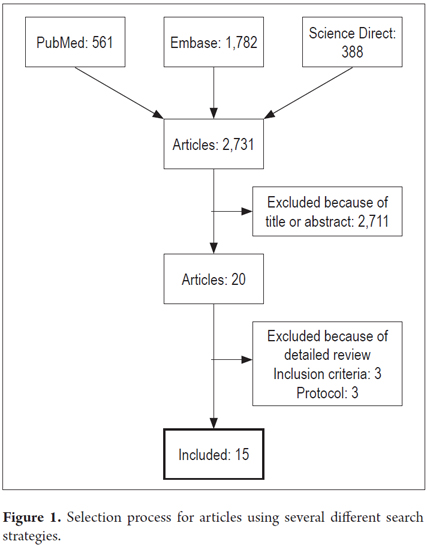
The search yielded a total of 2,731 articles which were initially assessed on the basis of their titles and abstracts. The evaluation was conducted independently by two reviewers (RC, AT). Twenty articles were chosen for the study after discarding 2,711 on the basis of the criteria for inclusion. After evaluation of the methodology, fifteen of these articles were finally included in the analysis with RevMan 5 (Figure 1).
Quality assessment
Once these studies had been selected, data were extracted and entered into a standardized format and evaluated using the Scottish Intercollegiate Guidelines Network (SIGN) tool for controlled clinical trials and prospective studies (18). The risks of bias and the quality of each study were assessed according to SIGN recommendations:
High quality (++): Most of the criteria are met. The study has little or no risk of bias, and outcomes are unlikely to be changed by other research.
Acceptable quality (+): Most of the criteria are met, there are some design defects associated with risk of bias, and the conclusion could change with further research.
Low quality (0): Most criteria are not met, there are significant flaws in key aspects of the design, and the conclusion is likely to change in future studies.
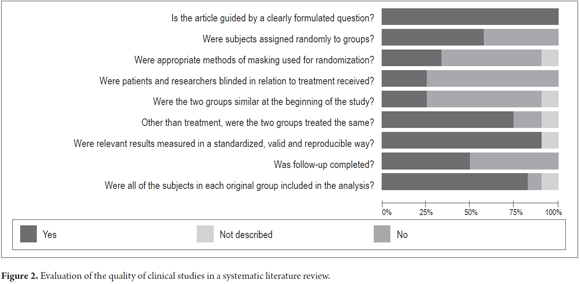
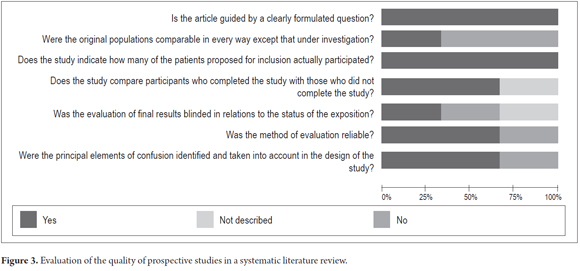
The results of each study were entered into Review Manager (RevMan) 5 from the Cochrane Library. The checklists of the research questions that were used for the study selection on this article are shown in Figures 2 and 3.
Finally, disagreements were resolved according to the criteria with the help of two experts. The GRADE system for assessing the level of evidence and strength of recommendations was used (19, 20). In this system, the level of evidence can be ranked as high, moderate, low, or very low. High means that is unlikely that further studies will modify confidence in the estimate of effect. Moderate means that new studies are likely to have a significant impact on confidence in the estimate of effect and that they may change the outcome. Low means that it is likely that new studies will have an important impact on confidence in the estimated result and that they may change the outcome. Very low means that any estimate of effect is very uncertain. The strength of a recommendation is rated as "b" when the desired effect of the intervention clearly exceeded undesirable effects but is rated "weak" when benefits, risks and undesirable effects are in close balance, or there is substantial uncertainty about the magnitudes of the benefits and risks.
RESULTS
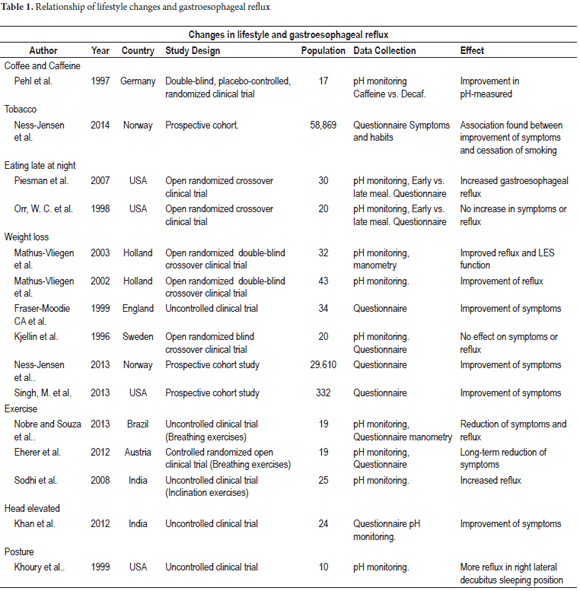
Figure 1 summarizes the Search and inclusion processes. Of the 2,731 articles initially found, only 15 items studied changes in lifestyle. The study types finally selected and the corresponding patient numbers are shown in Table 1. Available evidence about each recommendation for lifestyle change will be described separately. Some pathophysiological issues involved in lifestyle changes and studies that generated hypotheses associating risk factors and GERD are also described.
Fatty Food
Several studies have tried to link the consumption of foods that are high in fat to GERD, but this association remains controversial. One controlled clinical trial compared sensitivity to an acidic substance after an infusion of saline infusion with a 20% lipid solution (21). After two days there were no differences in starting times or intensity of symptoms (21). Another study compared the consumption of foods that are high in fact with foods that are low in fat. At the end of the study, no association between fat content and worsening of GERD as measured by pH could be found (22).
The relative effects that foods with high proportions of fat and low proportions of fat have also been calculated for pressure on the lower esophageal sphincter (LES), transient LES relaxations, reflux episodes, and the amount of time that pH is less than 4.0. One clinical trial compared high and low fat content in meals in twenty healthy individuals and found no significant difference (23). In contrast to other studies, Penagini et al. found no differences in episodes of reflux, exposure to abnormal levels of esophageal acid, the rate of transient LES relaxations or LES pressure related to high and low concentrations of fat in food (24). Meyer et al. have found increased sensitivity to acid after consumption of fat (25). Although Holloway et al. found no significant effect of fatty food on LES pressure, they did find that people with GERD have more frequent episodes of reflux and transient LES relaxations than do healthy people (26). A more recent study of healthy subjects found that intraduodenal infusions of fat decreased LES pressure and increased exposure to acid more than did infusions proteins or carbohydrates (27). Another clinical trial that evaluated different concentrations of fat and calories found that reflux is related to the amount of calories but not to fat concentration (28). Based on the available research, there is no evidence to support lowering the fat content in meals eaten by patients with GERD. In addition, there are no studies that specifically evaluate the impact of reduced dietary fat. Recommendation: Weak. Level of evidence: Low.
Spicy food
Spicy food has been very frequently linked to precipitation of GERD. This link was initially mentioned by Nebel et al (29). In Brazil, an observational study reported that spicy food hastened the onset of reflux in 11.7% of the people with GERD (30). Two studies in Pakistan found that spicy food precipitated reflux in most patients (31, 32). In Korea, Song JH et al. evaluated the effect of food on GERD with a questionnaire and found a risk of 9% (OR 1.09, 95% CI: 1.02-1.16) (33). Unlike these studies, Pandeya et al. found no association between spicy food and GERD (34). The global analysis indicates that there is no consistent relation between the consumption of spicy food and GERD, so we cannot recommend that GERD patients stop eating spicy food to improve their symptoms. Recommendation: Weak. Level of evidence: Low.
