Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.31 no.4 Bogotá Oct./Dec. 2016
Hepatotoxicity due to Isoniazide in a Patient with Crohn's Disease: Case Report and Literature Review
Gustavo Adolfo Reyes M. MD (1), Germán David Carvajal P. MD (2), Mónica Lorena Tapias M. MD (3), Luis Carlos Sabbagh S. MD (4)
(1) Internist and Gastroenterologist at Clínica Universitaria Colombia, Associate Professor of Gastroenterology at Fundación Universitaria Sanitas, Coordinator of Excellence in Digestive Endoscopy for the Colombian Association of Digestive Endoscopy in Bogotá, Colombia
(2) General Surgeon and Gastroenterology and Digestive Endoscopy Resident at Fundación Universitaria Sánitas in Bogotá, Colombia
(3) Internist and Hepatologist attached to Colsanitas from Fundación Santa Fe de Bogotá in Bogotá Colombia
(4) Internist and gastroenterologist, Chief of Gastroenterology at Colsanitas, President of the Pan-American Organization of Gastroenterology, Former President of the Colombian Association of Gastroenterology, Former President of the Colombian Association of Digestive Endoscopy, Director of the Postgraduate Program in Gastroenterology at Fundación Universitaria Sanitas
Received:Â Â Â 16-03-16Â Accepted:Â Â Â 01-11-16
Abstract
Isoniazid is used for treatment or prophylaxis of tuberculosis but may be associated with adverse hepatic reactions. Clinically manifest hepatitis occurs in 0.5%-1% of patients who receive isoniazid as monotherapy. This article describes the case of a patient with Crohn's disease who experienced severe hepatotoxicity due to isoniazid. It also reviews the literature.
Keywords
Isoniazid, hepatotoxicity, DILI (idiosyncratic drug-induced liver injury), Crohn's disease.
Introduction
Idiosyncratic drug-induced liver injury (DILI) is characterized by alterations in the liver's biochemical profile indicating an adverse reaction to a medication used at appropriate doses for prophylaxis or treatment of a disease (1).
Its clinical presentation ranges from asymptomatic, almost always self-limited, elevation of the hepatic biochemical profile to jaundice and life-threatening acute liver failure. Infrequently, it causes chronic liver disease (2). Its incidence ranges from 1 case in every 10,000 cases 1 case in 100,000 (3).
Idiosyncrasy refers to the individual differences in responses to a stimulus, in this case to a drug, due to genetic and environmental factors (4). In India, antituberculosis drugs are the leading cause of acute liver failure while in Western countries the main cause is acetaminophen, followed by antimicrobials (5, 6).
The pathophysiology of DILI is not fully understood. One hypothesis is that an inflammatory reaction results in release of bacterial lipopolysaccharides which act in conjunction with drug metabolites to cause DILI. Other factors are mitochondrial damage and autoimmunity (7). Risk factors include patient ages over 55 years, female sex, alcohol, polypharmacy, malnutrition, HIV, viral hepatitis B, viral hepatitis C and genetic factors (4, 7).
From the histopathological point of view, DILI manifests as acute liver damage but with patterns that vary from acute hepatocellular injury to acute steatosis, acute cholestasis and mixed cholestatic-necroinflammatory pattern (8).
Clinical Case
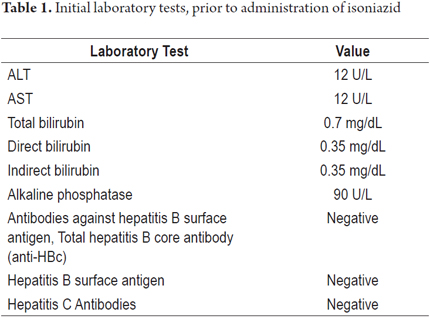
The patient is a 64-year-old man who had had ileocolitis for 10 years. Due to intolerance to immunomodulators, administration had been suspended 9 months previously, but since the disease remained clinically and endoscopically active disease, this patient became a candidate for biological therapy. The patient said that he did not consume alcohol and had completed a budesonide cycle two months previously. His initial hepatic biochemical profile was normal, and blood tests for hepatitis B and C were negative (Table 1). A PPD tuberculosis test was positive at 12 millimeters (normal is up to 5 millimeters). Prophylaxis against latent tuberculosis was indicated.
