Introduction
Coffee represents one of the most important agricultural products of Colombia (Barjolle et al., 2017). Currently, Colombia has 844,700 ha planted with coffee, 84% corresponding to Colombia, Castillo®, Cenicafé 1® and Tabi ®, cultivars developed by Cenicafe, which are resistant to the coffee leaf rust disease (FNC, 2021). These coffee cultivars are highly productive and have an outstanding beverage quality (Echeverry-Giraldo et al., 2020). The major concern for coffee growers is to maintain high production levels at a low cost; coffee contributes significantly to the national productivity and gross domestic product and supports approximately 540,000 families, mostly small farm-holders (CCGF, 2020).
Coffee plants have a high demand for phosphorus (P) during nursery stage and vegetative growth. These requirements are commonly satisfied by a high dose of soluble P fertilizers (Ávila et al., 2007; Sadeghian & González, 2012), which increase production costs and increase environmental concerns (Ni & Wang, 2015; Tian et al., 2017).
A solution to this problem is the biotechnological use of arbuscular mycorrhizal fungi (AMF), which can enhance plant P uptake (Cardoso et al., 2017) and growth of coffee seedlings (Rivillas & Dodd, 1996; Osorio & Habte, 2014), due to its capability to explore higher soil volume through extra-radical hyphae (Andrade et al., 2009). These effects are also positive under drought conditions, where AMF colonization efficiently makes use of water conditioning the plant stomatal opening and the leaf turgor (Augé, 2004).
AMF has an important role in organic/biological coffee system production where the use of chemical synthesis P fertilizers is restricted (Chiputwa et al., 2015; Sepúlveda et al., 2016). This bio-technology is environmentally friendly (Cardoso & Kuyper, 2006), cost-effective and contributes to reducing P fertilization (Jaramillo & Osorio, 2009; Rai et al., 2013), considering that the demand of phosphoric fertilizers has exceeded the supply and the prices have globally been increased (Williams, 2021), demonstrating, in a short time, high sensitivity on market (Alewell et al., 2020).
Several studies have been published about AMF colonization in different Coffea arabica varieties (Bolaños et al., 2000; Lebrón et al., 2012; Sewnet & Tuju, 2013; Franca et al., 2014; De Beenhouwer et al., 2015). In respect to the response of coffee plant growth during the nursery state to AMF applications, Orozco (1988) evaluated Gigaspora margarita (GM), Entrophospora colombiana (EC), Glomus manihotis (GMH), AMF native (N), and GM-EC-GMH-N combination, in a sterile soil (P-Bray II level: 151 mg kg-1) and found best results with GM and combining GM-EC-GMH-N. Recently, Sadeghian and Ospina (2021), working with an AMF containing 50 spores/g inoculum according to technical sheet of product and composed by Glomus sp., Entrophospora sp. and Scutellospora sp. (20 g/plant) at two levels of P (1 and 2 g P2O5/plant), did not find an additive effect of AMF. Hernández-Acosta et al. (2020) carried out an experiment in coffee seedlings cultivars Garnica, Catimor, Caturra and Catuaí growing in a sterilized substrate (P-Bray II level: 33 mg kg-1) and inoculated with different AMF (10 g/plant), with its quality previously verified (colonization between 57 and 65%). Plant height and dry biomass were positively affected by the joint application of Glomus claroides, Rhizophagus diaphanus, and Paraglomus albidum. Changes in the leaf P content varied among varieties, achieving highest levels when the AMF were applied in consortium (Hernández-Acosta et al., 2020).
Based on the last consideration, despite promising effects reported by using AMF, the response of coffee during the nursery stage to its application has been uncertain, and in some cases, inconsistent, due to variations in the experimental conditions, soil fertility status (sterile o non-sterile substrate, P level), species, formulations of AMF (single species application o joint application of various AMF species), dose per plant, as well as quality of AMF. In addition, few studies carried out verifications of AMF colonization at the end of the experimental goal to corroborate the real effect of AMF on plant growth under soil conditions.
Although more than 100 species of AMF establish association with coffee plants (Hernández-Acosta et al., 2021), in Colombia, there are few options based on AMF formulations as related to their quality for use in soil-coffee systems and to variability in soil fertility, particularly P availability in different soils.
