Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.35 no.1 Bogotá Jan./Mar. 2020
https://doi.org/10.22516/25007440.317
Case report
Leiomyosarcoma and ulcerative colitis: Case report
1 Servicio Aparato Digestivo, Hospital Universitario Getafe, Madrid, España.
2 Servicio Anatomía Patológica, Hospital Universitario Getafe, Madrid, España
Ulcerative colitis (UC) and Crohn’s disease (CD) patients present an increased risk of colorectal cancer (CRC) due to chronic inflammation, genetic susceptibility and environmental risk factors. In contrast, non-epithelial neoplasms are uncommon.
We discuss the case of an 83-year-old male with a long-standing UC, presenting with a polypoid lesion. Once resected, the lesion was identified as a high-grade leiomyosarcoma. A review of the literature revealed that only three previous cases of leiomyosarcoma among patients with UC have been published. Thus, this one would represent the fourth case where this rare non-epithelial neoplasm was detected in a patient with UC. The direct association of UC and leiomyosarcoma has not been well established; however, immunosuppression is suggested to be a risk factor for leiomyosarcoma in the literature.
Keywords: Leiomyosarcoma; inflammatory bowel disease; ulcerative colitis; dysplasia screening
Los pacientes con colitis ulcerosa (CU) y enfermedad de Crohn (EC) presentan un mayor riesgo de cáncer colorrectal (CCR), debido a la inflamación crónica, la susceptibilidad genética y los factores de riesgo ambientales. Sin embargo, las neoplasias no epiteliales son infrecuentes.
Presentamos el caso de un varón de 83 años con una CU de larga evolución, que presenta una lesión polipoide. Una vez resecada, se diagnosticó de un leiomiosarcoma de alto grado. En la literatura, únicamente se han publicado tres casos previos de leiomiosarcoma en pacientes con CU, por lo que este reporte representaría el cuarto caso. La asociación directa de la CU y el leiomiosarcoma no ha sido bien establecida. No obstante, se sugiere que la inmunosupresión y la inflamación crónica son factores de riesgo.
Palabras clave: Leiomiosarcoma; enfermedad inflamatoria intestinal; colitis ulcerosa (CU); detección de displasia
Introduction
Inflammatory bowel disease (IBD) is associated with increased risk of colorectal cancer (CRC) and has a cumulative incidence of 18% after 30 years of pathology. The high risk of CRC is attributed to three main factors: the carcinogenic effect of chronic mucosal inflammation, genetic predisposition, and environmental factors. 1,2
To prevent morbidity and mortality associated with CRC in patients with IBD, endoscopic screening for dysplasia should begin either 8 years after the diagnosis of left colitis, pancolitis, or Crohn’s disease (CD) with a third of the affected colon or at diagnosis of primary sclerosing cholangitis (PSC).
Frequency of endoscopic examinations depends on individual risk factors such as family history of CRC, diagnosis of PSC, inflammatory pseudopolyps, severe histological inflammation, or extensive mucosal involvement. Periodicity of examination is divided into two groups according to the underlying risk: low-risk patients must undergo endoscopy every 3 to 4 years while high-risk patients must undergo endoscopy every 1 to 2 years. 3,4
In contrast to CRC, the risk of non-epithelial colonic neoplasms has not been established among these patients. Only a small number of cases have been reported in the literature. Non-epithelial tumors of the gastrointestinal tract are rare diseases whose incidence is less than 2.6% of all gastrointestinal tumors.
Since 2000, only 31 cases of colorectal leiomyosarcoma have been reported. Differential diagnoses for this type of cancer should include lymphoma, Gastrointestinal Stromal Tumors (GIST), and inflammatory fibroid polyps. Only a histological study with immunohistochemistry can verify the type of malignancy. 2
Clinical case
We present the case of an 83-year-old man who was in functionally good condition but who had a history of recurrent pulmonary thromboembolism, chronic obstructive pulmonary disease (COPD), stable non-functional adrenal adenoma. He had been diagnosed with ulcerative colitis (UC), A3 S3 E3 according to the Montreal classification when he was 73 years old.
The patient was initially treated with steroids and mesalazine, but shortly after diagnosis he developed corticodependent criteria. He was started on azathioprine and achieved clinical and biological remission.
Six years after diagnosis, a review colonoscopy found quiescent UC with no data on acute activity. In addition, multiple histologically confirmed inflammatory polyps were found. The patient remained in clinical and biological remission with 200 mg of azathioprine and 2.4 g of mesalazine daily. Due to the long evolution of the disease and individual risk factors, we began endoscopic screening for dysplasia every 2 years.