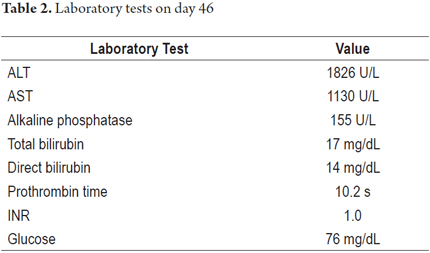
Treatment with orally administered isoniazid was initiated at 300 mg/day. By the 46th day, the patient had developed asthenia, adynamia, choluria, acholia, jaundice and nausea. He came to the ER where they found the patient to be in good general condition, with stable vital signs, marked jaundice, mild pain on deep palpation of the right hypochondrium, but no signs of peritoneal irritation and without neurological alteration. Paraclinical tests (Table 2) showed aminotransferases over ten times normal levels and hyperbilirubinemia, but there were no clinical or paraclinical signs of hepatic impairment. Hepatobiliary ultrasound was normal, and the patient tested negative for viral hepatitis.
Isoniazid was suspended, and the patient was discharged with a diagnosis of hepatitis without hepatic insufficiency under study. The patient evolved satisfactorily with improvement of symptoms and disappearance of jaundice two weeks after discharge. The patient restarted isoniazid on his own, and developed jaundice, choluria, asthenia and adynamia three days later. On his own, he suspended the medication and came to the emergency department which found him to be in good general condition, with normal vital signs, jaundice, a normal abdominal examination, and no neurological abnormalities. Paraclinical tests (Table 3) again showed aminotransferases of over ten times normal levels, marked hyperbilirubinemia, normal PT and glycemia. Hepatobiliary ultrasound was normal.
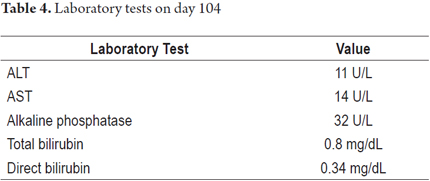
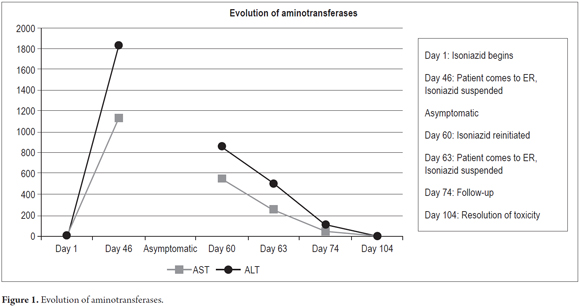
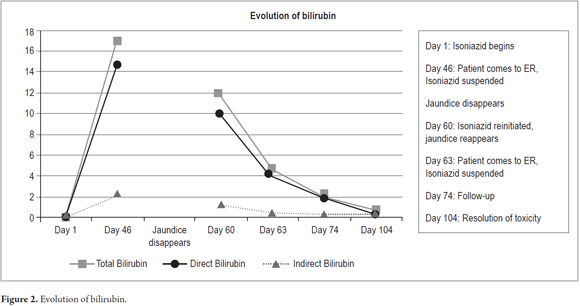
The patient evolved satisfactorily and was discharged with instructions not to reinitiate any medication until a prescription had been issued. Weekly outpatient follow-ups were scheduled. Improvement of his clinical picture was observed with progressive decreases of the hepatic biochemical profile until it became normal. The patient tested negative for autoimmune hepatitis and hemochromatosis (Table 4). Figures 1 and 2 show the evolution of aminotransferases and bilirubin during clinical presentation.
The patient received prophylaxis for tuberculosis with rifampicin and tolerated it well. Then treatment with adalimumab began, and the patient showed clinical improvement and remission of symptoms caused by Crohn's disease. At the moment of this writing, the patient has had one year free of any abnormalities in the biochemical profile of his liver.
Discussion
DILI is diagnosed through exclusion of other possible causes of liver injury such as infectious hepatitis, NASH, biliary obstruction, vascular lesions, hepatic neoplasms, Wilson's disease and hemochromatosis (Table 5). All of these must be ruled out before this diagnosis can be made.