Taking in account these issues, the hypothesis of this study was: plant P uptake and growth promotion of coffee seedlings due AMF inoculation depend on the soil P availability. Thus, our aim was to evaluate the response of coffee seedlings of C. arabica cv. Colombia to the inoculation of the AMF Rhizoglomus fasciculatum under different levels of P in the soil solution.
Materials and methods
The study was conducted under greenhouse conditions in the Universidad Nacional de Colombia in Medellín (6°15' N, 75°35' W, 1495 m a.s.l.). A sub-superficial (30-50 cm) soil sample from a Paleudult - Bt horizon (P-Bray II: 1 mg kg-1) was air-dried, passed through a 4 mm sieve, mixed with quartz sand (soil:quartz sand ratio w/w of 2:1), and autoclaved twice at 120°C and 0.1 MPa for 1 h. Based on a soil test, the following fertilizers were applied to 1 kg of the soil-sand mixture: 2 g of calcium carbonate, 436 mg of ammonium sulfate, 1550 mg of calcium sulfate, 980 mg of magnesium sulfate, 5 mg of Fe-EDTA, 5 mg of Cu-EDTA, 5 mg Zn-EDTA, and 5 g Borax.
A soil P adsorption isotherm was conducted following the procedure proposed by Fox and Kamprath (1970) to determine the P requirement and to achieve three levels of P in soil solution: 0.002, 0.02, and 0.2 mg L-1. Accordingly, KH2PO4 was applied at three doses: 0.950, and 2,800 mg kg-1, respectively, and mixed thoroughly. To balance the level of potassium added, potassium sulfate was applied to the first two levels (1,533 and 1,066 mg kg-1). The substrate was transferred into black plastic bags of 17x23 cm with capacity of 1.8 kg per bag, dry basis, the recommended conditions to grow coffee seedlings at the nursery stage. Then, the substrate was left uninoculated (control) or inoculated with 50 g of a crude mycorrhiza containing 250 spores of R. fasciculatum per g and then mixed throughout. The mycorrhizal inoculum was previously multiplied in maize roots under controlled conditions in the Soil Microbiology Laboratory of the Universidad Nacional de Colombia (Habte & Osorio, 2001). The uninoculated bags received 20 ml of a 10% mycorrhizal suspension, which was previously filtered through a No. 42 Whatman filter paper.
Seeds of coffee C. Arabica, cv. Colombia, were germinated in sterile sand and grown for 60 d and then transplanted into the P-amended and inoculated/uninoculated substrate. A thin layer of fine quartz sand was applied on the surface of each pot to prevent cross contamination. The seedlings grew for 150 d under sunlight exposure. The substrate was watered with distilled water to maintain it at 50-60% of the maximum water holding capacity. To prevent plant nutrient deficiencies, 50 cm3 of P-free Hoagland solution were supplied to each bag once a week.
The experimental design was completely randomized. Treatments had a factorial arrangement 2x3, two levels of mycorrhizal inoculation (inoculated and uninoculated) and three levels of soil solution P (0.002, 0.02, and 0.2 mg L"1). Each treatment had five replicates. The foliar P content was monitored at 30, 50, 70, 92, 110, 130, and 150 d after transplanting; for this purpose, a leaf-disk of 6 mm in diameter was collected from the newest fully extended mature leaf of each plant, then oven-dried, weighed, and ashed in a muffle furnace (500°C, 3 h) (Aziz & Habte, 1987). The ashes were dissolved in 10 ml of distilled water and the P concentration was measured by the molybdenum blue method (Murphy & Riley, 1962). At the time of harvesting at 150th d, the mycorrhizal colonization was determined in fine root fragments, which were washed with water, cleared by immersion in 10% KOH for 24 h and then acidified with 10% HCl for 5 min. After that, the root samples were stained with 0.15% of fuchsine acid (Kormanikef al., 1980). The extent of root colonization was determined by the grid-line intersection method (Giovannetti & Mosse, 1980). The shoot dry weight (SDW) was determined after oven-dry the samples at 60°C for 96 h.
The data were subjected to ANOVA tests and to the Duncan multiple range test for mean separation. In both cases, a level of significance P<0.05 was used. Both tests were performed with the statistical software Statgraphics.
Results and discussion
The variables under study were affected by the treatments. For instance, mycorrhizal colonization was only detected in those plants grown in the inoculated substrate, regardless of the soil solution P level (Fig. 1). Mycorrhizal colonization did not differ between 0.002 mg L-1 and 0.02 mg L-1 P levels (54% and 49%, respectively), but these two were statistically different (P<0.05) from that at 0.2 mg L-1 P level, which had the lowest value (9%). These results indicate that, in young coffee seedlings, mycorrhizal colonization depended on the P level in the soil solution.