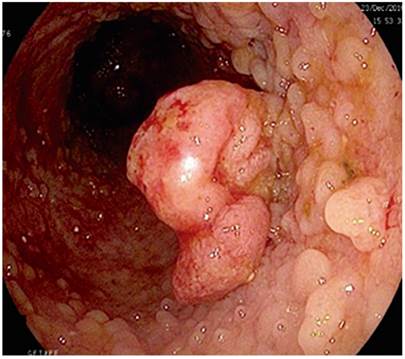
Eight years after diagnosis, a screening colonoscopy identified 15 mm sessile polyp (0-Is, Paris classification) that was eroded at its apex. Biopsies were taken and histology showed a tubular adenoma with pseudosarcomatous changes on chronically inflamed mucosa.
A second colonoscopy was performed to resect the polyp (Figure 1). The histological study showed a high-grade leiomyosarcoma with affected margins. Immunohistochemistry was positive for vimentin and negative for c-kit and CD34 (Figures 2A and 2B).
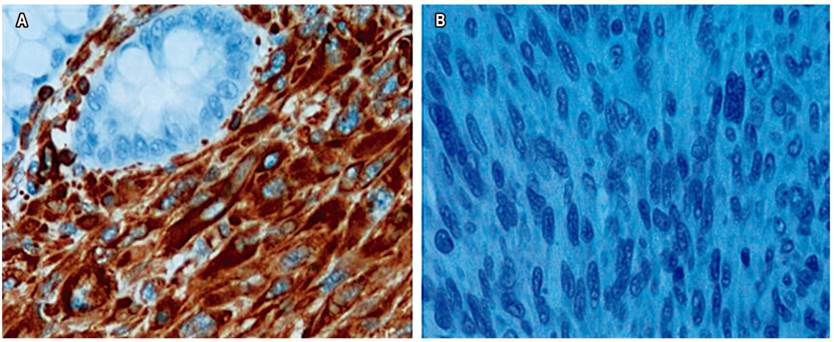
Figure 2A Immunohistochemistry was positive for vimentin. B. Immunohistochemistry was negative for c-kit and CD34.
An abdominal computed tomography (CT) scan found no local, regional or distant disease (T2N0M0, stage IIB). The case was presented in a multidisciplinary session and, given the long-standing chronic inflammation and the extension of the disease, we decided to perform a proctocolectomy.
In February 2017, the patient underwent a total proctocolectomy with a Brooke ileostomy. During the postoperative period, he presented hemoperitoneum secondary to the reintroduction of anticoagulation due to the high thrombotic risk. The patient was admitted to the intensive care unit (ICU), his evolution was torpid, and he died in April of that year.
Discussion
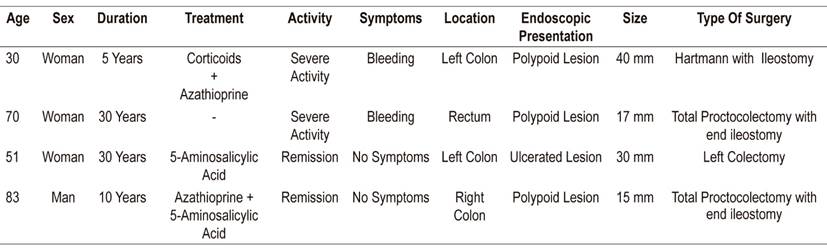
UC patients are at high risk of developing CRC. However, the risk of non-epithelial malignancies in these patients appears to be low although it has not been established. In fact, there were only three reported cases of leiomyosarcoma in UC patients (Table 1). In addition, there are five reported cases of this type of tumors (leiomyosarcoma, GIST, and gastrointestinal neuromas) associated with CD. 5
Leiomyosarcoma accounts for 10% to 20% of all soft tissue sarcomas. The most frequent locations are the uterus, the retroperitoneum and the gastrointestinal tract. Classic gastrointestinal complaints are in the stomach (50%), the small intestine (30%), the colorectal (15%) and the esophagus (5%). However, with the reclassification of mesenchymal stoma tumors in 2000, these percentages may have changed. Colorectal sarcomas account for 0.1% of all malignant colorectal tumors. 4,6
Leiomyosarcoma originates in smooth muscle fiber and is characterized by a high mitotic index and marked cellular atypia. Risk factors include advanced age, exposure to radiation and genetic predisposition. Immunosuppression and chronic inflammation of the gastrointestinal tract have also been implicated. 2,3,7
In fact, three of the four patients with leiomyosarcoma and UC were over 50 years old, and 75% were women. Most of them had long-standing diseases, and one was being treated with high doses of corticosteroids. Half were in remission. The most frequent endoscopic lesion was polypoid. One patient underwent a partial colectomy, while another underwent a Hartmann and an end ileostomy. Our patient is the oldest reported and the first with a right location (Table 1).
The age of onset of these tumors ranges from 40 to 60 years and they are more frequent in men than women and more frequent in the African-American population than in Caucasians. Treatment is usually complete surgical resection. Local recurrence rates range from 38% to 80%, and hematogenous spread occurs in 55% to 71% of cases.