Carbonated beverages
Carbonated beverages are considered to precipitate GERD, although this is controversial. Several studies of healthy volunteers have found that these beverages reduce LES pressure below the pressure found in relation to water or other beverages and that they increase transitory relaxation (35, 36). Descriptive studies based on people diagnosed with GERD have also found associations between consumption of carbonated beverages and GERD (37, 38). Another study of healthy subjects assessed the effects of beverages with different concentrations of carbon dioxide, but did not find levels of reflux that were higher than in subjects who consumed beverages that are not carbonated (39). There are no published studies evaluating the effects of suspending consumption of carbonated beverages to improve GERD. To date, the data has not been consistent enough to support advice that patients avoid consumption. Recommendation: Weak. Level of evidence: Low.
Mint
Mint is used as a flavoring and has been thought to be related to gastroesophageal reflux, but studies have failed to demonstrate any particular association. Bulat et al. investigated the effects of different doses of spearmint on the LES in healthy individuals and found no changes in LES pressure or in reflux (40). There is no evidence available that would support the recommendation to suspend the use of mint to control GERD. Recommendation: Weak. Level of evidence: Low.
Chocolate
One of the first studies that evaluated the effects of chocolate on reflux was conducted in 1975 on 9 healthy volunteers. After volunteers ingested 120 ml of chocolate, LES pressure was measured, and significant decreases from baseline pressure were found: 14.6 mmHg +/- 1.1 mmHg to 7.9 mmHg +/- 1.3 mmHg (P <0.01) (41). Another study with 6 volunteers found that the length of the LES was affected after consumption of chocolate (42). More recently, Murphy et al. evaluated the effect of chocolate consumption on esophageal pH. They found a significant increase of abnormal acid exposure over what occurred with other beverages (43). Nevertheless, two other studies of patients with GERD did not find that chocolate was a risk factor for GERD symptoms (38, 44). In addition, we found no study that assessed effects of suspending chocolate consumption in patients with GERD. Recommendation: Weak. Level of evidence: Low.
Citrus Juices
Several studies have found that consumption of citrus juices increases heartburn in patients with GERD. Citrus juice has also been recognized as a precipitant of symptoms in some populations (29, 45, 46). Another study found that the length of the LES decreased following consumption of 240 mL of orange juice by healthy individuals (42). In contrast, other research found that neither symptoms nor the length of the LES is affected by consumption of citrus juice by patients with GERD (44, 47). One study also found that consumption of orange juice led to symptoms in patients and that they had neutral pH (48). To date no studies have evaluated suspension of consumption of citrus juices on GERD. Recommendation: Weak. Level of evidence: Low.
Alcohol
For decades, alcohol has been associated with occurrence of gastrointestinal symptoms. Hogan et al. were among the first to document a decrease in LES pressure after consumption of alcohol (49). Other effects have also been linked to alcohol. They include increased acid secretion, reduction of LES length, increased spontaneous relaxations of the LES, alterations of esophageal motility and alterations of gastric emptying (50). Studies of healthy people have shown increased symptoms and decreased esophageal pH (51-54). Associations between alcohol consumption and GERD have also been found (55-63). Recently, a relationship between increased alcohol consumption and increased pyrosis has been found among patients who have used NSAIDs (64). However, other observational studies have not found that alcohol consumption is a risk factor for GERD (65-67). Only one prospective study has evaluated suspension of consumption. After 6 months of suspension, improvements in esophageal motility disorders were found, but the alterations of esophageal pH and symptoms persisted without change (68). On the basis of current evidence, it is not possible to recommend abstinence from alcohol in order to improve GERD. Recommendation: Weak. Level of evidence: Low.
Coffee/Caffeine
Like other substances, caffeine is considered to be a precipitant of symptoms in patients with GERD. Feldmann et al. found that postprandial heartburn had a significant association with consumption of coffee (45). An association between coffee and the onset of symptoms has also been found in patients with esophagitis (48). Thomas et al. found that, for healthy study participants and participants with GERD, drinking coffee on an empty stomach or after consumption of food caused a significant drop in LES pressure (69). When this same parameter was evaluated by Salmon et al., they did not find the same decrease in LES pressure when coffee was consumed on an empty stomach. They found that the only significant change in LES pressure occurred after coffee consumption following consumption of food. They could not exclude the possibility that the effect was the result of food ingestion (70). A prevalence study conducted by Nilsson et al. in a Norwegian population found a protective effect even for people who drank more than 7 cups per day compared with those who only drank one cup per day (OR 0.6, 95% CI: 0.4 to 0.7) (65). Some surveys have failed to find any association (63, 66, 71). A recent study in Japan of 8,013 people, of whom 5,451 were coffee drinkers, found an association with esophagitis (OR: 0.84, 95% CI: 0.70-1.01, p <0.057) and non-erosive GERD (OR: 0.93, 95% CI: 0.79-1.10, p <0.408) (72). A clinical trial with a group of patients with reflux and a healthy control group in which coffee consumption was compared with consumption of hot water found no difference in the onset of symptoms, in levels of esophageal pH or in parameters esophageal manometry (73).
The decaffeination process has also been studied. Initially, healthy people were studied. It was found that those who drank decaffeinated coffee had less reflux, but this finding was not reproduced in those who decaffeinated drank tea which led to speculation that other components involved in the physiopathology of coffee were involved (74). Subsequently, a randomized clinical trial conducted with patients who had GERD found that the time esophageal pH was below 4.0 was lower in those who drank decaffeinated coffee than in those who drank regular coffee (74). This study was of very short duration and had limitations in controlling all confounding factor so that it is not possible to estimate the effect of the beverage on the behavior of GERD.
Because the findings are not very conclusive, a conclusion about any recommendation involving coffee consumption cannot be made. Better quality studies with longer follow-up times are needed before a conclusion can be reached about whether suspension of coffee or caffeine improves symptoms in patients with gastroesophageal reflux. Recommendation: Weak. Level of evidence: Low.
Smoking
Several mechanisms by which smoking might generate gastroesophageal reflux have been described. Some studies have shown that smoking can reduce the LES pressure (75-77). Others have shown that secretion of bicarbonate in the saliva decreases and that this could lead to lower levels of intra-esophageal neutralization of acid (78, 79). Smoking may generate gastroesophageal reflux directly in some patients due to increases in intra-abdominal pressure from coughing or inhaling deeply (80). Smokers have higher risks of symptomatic gastroesophageal reflux than non-smokers according to several population studies (81-87). A survey by Wang et al. found that smokers were more likely than non-smokers to have GERD (OR = 1.27; 95% CI: 1.17-1.38). This was even more likely for those who smoked more than one pack of cigarettes per day (OR = 4.94; 95% CI: 3.70-6.61) (63). A prospective study by Nilsson et al. found a dose-dependent association and that those who smoked daily for more than 20 years had a higher association than those who had smoked for less than 1 year (OR 1.7; 95% CI 1.5 - 1.9). In addition, those who had smoked cigarettes more than 50,000 had greater association than those who had smoked less than 100 cigarettes (OR 1.6; 95% CI 1.4 - 1.8) (6). However, some studies have reported different results. Fulani et al. compared smokers, including some who abstained from smoking during the study, to nonsmokers. Differences were measured by pH monitoring and through a questionnaire. The study found no differences among the three groups (those who kept smoking, those who abstained from smoking, and non-smokers) in episodes of reflux or in the amount of time that esophageal pH was less than 4.0 (88). Three prospective studies have shown no associations between smoking and increased gastroesophageal reflux (89-91). One study of 14 smokers who abstained from smoking for 48 hours found an increase in reflux episodes after restarting the smoking habit (92).
Recently a prospective study of the effect of stopping smoking on gastroesophageal reflux symptoms was performed (93). Initially, 58,869 people were surveyed. Eleven years later a second survey including 44,997 was conducted. 29,610 people participated in both surveys. 1,553 patients who had severe symptoms in the first survey, and the 486 of these who also smoked, were included. Patients who continued to smoke were compared to those who had stopped smoking (exposed patients). At the end, a correlation between cessation of smoking and improvement of symptoms was found, but only in those who took at least one anti-reflux medication each week and who had normal body mass indexes (OR 5.67; 95% CI: 1.36 - 23.64) (93).
This is the only study that has prospectively analyzed the effect of suspending smoking on reflux symptoms, but because it is an observational study, a causal link still cannot be made. Recommendation: Weak. Level of evidence: Low.