The period of latency between drug exposure and development of DILI varies from five to 90 days. When drug suspected of causing the symptoms is suspended, levels of aminotransferases should decrease by at least 50% within the following weeks. Re-exposure to medication as a diagnostic confirmation may be considered unethical, except when there is no other therapeutic option. Reported cases of involuntary re-exposure confirm whether a given drug is hepatotoxic. Elevation of aminotransferases or alkaline phosphatase alone without hyperbilirubinemia or jaundice is considered an indication of mild disease. Jaundice or hyperbilirubinemia over 2 mg/dL indicates severe disease, as in the case presented here. If jaundice is associated with an INR of over 1.5, encephalopathy or ascites, the risk of mortality is as high as 21% in the case of DILI due to antituberculosis drugs. (7)
Isoniazid is used in combination therapy for management of active tuberculosis or as a monotherapy for latent tuberculosis. Isoniazid can cause adverse hepatic reactions in the early stages of treatment, from one week to six months. These appear as mild hepatitis in 10% to 20% of patients but are typically asymptomatic and self-limiting. ALT is less than three times normal levels, (9) and generally normalizes despite continuing treatment. This phenomenon is called adaptation. (10) Symptomatic hepatitis is another form of presentation which occurs in 0.5% to 1% of patients and which is associated with gastrointestinal symptoms, jaundice and risk of progression to liver failure (0.01%). (9, 11)
Risk factors include patient age over 35 years, female gender, slow acetylating phenotypes, concomitant treatment with other anti-TB drugs or acetaminophen, daily dosage of isoniazid greater than 50 mg/kg, ethanol consumption and cytochrome P- 450 2E1 c1/c1. (12)
Mechanisms that have been proposed for isoniazid hepatotoxicity include the following: (12-14)
- Acetylhydrazine and hydrazine: These toxic metabolites are generated by the hepatic metabolism of isoniazid. Patients who acetylate slowly accumulate greater amounts of these metabolites.
- Cytochrome P450 2E1 c1/c1 genotype: Increased activity of this cytochrome produces greater amounts of hepatotoxic metabolites.
- Oxidative stress: Isoniazid and its metabolite hydrazine decrease glutathione levels and activity of glutathione-S-transferase (antioxidants) which leads to an increase in oxidative mechanisms and, therefore, oxidative stress.
- Isoniazid-Induced Immune Response: Antibodies directed against isoniazid and cytochrome P450 have been found in patients with hepatic insufficiency caused by the drug.
Conclusions
This case of a 64-year-old patient with severe idiosyncratic hepatotoxicity due to isoniazid (acute hepatitis-marked hyperbilirubinemia) was confirmed because there was an improvement when isoniazid was discontinued and then reactivated by involuntary re-exposure to the medication (not indicated by the physician) with adequate evolution after discontinuation of the drug. Due to increased incidence of inflammatory bowel disease in our environment, where there is a high exposure to tuberculosis, physicians are increasingly faced with the need to provide anti-tuberculosis prophylaxis prior to the use of biological drugs. It is important to take into account the possibility of isoniazid hepatotoxicity and to carry out adequate follow-up for these patients.
Financing
None
Conflicts of interest
None
References
1. Edwards RI, Aronson JK. Adverse drug reactions: definition, diagnosis and management. Lancet. 2000;356:1255-9. [ Links ]
2. Navarro VJ, Senior JR. Drug related hepatotoxicity. N Eng J Med. 2006;354:731-9. [ Links ]
3. Chalasani N, Fontana RJ, Bonkovsky HL, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924-34. [ Links ]
4. Stirnimann G, Kessebohm K, Lauterburg B. Liver injury caused by drugs: an update. Swiss Med Wkly. 2010;140:w13080. [ Links ]
5. Kumar R, Shalimar, Bhatia B, et al. Antituberculosis therapy-induced acute liver failure: magnitude, profile, prognosis, and predictors of outcome. Hepatology. 2010;51:1655-74. [ Links ]
6. Reuben A, Koch DG, Lee WM. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065-76. [ Links ]
7. Devarbhavi H. An update on drug-induced liver injury. J Clin Exp Hepatol. 2012;2:247-59. [ Links ]
8. López R. Aspectos morfológicos de la enfermedad hepática inducida por drogas. Rev Col Gastroentero. 2014;29(4):449-60. [ Links ]
9. James L, Roberts D. Isoniazid hepatotoxicity: progress in understanding the immunologic component. Editorials Hepatology. 2014;59(3):746-8. [ Links ]
10. Watkins PB. Idiosyncratic liver injury: challenges and approaches. Toxicol Pathol. 2005;33:1-5. [ Links ]
11. Devarbhavi H, Andrade RJ. Drug-induced liver injury due to antimicrobials, central nervous system agents, and nonsteroidal anti-inflammatory drugs. Semin Liver Dis. 2014;34:145-61. [ Links ]
12. Metushi IG, Cai P, Zhu X, et al. A fresh look at the mechanism of isoniazid-induced hepatotoxicity. Clin Pharmacol Ther. 2011;89:911-4. [ Links ]
13. Tostmann A, Boeree MJ, Aarnoutse RE, et al. Antituberculosis drug-induced hepatotoxicity: concise up-to-date review. J Gastroenterol Hepatol. 2008;23(2):192-202. [ Links ]
14. Metushi IG, Sanders C, The Acute Liver Study Group, et al. Detection of anti-isoniazid and anti-cytochrome p450 antibodies in patients with isoniazid-induced liver failure. Hepatology. 2014;59(3):1084-93. [ Links ]











 text in
text in