The liver is organ which is most commonly affected, followed by the lungs and bones. Leiomyosarcoma’s endoscopic appearance is not specific, and differential diagnosis should be made from lymphoma, GIST, and inflammatory fibrous polyp. Abdominal CT scans and/or nuclear magnetic resonance imaging are useful for evaluating disease stage and for postoperative follow-up. 9
The diagnosis is based on histology and immunohistochemistry. Cells have fusiform appearance with a characteristic cigar shaped nucleus. The histological grade depends on the number of mitoses, the degree of differentiation, and the percentage of necrosis. Immunohistochemistry is positive for smooth muscle cell markers such as vimentin, smooth muscle specific actin (Smooth Muscle Actin, SMA), HHF-35 and desmin, and negative for c-kit (CD 117), CD 34, AE1/AE3 and S-100.
The cornerstone of treatment is surgery with curative intent. For patients with marked inflammatory activity and extensive involvement, a panproctocolectomy is considered the surgery of choice. For those who are in remission, partial resection appears to be an appropriate technique.
Leiomyosarcoma has a poor prognosis, with a 5-year survival rate of 30% in the minority of patients without associated risk factors. Poor prognostic factors include tumors larger than 5 cm, a high histologic grade, age over 60 years, and affected surgical margins.
Our patient presented two factors indicating poor prognosis, in addition to the inherent risks of colorectal surgery and the multiple comorbidities that conditioned its outcome. Therefore, it is important to handle each case individually.
Recent studies of the therapeutic effects of tyrosine kinase inhibitors in advanced stages have not demonstrated any significant benefits. 10
Conclusions
Leiomyosarcoma of the colon is a rare and aggressive tumor whose prognosis is very poor. Since a direct association of UC and leiomyosarcoma is not well established, we cannot rule out incidental concomitance of these diseases. Nevertheless, immunosuppression and chronic inflammation may be risk factors. Further prospective studies with larger sample sizes are needed to establish whether there is a causal relationship or whether it is incidental concomitance.
Referencias
1. Clark MA, Fisher C, Judson I, Thomas JM. Soft-tissue sarcomas in adults. N Engl J Med. 2005;353(7):701-11. http://dx.doi.org/10.1056/NEJMra041866 [ Links ]
2. Hilal L, Barada K, Mukherji D, Temraz S, Shamseddine A. Gastrointestinal (GI) leiomyosarcoma (LMS) case series and review on diagnosis, management, and prognosis. Med Oncol. 2016;33(2):20. http://dx.doi.org/10.1007/s12032-016-0730-3 [ Links ]
3. Singh P, Bello B, Weber C, Umanskiy K. Rectal leiomyosarcoma in association with ulcerative colitis: a rare condition with an unusual presentation. Int J Colorectal Dis. 2014;29(7):887-8. https://doi.org/10.1007/s00384-014-1873-3 [ Links ]
4. Scarpa M, Castagliuolo I, Castoro C, Pozza A, Scarpa M, Kotsafti A, et al. Inflammatory colonic carcinogenesis: a review on pathogenesis and immunosurveillance mechanisms in ulcerative colitis. World J Gastroenterol. 2014;20(22):6774-6785. https://doi.org/10.3748/wjg.v20.i22.6774 [ Links ]
5. Jayakumar R, Basu PP, Huang T, Axiotis CA. Postirradiation Leiomyosarcoma of Rectum Presenting as a Polyp: Case Report and Review of the Literature. Int J Surg Pathol. 2016;24(2):163-9. https://doi.org/10.1177/1066896915617025 [ Links ]
6. Granero-Peiró L, Martínez-Ortega P, Sánchez-Justicia C, Hernández-Lizoáin JL. Leiomyosarcoma of the ascending colon: a rare tumor with poor prognosis. Rev Esp Enferm Dig. 2015;107(9):584-5. [ Links ]
7. Akutsu D, Mizokami Y, Suzuki H, Terasaki M, Narasaka T, Kaneko T, et al. A Rare Case of Colonic Leiomyosarcoma in Association with Ulcerative Colitis. Intern Med. 2016;55(19):2799-2803. https://doi.org/10.2169/internalmedicine.55.6770 [ Links ]
8. Yamamoto H, Handa M, Tobo T, Setsu N, Fujita K, Oshiro Y, et al. Clinicopathological features of primary leiomyosarcoma of the gastrointestinal tract following recognition of gastrointestinal stromal tumours. Histopathology. 2013;63(2):194-207. https://doi.org/10.1111/his.12159 [ Links ]
9. Magro F, Gionchetti P, Eliakim R, Ardizzone S, Armuzzi A, Barreiro-de Acosta M, et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J Crohns Colitis. 2017;11(6):649-670. https://doi.org/10.1093/ecco-jcc/jjx008 [ Links ]
10. Butcher RO, Titi S, Limdi JK. Mesenchymal tumours, immunosuppression and inflammatory bowel disease: rare, real or fortuitous? J Crohns Colitis. 2011;5(6):645-6. https://doi.org/10.1016/j.crohns.2011.07.016 [ Links ]
Received: November 18, 2018; Accepted: January 28, 2019











 text in
text in