Eating late at night
A case-control study by Fujiwara et al. examined the association between GERD and eating just before going to sleep (94). The odds ratio was adjusted for BMI, smoking and drinking to present reflux in those who ate dinner less than three hours before bedtime. Data for this group were compared to data from a control group who ate at least four hours before bedtime. The resulting OR was 7.45 (95% CI: 3.38-16.4) (94). Two pilot studies have tried to evaluate the effects of eating at different times on GERD. Orr and Harnish conducted a clinical trial with 20 people who had GERD with heartburn at least 4 times a week and who had had at least one episode of symptoms at night during the previous two months. They spent two nights in a sleep laboratory with each stay separated by no more than 3 weeks from the other. On one of the nights they were instructed to consume the usual food before 1900, and on the other night they were given a standardized meal at 2100 hours. They went to bed at 2300 and were awakened at 0600. Polysomnography measured sleep disturbances and patients' pH was monitored. The average number of episodes of reflux experienced in the two groups did not differ significantly. It was 3.1 for early diners, and 4.0 for later diners (p = 0.30). There were no significant differences in durations of reflux episodes (6.9 min vs. 10.8 min, p = 0.14) or the total time that pH was less than 4.0 (14.8 min vs. 21.3 min, p = 0.15). There were also no significant differences in polysomnographic parameters between the two groups. It should be kept in mind that meals prior to 1900 were not standardized, and that patients were allowed to go to the lab on two separate nights up to three weeks apart which allows for great variability (95). A randomized clinical trial conducted by Piesman et al. assigned some patients to eat either early or late one night (1700 vs. 2100) and then to reverse their eating times the next day. They were instructed to go to sleep at 2300 and to wake up 0600 on both days. pH was monitored for the entire 48 hours of the study. 30 patients were included in the analysis. The average amount of time between eating and going to bed for nights subjects ate late was 93 minutes, while the average amount of time between eating and going to bed for nights subjects ate early was 330 minutes. Eating late was associated with a significant increase in the percentage of time with pH less than 4.0 while subjects were in supine positions (mean change, 5.2 +/- 1.6, p = 0.002). There were also significantly longer episodes (mean change 4.8 +/- 2.3, p = 0.021). An analysis of subgroups showed that the patients with esophagitis (11/30) and those with hiatal hernias (14/30) had significantly more supine reflux. There were no significant differences in symptoms. Although lunchtime was early, lunch may have interfered with dinner and caused more distension and reflux (96). These two studies do not allow the conclusion that the dinner schedule may influence symptoms in patients with GERD, therefore we cannot recommend this behavior as a therapeutic measure. Recommendation: Weak. Level of evidence: Low.
Obesity and weight loss
Multiple hypotheses have been advanced about the role that obesity plays in GERD. The most important of all is that obese people experience more episodes transient LES relaxations. This has been seen very frequently in people with high BMI (97, 98). Another mechanism that has been described is increased intra-abdominal pressure which could in turn cause increased intragastric pressure which may make obese people and susceptible to development of hiatal hernias (99-102). Changes in esophageal motility have been observed in overweight and obese patients (103-106).
Multiple studies have looked at whether there is a relationship between GERD and obesity. Many have found a significant association between high BMI or waist circumference and high levels of gastroesophageal reflux (65, 71, 82, 107-110), including studies looked at by two metaanalyses (111, 112). Nevertheless, other studies have found no such relationship (113-116). Six studies have evaluated the effect of weight reduction on improvement of gastroesophageal reflux, and there have been four clinical trials and two prospective studies.
Mathus-Vliegen et al. conducted a controlled clinical trial which included 32 obese patients (BMI 44.3 +/- 1.3 kg/m2). They were divided into two groups, one consisting of patients who were going to receive treatment with intragastric balloons for 13 weeks, and another with patients who received false balloons. Patients were also instructed to change their diets and exercise regimes to reduce weight. The study was double-blinded for the first 13 weeks. pH was monitored and patients underwent manometry during the study. After 13 weeks of treatment, the group that was treated with false balloons showed a weight reduction of 9.7 +/- 3.9%, a significant increase in the length of the LES (3.0 cm +/- 0.7 to 3.6 +/- 0.7, p <0.05), and decreased duration of pH below 4.0 with patients in standing position (8.0% +/- 3.9 to 5.5% +/- 4.1). After 13 weeks, all patients were treated with intragastric balloons. The study was designed to compare data within groups rather than between groups, especially before treatment and after treatment data from the group treated with false balloons. One cannot exclude the effect of other factors on the improvement of reflux, such as consumption of low fat diets (117).
Mathus-Vliegen et al. conducted a similar second study with 43 patients who had GERD and were obese. The patients were randomly assigned to two groups, one of which was treated with intragastric balloons for 13 weeks, and another which received false balloons. pH was monitored before and after treatment. Initially, they were able to record data of 42 patients whose average BMI was 43.4 kg/m2 and whose average age was 41.4 years. Twenty-two exhibited some evidence of reflux. The total amount of time that pH was less than 4.0 (total time including standing and supine) for these obese patients was significantly greater than the reference values. Nevertheless, reflux had no significant correlation with weight. After 13 weeks of treatment, the patients who had been treated with false gastric balloons had lost 9.7% of their weight and experienced a significant reduction in the time that pH was less than 4.0 (5.60% at baseline and 3.72% at 13 weeks <0.05) (118). You cannot exclude the effect of low- fat meals with small volumes for improving reflux parameters, plus there was no comparison with a control group.
An uncontrolled study by Fraser-Moodie et al. followed 34 patients who had had symptoms of gastroesophageal reflux within the previous 6 months. Average patient weight was 83.4 kg, and average BMI was 23.5 kg/m2. Patients were given recommendations on diet without any other changes in lifestyle. After 6 weeks of follow-up, average weight had decreased to 80.6 kg and average BMI had decreased to 21.8 kg/m2. The symptoms that were initially present in all patients went from an average score of 5.4 (3-10) to 1.8 (p <0.001). Twenty-seven (80%) patients had decreased their weights (4.0 Kg) and had improvements in their symptoms (75%) (119).
Recently a cohort study by Mandeep et al. included 332 patients in a structured weight loss program. Of the total number, 124 (37%) had GERD, average age was 46 years, average patient weight was 101 kg (+/- 18), average BMI was 35 (+/- 5 ) kg/m2 and average waist circumference was 103 cm (+/- 13). Weight reduction strategies included dietary modifications, increased physical activity and other behavioral changes. After 6 months follow-up, 97% of these people had reduced their weights. Among those who had GERD, 81% had reduced their symptom Scores (65% full resolution, and 15% partial resolution). There was a positive correlation between the degree of weight loss and changes in reflux symptom Scores at 6 months follow-up, and there was a significant improvement in reflux symptom Scores as the percentage of weight loss increased (Pearson product-moment correlation coefficient, r = 0.17, P <0.05). In this study there was no control group of patients, since almost all (97%) decreased weight. Only 37% of these people had GERD. The program included several measures such as changes in diet, exercise and behavioral changes, so the contribution of each measure to changes in symptoms could not be evaluated (120).
Kjellin et al. tested the hypothesis with 20 patients who had GERD. They were randomized into two groups, Group A received a very low calorie diet with continuous monitoring six weeks while Group B received only general recommendations. Patients were monitored with performed manometry, endoscopy, pH tests and a standardized reflux symptoms questionnaire. After 6 months there was a significant weight reduction in Group A (10.8 +/- 1.4 kg) but not in Group B (-0.6 +/- 0.7 kg). There were no significant differences in changes of symptoms. There was no reduction in reflux according to pH testing (121). This study found no significant objective or subjective differences related to reflux after weight reduction. It should be noted that patients had free access to medications during the study and that the number of people included was small.
Recently the results of a prospective study that was part of the HUNT study were published. Two surveys were conducted. In the first, 58,869 people were surveyed while in the second, 44,997 people were surveyed. 29,610 people participated in both surveys. At the beginning 9,299 reported some degree of reflux, and 1,553 (5.2%) reported severe reflux. Participants were stratified according to use of anti-reflux medications (less than once a week and at least once a week). An analysis of this data found that the adjusted odds ratio of those who did not use anti-reflux medications and those who used them less than 1 time per week increased for improving to the point that they reduced their BMI by more than 3.5 units whereas others had only minor changes of 0.5 units (OR 1.98, 95% CI: 1.45 - 2.72). The increase in the OR was higher for those taking medications at least once per week (OR 3.95, 95% CI 2.03 - 7.65). In assessing the cohort of people with severe reflux, there was a significant increase in the adjusted OR for absence of severe reflux symptoms at follow-up among those who consumed medications at least once per week and who had reduced BMI by more than 3.5 units whereas others had no reduction (OR 3.11, 95% CI 1.13 - 8.58) (122). Several biases may have been built into the study design. Due to the long follow-up time, fluctuations in symptoms between the two surveys cannot be assessed. Moreover, there was a great loss patients. Some patients were included after a survey was sent to their homes, and they reported their own weights and heights with the likelihood that some data was erroneous. The results on this topic are controversial because of data from studies with methodological limitations. Recommendation: Weak. Level of evidence: Moderate.
Exercise
Three studies that have evaluated the effect of exercise on symptoms of reflux were included. Two of them investigated whether the training of the muscles involved in breathing could improve symptoms, while the other evaluated the effects of inclination exercises on reflux.
Sodhi et al. investigated the effects of inclination exercises in which the patients touched their feet while in three different positions: sitting, standing and lying (123). Twenty-five patients with GERD were included. They competed a questionnaire about symptoms, and then their esophageal pH was monitored for 24 hours prior to the test and 24 hours on the day of the exercises. Patients also underwent esophageal manometry and upper digestive tract endoscopy. Of the 25 patients, 14 had reflux while in a standing position, four had reflux only in supine positions, and seven had reflux in both positions. There were no differences in measured pH before and during exercise. Reflux time, the amount of time pH was below 4.0, was 0.0 (0-60) and 0.0 (0-80) [p = 0.71] in those with reflux only in a standing position. For those who had reflux while supine, reflux time was 13% (0-53) while for those who had reflux in both positions, reflux time was 0.0% (0-42). It is worth noting that patients with reflux in both positions had lower LES pressure 7.0 (SD 2.8) mmHg than those who had reflux only in a standing position 19.6 (SD 6.8) mmHg (p = 0.001). This may explain other differences between these two groups. There was a low correlation between symptoms and episodes of reflux (123).
A training program for breathing muscles has been evaluated for patients with non-erosive gastroesophageal reflux disease. Initially, 19 people were included. They were randomized into two groups, one of which received training in breathing exercises for four weeks, and the other of which did not participate in the training (124). All patients underwent upper gastrointestinal tract endoscopy, pH monitoring and manometry before starting the protocol. After finishing their pH was against tested and manometry was repeated. Exercise training was overseen by experts who instructed patients in thoracic and abdominal breathing to increase contractions of the diaphragm. There was no difference in the fraction of time that pH was less than 4.5 (9.1 ± 1.3% vs. 10.7 ± 1.8%) between the 2 groups. After the first month there was a significant decrease in the fraction of time that pH was less than 4.5% (4.7 ± 0.9%, P <0.05) in the group that received training while the control group was unchanged. Nevertheless, the comparison between the two groups was not significant. Patients were allowed to use proton-pump inhibitors on demand. There were no differences between manometry measurements before and after training. Upon completion of the first four weeks, all 19 patients were taught to perform the exercises. After 9 months follow-up, only 11 patients continued to follow the recommendations. Among patients who continued training there was a significant decrease in the symptom scores which were, on average, 15.1 ± 2.2 before training, but which fell to 9.7 ± 1.6 (P <0.05) after training. There were no decreases among the eight patients who did not continue training. The use of PPIs decreased from 98 ± 34 to 25 ± 12 mg/week (P <0.05) in the group that continued training after 9 months training. Those who did not continue training had no such change. Average PPI use before had been 179 ± 31. This declined slightly to 144 ± 40 after (124). This study's limitations include the small number of patients and the fact that the study could not be blinded.
Another study of 12 patients with GERD and seven healthy volunteers evaluated whether training breathing muscles could improve motor function of the gastroesophageal junction and gastroesophageal reflux. After patients were checked with manometry and pH tests, they began a two month breathing muscle training program. Upon completion of the training the heartburn score of the group of patients with GERD decreased from 3(3-4) to 0 (0-0.7) (P <0.003) and their regurgitation scores decreased from 2.5 (1-3.7) to 0 (0-0) (P <0.008). The number of transient also LES relaxations decreased from 8.5 events/hour (4 -17) to 7 events/hour (2-13) (P <0.032). The total times of abnormal acid exposure in the proximal esophagus were similar before and after training: 10.4 ± 4.4 minutes vs. 12.5 ± 4.1 minutes (P <0.751). The total times of abnormal acid exposure in the distal esophagus were also similar before and after training: 50.9 ± 15.1 minutes vs. 56.9 ± 13.1 minutes (P ± 0.765). There was also a reduction in the progression of proximal reflux after training from -8 (-16 to 5) to -10 (-28 to -3) (P <0.04) (125). Because this study was conducted with patients diagnosed with GERD and a few healthy volunteers, treatments was not compared between two groups with similar characteristics. In addition, this study was not blinded. Recommendation: Weak. Level of evidence: Low.
Elevating the head of the bed and posture management
This measure has been taken into account, since it is considered that different positions, such as supine may worsen GERD by facilitating the passage of the gastric contents into the esophagus. By 1977, an association between sleeping with the head of the bed elevated and improved reflux had been found (126). Khan et al. conducted a clinical trial which included 71 people with nocturnal symptoms of reflux (127). Only those with reflux verified by esophageal pH monitoring were included in the study (24). Patients were instructed to put a 20 cm high block of wood underneath the headboard to elevate the bed for seven days (two in the hospital and five at home). Twenty continued to follow the recommendation until the end of the period. The mean (SD) amount of time that pH was below 4.0 was 15.0 (8.4) on day one and 13.7 (7.2) on day seven (P = 0.001). On day one, 14 patients had moderate heartburn, five had moderate to severe heartburn, and one had severe heartburn. After completing seven days, 12 had moderate heartburn, seven had mild heartburn, and in one case, the heartburn had completely resolved (127).
Khoury et al. have investigated the effect of postural management on gastroesophageal reflux (128). They included ten patients who had been diagnosed with GERD in a study. Patients were monitored overnight with a motion sensor, and patients pH was also monitored overnight. The percentage of time pH was less than 4.0 was higher when patients were in right lateral decubitus (median 18.1, range 7.4 - 44.4) (p <0.003) than when patients were in left lateral decubitus (median 0.9, range 0.0 - 4.5) or prone position (median 1.4, range 0.0 to 4.5). Also, the time it took to clear esophageal acid was greater in right lateral decubitus than in the other positions. The number of reflux episodes per hour was higher in supine position than in the other positions. There was no assessment of symptoms during the study (128).
To date, there have been no randomized clinical trials that have successfully demonstrated that these measures impact patients' GERD symptoms. For this reason, recommendation of these behaviors in the long term is difficult considering that they could significantly interfere in patients' quality of life. Recommendation: Weak. Level of evidence: Low.
DISCUSSION
Gastroesophageal reflux has high prevalence in the adult population and has been classically related to certain foods, eating habits and customs. Multiple studies have looked at the associations between this disease and various triggers, but the information obtained shows no b associations. Similarly, whether changes in lifestyle can improve GERD has also been investigated in the hope of creating an alternative to pharmacological management and providing financial relief for health systems.
Studies of some dietary factors including consumption of carbonated beverages, peppermint, chocolate, citrus fruits, fatty foods and spicy foods have not been properly designed. Consequently, the evidence does not support the conclusion that suspending consumption of these items has a positive effect on a person with GERD. Studies of coffee and caffeine have yielded conflicting results regarding whether or not they provoke GERD symptoms, so there is not sufficient evidence to recommend that patients suspend consumption of coffee or caffeine. Quitting smoking has been evaluated in a prospective study that found a relationship between quitting and decreases of severe symptoms only in people with normal BMI who were taking reflux medication at least once a week (93).
Because of that study's design methodology, we cannot conclude that there is a causal relationship between smoking cessation and improvement of symptoms. Eating schedules have been investigated, but the studies have conflicting results. While one study shows no significant difference between going to sleep soon after eating or going to sleep later (95), another study has found higher levels of reflux among those who dined near bedtime. Nevertheless, in that study there were no changes in symptoms (96). Since these findings are contradictory, we cannot conclude that there is a relationship between meal schedule and improvement of reflux symptoms. We also found no conclusive evidence about other measures such as raising the head of the bed. We found only one uncontrolled clinical trial that showed that symptoms improved (127).
Another measure that has been studied is weight reduction since the proportion of people who are overweight or obese who suffer from GERD has been well studied, and positive associations between the conditions have been found frequently. Six studies, four clinical trials and two prospective cohort studies, have looked at possible association between weight reduction and improvements in gastroesophageal reflux. Two clinical trials that compared participants' reflux before and after weight reduction found improvements (117, 118), but another similar clinical trial found no association between improvement of symptoms and weight loss (121). Two clinical trials that evaluated symptoms obtained opposing results (119, 121). Two prospective studies found that the symptoms of those who lost weight improved (120, 122). These clinical trials had various methodological problems: some were uncontrolled, and some had numbers of participants that were too small to draw clear conclusions. Similarly, the prospective cohort studies may have suffered from information selection bias, and may not have had enough power to show a causal association.
Exercising respiratory muscles has also been evaluated. One study suggests that symptoms could improve by continued training over the long term while another study suggest that short term training may reduce symptoms (124, 125). Exercises that that require inclination of the body may also help by increasing intra-abdominal pressure (123).
An open uncontrolled clinical trial found that a right lateral decubitus sleeping position is related to higher levels of reflux than other sleeping positions, but found no differences in symptoms (128).
Our study has some limitations. The online search of publications included only articles in English and Spanish, and it only covered the last 20 years. This could limit our findings and evidence, but the studies with that were the least rigorous in their diagnostic methods and studies of low quality were omitted. Other important limitations are the small number of controlled clinical trials and the fact that many of them are of moderate or low quality.
CONCLUSIONS
Gastroesophageal reflux is a disease that has a significant impact on patient quality of life (129). Despite the fact that in the literature the evidence that lifestyle changes can improve GERD symptoms is poor, changes in lifestyle continue to be recommended as a way to obtain clinical improvement of the diseas (17, 130). The consumption of some foods and beverages has been linked to higher levels of gastroesophageal reflux symptoms, but - in the specific cases of carbonated beverages, peppermint, chocolate, citrus, fatty foods and spicy foods there have been no studies that support the idea that suspension of these foods and beverages can, by themselves, result in clinical improvement of the disease. Similarly, there is not enough evidence to recommend to recommend suspension of other habits such as consuming coffee and caffeine and smoking. Neither is there sufficient evidence to recommend that patients do not eat close to bed time, or that patients exercise or manage their postures to control GERD. There is evidence that weight reduction may help, but the studies containing this evidence have methodological flaws in their designs which prevents us from making b recommendations. More controlled clinical trials are needed to define the actual role that changes in lifestyle may have for clinical improvement of GERD.
Conflicts of interest
None. The costs of this investigation were assumed by the authors.
REFERENCES
1. Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101,1900-20. [ Links ]
2. Kinoshita Y, Adachi K, Hongo M. Haruma K. Systematic review of the epidemiology of gastroesophageal reflux disease in Japan. J Gastroenterol. 2011;46:1092-103. [ Links ]
3. Dent J, El-Serag HB, Wallander M. Johansson S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005;54:710-7. [ Links ]
4. Salis G. Revisión sistemática: epidemiología de la enfermedad por reflujo gastroesofágico en Latinoamérica. Acta Gastroenterológica Latinoam. 2011;41:60-69. [ Links ]
5. Ronkainen J, Aro P, Storskrubb T, Lind T, Bolling-Sternevald E, Junghard OJ. et al. Gastro-oesophageal reflux symptoms and health-related quality of life in the adult general population--the Kalixanda study. Aliment. Pharmacol Ther. 2006;23:1725-33. [ Links ]
6. Becher A, El-Serag H. Systematic review: the association between symptomatic response to proton pump inhibitors and health-related quality of life in patients with gastro-oesophageal reflux disease. Aliment. Pharmacol Ther. 2011;34:618-27. [ Links ]
7. Vakil NB, Traxler B. Levine D. Dysphagia in patients with erosive esophagitis: prevalence, severity, and response to proton pump inhibitor treatment. Clin Gastroenterol Hepatol. 2004;2:665-8. [ Links ]
8. Rubenstein JH, Scheiman JM, Sadeghi S, Whiteman D. Inadomi JM. Esophageal adenocarcinoma incidence in individuals with gastroesophageal reflux: synthesis and estimates from population studies. Am J Gastroenterol. 2011;106:254-60. [ Links ]
9. Havemann BD, Henderson CA, el-Serag HB. The association between gastro-oesophageal reflux disease and asthma: a systematic review. Gut. 2007;56:1654-64. [ Links ]
10. el-Serag HB, Sonnenberg A. Comorbid occurrence of laryngeal or pulmonary disease with esophagitis in United States military veterans. Gastroenterology. 1997;113:755-60. [ Links ]
11. Irwin RS, Curley FJ, French CL. Chronic cough. The spectrum and frequency of causes, key components of the diagnostic evaluation, and outcome of specific therapy. Am Rev Respir Dis. 1990;141:640-7. [ Links ]
12. Labenz J, Jaspersen D, Kulig M, Leodolter A, Lind T, Meyer-Sabellek W. et al. Risk factors for erosive esophagitis: a multivariate analysis based on the ProGERD study initiative. Am J Gastroenterol. 2004;99:1652-6. [ Links ]
13. Ford AC, Forman D, Bailey AG, Axon AT, Moayyedi P. The natural history of gastro-oesophageal reflux symptoms in the community and its effects on survival: a longitudinal 10-year follow-up study. Aliment Pharmacol Ther. 2013;37:323-31. [ Links ]
14. Mittal RK, Holloway RH, Penagini R, Blackshaw LA, Dent J. Transient lower esophageal sphincter relaxation. Gastroenterology. 1995;109:601-10. [ Links ]
15. Bredenoord AJ, Pandolfino JE, Smout AJPM. Gastro-oesophageal reflux disease. Lancet. 2013;381:1933-42. [ Links ]
16. Beaumont H, Bennink RJ, de Jong J. Boeckxstaens GE. The position of the acid pocket as a major risk factor for acidic reflux in healthy subjects and patients with GORD. Gut. 2010;59:441-51. [ Links ]
17. Kaltenbach T, Crockett S, Gerson L. Are Lifestyle Measures Effective in Patients With Gastroesophageal Reflux Disease? Arch Intern Med. 2006;166:965-971. [ Links ]
18. Scottish Intercollegiate Guidelines Network (SIGN). Accesado Septiembre 5, 2014. [ Links ]
19. Atkins D, Eccles M, Flottorp S, Guyatt GH, Henry D, Hill S. et al. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv Res. 2004;4:38. [ Links ]
20. Atkins D, Briss PA, Eccles M, Flottorp S, Guyatt GH, Harbour RT, et al. Systems for grading the quality of evidence and the strength of recommendations II: pilot study of a new system. BMC Health Serv Res. 2005;5:25. [ Links ]
21. Mangano M, Colombo P, Bianchi PA, Penagini R. Fat and esophageal sensitivity to acid. Dig Dis Sci. 2002;47:657-60. [ Links ]
22. Becker DJ, Sinclair J, Castell DO, Wu WC. A comparison of high and low fat meals on postprandial esophageal acid exposure. Am J Gastroenterol. 1989;84:782-6. [ Links ]
23. Pehl C, Waizenhoefer A, Wendl B, Schmidt T, Schepp W, Pfeiffer A. et al. Effect of low and high fat meals on lower esophageal sphincter motility and gastroesophageal reflux in healthy subjects. Am J Gastroenterol. 1999;94:1192-6. [ Links ]
24. Penagini R, Mangano M, Bianchi PA. Effect of increasing the fat content but not the energy load of a meal on gastro-oesophageal reflux and lower oesophageal sphincter motor function. Gut. 1998;42:330-3. [ Links ]
25. Meyer JH, Lembo A, Elashoff JD, Fass R, Mayer EA. Duodenal fat intensifies the perception of heartburn. Gut. 2001;49:624-8. [ Links ]
26. Holloway RH, Lyrenas E, Ireland A, Dent J. Effect of intraduodenal fat on lower oesophageal sphincter function and gastro-oesophageal reflux. Gut. 1997;40:449-53. [ Links ]
27. Lacy BE, Carter J, Weiss JE, Crowell MD. The effects of intraduodenal nutrient infusion on serum CCK, LES pressure, and gastroesophageal reflux. Neurogastroenterol. Motil. 2011;23:631-6. [ Links ]
28. Fox M, Barr C, Nolan S, Lomer M, Anggiansah A, Wong T. et al. The effects of dietary fat and calorie density on esophageal acid exposure and reflux symptoms. Clin Gastroenterol Hepatol. 2007;5:439-44. [ Links ]
29. Nebel OT, Fornes MF, Castell DO. Symptomatic gastroesophageal reflux: incidence and precipitating factors. Am J Dig Dis. 1976;21:953-6. [ Links ]
30. Moraes-Filho JPP, Chinzon D, Eisig JN, Hashimoto CL, Zaterka S. Prevalence of heartburn and gastroesophageal reflux disease in the urban Brazilian population. Arq Gastroenterol. 2005;42:122-7. [ Links ]
31. Jafri N, Jafri W, Yakoob J, Islam M, Manzoor S, Jalil A. et al. Perception of gastroesophageal reflux disease in urban population in Pakistan. J Coll Physicians Surg Pak. 2005;15:532-4. [ Links ]
32. Butt AK, Hashemy I. Risk factors and prescription patterns of gastroesophageal reflux disease: HEAL study in Pakistan. J Pak Med Assoc. 2014;64:751-7. [ Links ]
33. Song JH, Chung SJ, Lee JH, Kim YH, Chang DK, Son HJ. et al. Relationship between gastroesophageal reflux symptoms and dietary factors in Korea. J Neurogastroenterol Motil. 2011;17:54-60. [ Links ]
34. Pandeya N, Green AC, Whiteman DC. Prevalence and determinants of frequent gastroesophageal reflux symptoms in the Australian community. Dis Esophagus. 2012;25:573-83. [ Links ]
35. Hamoui N, Lord RV, Hagen JA, Theisen J, Demeester TR, Crookes PF. et al. Response of the lower esophageal sphincter to gastric distention by carbonated beverages. J Gastrointest Surg. 2006;10:870-7. [ Links ]
36. Shukla A, Meshram M, Gopan A, Ganjewar V, Kumar P, Bhatia SJ. et al. Ingestion of a carbonated beverage decreases lower esophageal sphincter pressure and increases frequency of transient lower esophageal sphincter rela xation in normal subjects. Indian J Gastroenterol. 2012;31:121-4. [ Links ]
37. Fass R, Quan SF, O'Connor GT, Ervin A, Iber C. Predictors of heartburn during sleep in a large prospective cohort study. Chest. 2005;127:1658-66. [ Links ]
38. Zeidan RK, Kansour NB, Abboud C, Al Hajje A, Rachidi S, Zein S, et al. Gastro-esophageal reflux disease in Lebanese adults: Effects on quality of life and correlates. J Popul Ther Clin Pharmacol. 2013;20:e289-e290. [ Links ]
39. Cuomo R, Savarese MF, Sarnelli G, Vollono G, Rocco A, Coccoli P. et al. Sweetened carbonated drinks do not alter upper digestive tract physiology in healthy subjects. Neurogastroenterol Motil. 2008;20:780-9. [ Links ]
40. Bulat R, Fachnie E, Chauhan U, Chen Y, Tougas G. Lack of effect of spearmint on lower oesophageal sphincter function and acid reflux in healthy volunteers. Aliment Pharmacol Ther. 1999;13:805-12. [ Links ]
41. Wright LE, Castell DO. The adverse effect of chocolate on lower esophageal sphincter pressure. Am J Dig Dis. 1975;20:703-7. [ Links ]
42. Babka JC, Castell DO. On the genesis of heartburn. The effects of specific foods on the lower esophageal sphincter. Am J Dig Dis. 1973;18:391-7. [ Links ]
43. Murphy DW, Castell DO. Chocolate and heartburn: evidence of increased esophageal acid exposure after chocolate ingestion. Am J Gastroenterol. 1988;83:633-6. [ Links ]
44. Terry P, Lagergren J, Wolk A, Nyrén O. Reflux-inducing dietary factors and risk of adenocarcinoma of the esophagus and gastric cardia. Nutr Cancer. 2000;38:186-91. [ Links ]
45. Feldman M, Barnett C. Relationships between the acidity and osmolality of popular beverages and reported postprandial heartburn. Gastroenterology. 1995;108:125-31. [ Links ]
46. Oliveria SA, Christos PJ, Talley NJ, Dannenberg AJ. Heartburn Risk Factors, Knowledge, and Prevention Strategies. Arch Intern Med. 1999;159:1592-8. [ Links ]
47. Cranley JP, Achkar E, Fleshler B. Abnormal lower esophageal sphincter pressure responses in patients with orange juice-induced heartburn. Am J Gastroenterol. 1986;81:104-6. [ Links ]
48. Price SF, Smithson KW, Castell DO. Food sensitivity in reflux esophagitis. Gastroenterology. 1978;75:240-3. [ Links ]
49. Hogan WJ, Viegas de Andrade SR, Winship DH. Ethanol-induced acute esophageal motor dysfunction. J Appl Physiol. 1972;32:755-60. [ Links ]
50. Bujanda L. The effects of alcohol consumption upon the gastrointestinal tract. Am J Gastroenterol. 2000;95:3374-82. [ Links ]
51. Kaufman SE, Kaye MD. Induction of gastro-oesophageal reflux by alcohol. Gut. 1978;19:336-8. [ Links ]
52. Vitale GC, Cheadle WG, Patel B, Sadek SA, Michel ME, Cuschieri A. et al. The effect of alcohol on nocturnal gastroesophageal reflux. JAMA. 1987;258:2077-9. [ Links ]
53. Rubinstein E, Hauge C, Sommer P, Mortensen T. Oesophageal and gastric potential difference and pH in healthy volunteers following intake of coca-cola, red wine, and alcohol. Pharmacol Toxicol. 1993;72:61-5. [ Links ]
54. Grande L, Manterola C, Ros E, Lacima G, Pera C. Effects of red wine on 24-hour esophageal pH and pressures in healthy volunteers. Dig Dis Sci. 1997;42:1189-93. [ Links ]
55. Rosaida MS, Goh KL. Gastro-oesophageal reflux disease, reflux oesophagitis and non-erosive reflux disease in a multiracial Asian population: a prospective, endoscopy based study. Eur J Gastroenterol Hepatol. 2004;6:495-501. [ Links ]
56. O'Leary C, McCarthy J, Humphries M, Shanahan F, Quigley E. The prophylactic use of a proton pump inhibitor before food and alcohol. Aliment Pharmacol Ther. 2003;17:683-6. [ Links ]
57. Avidan B, Sonnenberg A, Schnell TG, Sontag SJ. No association between gallstones and gastroesophageal reflux disease. Am J Gastroenterol. 2001;96:2858-62. [ Links ]
58. Talley NJ, Piper DW. Comparison of the clinical features and illness behaviour of patients presenting with dyspepsia of unknown cause (essential dyspepsia) and organic disease. Aust N Z J Med. 1986;16:352-9. [ Links ]
59. Niu CY, Zhou YL, Yan R, Mu NL, Gao BH, Wu FX, et al. Incidence of gastroesophageal reflux disease in Uygur and Han Chinese adults in Urumqi. World J Gastroenterol. 2012;18:7333-40. [ Links ]
60. Minatsuki C, Yamamichi N, Shimamoto T, Kakimoto H, Takahashi Y, Fujishiro M, et al. Background factors of reflux esophagitis and non-erosive reflux disease: a cross-sectional study of 10,837 subjects in Japan. PLoS One. 2013;8:e69891. [ Links ]
61. Friedenberg FK, Makipour K, Palit A, Shah S, Vanar V, Richter JE, et al. Population-based assessment of heartburn in urban Black Americans. Dis. Esophagus. 2013;26:561-9. [ Links ]
62. Islami F, Nasseri-Moghaddam S, Pourshams A, Poustchi H, Semnani S, Kamangar F, et al. Determinants of gastroesophageal reflux disease, including hookah smoking and opium use- a cross-sectional analysis of 50,000 individuals. PLoS One. 2014;9:e89256. [ Links ]
63. Wang JH, Luo JY, Dong L, Gong J, Tong, M. Epidemiology of gastroesophageal reflux disease: a general population-based study in Xi'an of Northwest China. World J Gastroenterol. 2004;10:1647-51. [ Links ]
64. Martín-de-Argila C, Martínez-Jiménez P. Epidemiological study on the incidence of gastroesophageal reflux disease symptoms in patients in acute treatment with NSAIDs. Expert Rev Gastroenterol Hepatol. 2013;l7:27-33. [ Links ]
65. Nilsson M, Johnsen R, Ye W, Hveem K, Lagergren J. Lifestyle related risk factors in the aetiology of gastro-oesophageal reflux. Gut. 2004;53:1730-5. [ Links ]
66. Stanghellini V. Relationship between upper gastrointestinal symptoms and lifestyle, psychosocial factors and comorbidity in the general population: results from the Domestic/International Gastroenterology Surveillance Study (DIGEST). Scand J Gastroenterol. 1999;231(Suppl):29-37. [ Links ]
67. Talley NJ, Zinsmeister AR, Schleck CD, Melton LJ. Smoking, alcohol, and analgesics in dyspepsia and among dyspepsia subgroups: lack of an association in a community. Gut. 1994;35:619-24. [ Links ]
68. Grande L, Monforte R, Ros E, Toledo-Pimentel V, Estruch R, Lacima G. et al. High amplitude contractions in the middle third of the oesophagus: a manometric marker of chronic alcoholism? Gut. 1996;38:655-62. [ Links ]
69. Thomas FB, Steinbaugh JT, Fromkes JJ, Mekhjian HS, Caldwell JH. Inhibitory effect of coffee on lower esophageal sphincter pressure. Gastroenterology. 1980;79:1262-6. [ Links ]
70. Salmon PR, Fedail SS, Wurzner HP, Harvey RF, Read AE. Effect of coffee on human lower oesophageal function. Digestion. 1981;21:69-73. [ Links ]
71. Zheng Z, Nordenstedt H, Pedersen NL, Lagergren J, Ye W. Lifestyle factors and risk for symptomatic gastroesophageal reflux in monozygotic twins. Gastroenterology. 2007;132:87-95. [ Links ]
72. Shimamoto T1, Yamamichi N, Kodashima S, Takahashi Y, Fujishiro M, Oka M, et al. No association of coffee consumption with gastric ulcer, duodenal ulcer, reflux esophagitis, and non-erosive reflux disease: a cross-sectional study of 8,013 healthy subjects in Japan. PLoS One. 2013;8:e65996. [ Links ]
73. Boekema PJ, Samsom M, Smout AJ. Effect of coffee on gastro-oesophageal reflux in patients with reflux disease and healthy controls. Eur J Gastroenterol Hepatol. 1999;11:1271-6. [ Links ]
74. Wendl B, Pfeiffer A, Pehl C, Schmidt T, Kaess H. Effect of decaffeination of coffee or tea on gastro-oesophageal reflux. Aliment. Pharmacol Ther. 1994;8:283-7. [ Links ]
75. Chattopadhyay DK, Greaney MG, Irvin TT. Effect of cigarette smoking on the lower oesophageal sphincter. Gut. 1977;18:833-5. [ Links ]
76. Dennish GW, Castell DO. Inhibitory effect of smoking on the lower esophageal sphincter. N Engl J Med. 1971;284:1136-7. [ Links ]
77. Stanciu C, Bennett JR. Smoking and gastro-oesophageal reflux. Br Med J. 1972;3:793-5. [ Links ]
78. Kahrilas PJ, Gupta RR. The effect of cigarette smoking on salivation and esophageal acid clearance. J Lab Clin Med. 1989;114:431-8. [ Links ]
79. Trudgill NJ, Smith LF, Kershaw J, Riley SA. Impact of smoking cessation on salivary function in healthy volunteers. Scand J Gastroenterol. 1998;33:568-71. [ Links ]
80. Kahrilas PJ, Gupta RR. Mechanisms of acid reflux associated with cigarette smoking. Gut. 1990;31:4-10. [ Links ]
81. Tibbling L, Gibellino FM, Johansson KE. Is mis-swallowing or smoking a cause of respiratory symptoms in patients with gastroesophageal reflux disease? Dysphagia. 1995;10:113-6. [ Links ]
82. Nocon M, Labenz J, Willich SN. Lifestyle factors and symptoms of gastro-oesophageal reflux -- a population-based study. Aliment. Pharmacol Ther. 2006;23:169-74. [ Links ]
83. Isolauri J, Laippala P. Prevalence of symptoms suggestive of gastro-oesophageal reflux disease in an adult population. Ann Med. 1995;27:67-70. [ Links ]
84. Locke GR, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ. Risk factors associated with symptoms of gastroesophageal reflux. Am J Med. 1999;106:642-9. [ Links ]
85. Haque M, Wyeth JW, Stace NH, Talley NJ, Green R. Prevalence, severity and associated features of gastro-oesophageal reflux and dyspepsia: a population-based study. NZ Med J. 2000;113:178-81. [ Links ]
86. Wong WM, Lai KC, Lam KF, Hui WM, Hu WH, Lam CL, et al. Prevalence, clinical spectrum and health care utilization of gastro-oesophageal reflux disease in a Chinese population: a population-based study. Aliment Pharmacol Ther. 2003;18:595-604. [ Links ]
87. Watanabe Y, Fujiwara Y, Shiba M, Watanabe T, Tominaga K, Oshitani N, et al. Cigarette smoking and alcohol consumption associated with gastro-oesophageal reflux disease in Japanese men. Scand J Gastroenterol. 2003;38:807-11. [ Links ]
88. Pehl C, Pfeiffer A, Wendl B, Nagy I, Kaess H. Effect of smoking on the results of esophageal pH measurement in clinical routine. J Clin Gastroenterol. 1997;25:503-6. [ Links ]
89. Kuster E, Ros E, Toledo-Pimentel V, Pujol A, Bordas JM, Grande L, et al. Predictive factors of the long term outcome in gastro-oesophageal reflux disease: six year follow up of 107 patients. Gut. 1994;35:8-14. [ Links ]
90. Kay L, Jørgensen T. Epidemiology of upper dyspepsia in a random population. Prevalence, incidence, natural history, and risk factors. Scand. J Gastroenterol. 1994;29:2-6. [ Links ]
91. Schindlbeck NE, Klauser AG, Berghammer G, Londong W, Müller-Lissner SA. Three year follow up of patients with gastrooesophageal reflux disease. Gut. 1992;33:1016-9. [ Links ]
92. Kadakia SC, Kikendall JW, Maydonovitch C, Johnson LF. Effect of cigarette smoking on gastroesophageal reflux measured by 24-h ambulatory esophageal pH monitoring. Am J Gastroenterol. 1995;90:1785-90. [ Links ]
93. Ness-Jensen E, Lindam A, Lagergren J, Hveem K. Tobacco smoking cessation and improved gastroesophageal reflux: a prospective population-based cohort study: the HUNT study. Am J Gastroenterol. 2014;109:171-7. [ Links ]
94. Fujiwara Y, Machida A, Watanabe Y, Shiba M, Tominaga K, Watanabe T, et al. Association between dinner-to-bed time and gastro-esophageal reflux disease. Am J Gastroenterol. 2005;100:2633-6. [ Links ]
95. Orr WC, Harnish MJ. Sleep-related gastro-oesophageal reflux: provocation with a late evening meal and treatment with acid suppression. Aliment Pharmacol Ther. 1998;12:1033-8. [ Links ]
96. Piesman M, Hwang I, Maydonovitch C, Wong RKH. Nocturnal reflux episodes following the administration of a standardized meal. Does timing matter? Am J Gastroenterol. 2007;102:2128-34. [ Links ]
97. Wu JCY, Mui LM, Cheung CMY, Chan Y, Sung JJY. Obesity is associated with increased transient lower esophageal sphincter relaxation. Gastroenterology. 2007;132:883-9. [ Links ]
98. El-Serag H. Role of obesity in GORD-related disorders. Gut. 2008;57:281-4. [ Links ]
99. El-Serag HB, Tran T, Richardson P, Ergun G. Anthropometric correlates of intragastric pressure. Scand J Gastroenterol. 2006;41:887-91. [ Links ]
100. De Vries DR, van Herwaarden MA, Smout AJPM, Samsom M. Gastroesophageal pressure gradients in gastroesophageal reflux disease: relations with hiatal hernia, body mass index, and esophageal acid exposure. Am J Gastroenterol. 2008;103:1349-54. [ Links ]
101. Lambert DM, Marceau S, Forse RA. Intra-abdominal pressure in the morbidly obese. Obes Surg. 2005;15:1225-32. [ Links ]
102. Pandolfino JE, El-Serag HB, Zhang Q, Shah N, Ghosh SK, Kahrilas PJ. Obesity: a challenge to esophagogastric junction integrity. Gastroenterology. 2006;130:639-49. [ Links ]
103. Jaffin BW, Knoepflmacher P, Greenstein R. High prevalence of asymptomatic esophageal motility disorders among morbidly obese patients. Obes Surg. 1999;9:390-5. [ Links ]
104. Hong D, Khajanchee YS, Pereira N, Lockhart B, Patterson EJ, Swanstrom LL. Manometric abnormalities and gastroesophageal reflux disease in the morbidly obese. Obes Surg. 2004;14:744-9. [ Links ]
105. Suter M, Dorta G, Giusti V, Calmes JM. Gastric banding interferes with esophageal motility and gastroesophageal reflux. Arch Surg. 2005;140:639-43. [ Links ]
106. Koppman JS, Poggi L, Szomstein S, Ukleja A, Botoman A, Rosenthal R. Esophageal motility disorders in the morbidly obese population. Surg Endosc. 2007;21:761-4. [ Links ]
107. Nocon M, Labenz J, Jaspersen D, Meyer-Sabellek W, Stolte M, Lind T, et al. Association of body mass index with heartburn, regurgitation and esophagitis: results of the Progression of Gastroesophageal Reflux Disease study. J Gast roenterol Hepatol. 2007;22:1728-31. [ Links ]
108. Corley DA, Kubo A, Zhao W. Abdominal obesity, ethnicity and gastro-oesophageal reflux symptoms. 2007;56:756-62. [ Links ]
109. Jacobson BC, Somers SC, Fuchs CS, Kelly CP, Camargo CA. Body-mass index and symptoms of gastroesophageal reflux in women. N Engl J Med. 2006;354:2340-8. [ Links ]
110. Nandurkar S, Locke GR 3rd, Fett S, Zinsmeister AR, Cameron AJ, Talley NJ. Relationship between body mass index, diet, exercise and gastro-oesophageal reflux symptoms in a community. Aliment Pharmacol Ther. 2004;20:497-505. [ Links ]
111. Hampel H, Abraham NS, El-Serag HB. Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med. 2005;143:199-211. [ Links ]
112. Corley DA, Kubo A. Body mass index and gastroesophageal reflux disease: a systematic review and meta-analysis. Am J Gastroenterol. 2006;101:2619-28. [ Links ]
113. Zagari RM, Fuccio L, Wallander MA, Johansson S, Fiocca R, Casanova S. et al. Gastro-oesophageal reflux symptoms, oesophagitis and Barrett's oesophagus in the general population: the Loiano-Monghidoro study. Gut. 2008;57:1354-9. [ Links ]
114. Talley NJ, Howell S, Poulton R. Obesity and chronic gastrointestinal tract symptoms in young adults: a birth cohort study. Am J Gastroenterol. 2004;99:1807-14. [ Links ]
115. Lagergren J, Bergström R, Nyrén O. No relation between body mass and gastro-oesophageal reflux symptoms in a Swedish population based study. Gut. 2000;47:26-9. [ Links ]
116. Andersen LI, Jensen G. Risk factors for benign oesophageal disease in a random population sample. J Intern Med. 1991;230:5-10. [ Links ]
117. Mathus-Vliegen EMH, van Weeren M, van Eerten PV. Los function and obesity: the impact of untreated obesity, weight loss, and chronic gastric balloon distension. Digestion. 2003;68:161-8. [ Links ]
118. Mathus-Vliegen EMH, Tygat GNJ. Gastro-oesophageal reflux in obese subjects: influence of overweight, weight loss and chronic gastric balloon distension. Scand J Gastroenterol. 2002;37:1246-52. [ Links ]
119. Fraser-Moodie CA, Norton B, Gornall C, Magnago S, Weale AR, Holmes GK. Weight loss has an independent beneficial effect on symptoms of gastro-oesophageal reflux in patients who are overweight. Scand J Gastroenterol. 1999;34:337-40. [ Links ]
120. Singh M, Lee J, Gupta N, Gaddam S, Smith BK, Wani SB, et al. Weight loss can lead to resolution of gastroesophageal reflux disease symptoms: a prospective intervention trial. Obesity (Silver Spring). 2013;21:284-90. [ Links ]
121. Kjellin A, Ramel S, Rössner S, Thor K. Gastroesophageal reflux in obese patients is not reduced by weight reduction. Scand J Gastroenterol. 1996;31:1047-51. [ Links ]
122. Ness-Jensen E, Lindam A, Lagergren J, Hveem K. Weight loss and reduction in gastroesophageal reflux. A prospective population-based cohort study: the HUNT study. Am J Gastroenterol. 2013;108:376-82. [ Links ]
123. Sodhi JS, Zargar SA, Javid G, Khan MA, Khan BA, Yattoo GN, et al. Effect of bending exercise on gastroesophageal reflux in symptomatic patients. Indian J Gastroenterol. 2008;27,227-31. [ Links ]
124. Eherer AJ, Netolitzky F, Högenauer C, Puschnig G, Hinterleitner TA, Scheidl S, et al. Positive effect of abdominal breathing exercise on gastroesophageal reflux disease: a randomized, controlled study. Am J Gastroenterol. 2013;107:372-8. [ Links ]
125. Nobre e Souza MÂ, Lima MJ, Martins GB, Nobre RA, Souza MH, de Oliveira RB, et al. Inspiratory muscle training improves antireflux barrier in GERD patients. Am J Physiol Gastrointest Liver Physiol. 2013;305:G862-7. [ Links ]
126. Stanciu C, Bennett JR. Effects of posture on gastro-oesophageal reflux. Digestion. 1977;15:104-9. [ Links ]
127. Khan BA, Sodhi JS, Zargar SA, Javid G, Yattoo GN, Shah A. et al. Effect of bed head elevation during sleep in symptomatic patients of nocturnal gastroesophageal reflux. J Gastroenterol Hepatol. 2012;27:1078-82. [ Links ]
128. Khoury RM, Camacho-Lobato L, Katz PO, Mohiuddin MA, Castell DO. Influence of spontaneous sleep positions on nighttime recumbent reflux in patients with gastroesophageal reflux disease. Am J Gastroenterol. 1999;94:2069-73. [ Links ]
129. Gerson LB, Ullah N, Hastie T, Triadafilopoulos G, Goldstein M. Patient-derived health state utilities for gastroesophageal reflux disease. Am J Gastroenterol. 2005;100:524-33. [ Links ]
130. Katz PO, Gerson LB, Vela MF. Guidelines for the Diagnosis and Management of Gastroesophageal Refl ux Disease. Am J Gastroenterol. 2013;108:308-28. [ Links ]











 text in
text in