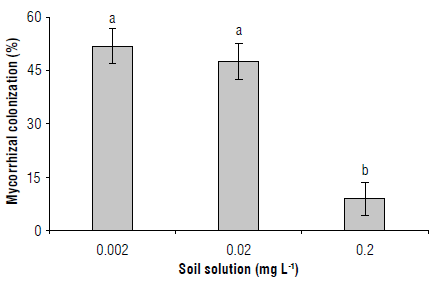
FIGURE 1 Mycorrhizal colonization in roots of C. arabica cv. Colombia, in a substrate inoculated with the mycorrhizal fungus Я fasciculatum at three levels of P in solution. Columns a are significantly different from b (P<0.05) according to the Duncan multiple range test. Bars represent the standard errors.
The AMF colonization was between 1.4x and 3.6x the results in Hernández-Acosta et al. (2020) in С. arabica varieties Catuaí and Carniça using 10 g/plant of AMF in consortium and between 0.5x and 1.5x the results in Cuervo (2017) in C. arabica cultivars Tabi® and Castillo® planted in an Andisol (Bw horizon) and inoculated with 50 g/plant of R. fasciculatum containing 500 spores/g. Orozco (1988) reported AMF concentration around 60% in C. arabica, cv. Colombia. Differences among the results could be explained by variations between AMF species and formulations, such as the dose of inoculum and the concentration of spores, although these effects are likely controlled by the AMF or coffee specie (Jaitieng et al, 2021).
Phosphorus concentration in soil is a key factor to explain AMF colonization; while Hernández-Acosta et al. (2020) and Cuervo (2017) worked under P concentration in soil corresponding to 33.0 and 2.0 mg kg"1, respectively, soil P concentration in our study was 1.0 mg kg"1. According to Moreira et al. (2019), AMF colonization decreased significantly (around 50%) as P applications increased up to 0.74 g kg"1 of soil in coffee Catuaí Vermelho IAC 99 growing in substrate without sterilization. These findings demonstrate that AMF symbiosis is activated as the defense of plants against combined stress conditions as documented by Rashad et al. (2021).
The shoot dry weight (SDW) was significantly affected by the soil solution P concentration, the AMF inoculation, and the interaction between both factors. At the 0.002 mg L-1 level of P, the AMF inoculation did not affect the SDW, which fluctuated between 0.39 and 0.52 g/plant (Fig. 2). By contrast, at the 0.02 mg L-1 level of P, the inoculation with R. fasciculatum significantly increased the SDW by 3.05x respect to the uninoculated control at 1.03 g/plant on average. Similarly, at 0.2 mg L-1 level, the SDW was significantly affected by the inoculation with R. fasciculatum (3.13 gl plant), while the uninoculated control was 2.07 g/plant.
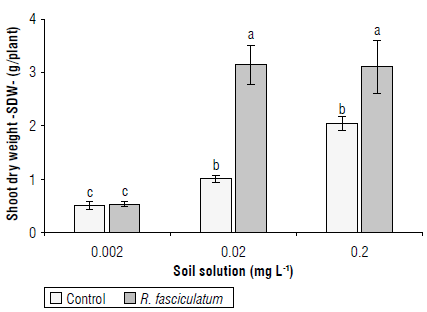
FIGURE 2 Shoot dry weight (SDW) of coffee cv. Colombia seedlings grown in a substrate either uninoculated (control) or inoculated with R. fasciculatum at three levels of P in solution. Different lowercase letters indicate significant differences according to the Duncan multiple test (P<0.05). Bars represent the standard errors.
The foliar P concentration was significantly affected by the interaction soil P level x AMF inoculation only at day 150. At the 0.002 mg L-1 level, the AMF inoculation did not increase the foliar P content at any time of evaluation; the values ranged from 0.07 to 0.15%. By contrast, at 0.02 mg L-1 the inoculation with R. fasciculatum significantly increased the foliar P concentration from 110 d after transplanting; the uninoculated plants had values between 0.10 and 0.13%, whereas the inoculated plants had between 0.17 to 0.19%, respectively. On the other hand, at 0.2 mg L-1 of P the AMF inoculation did not significantly affect the foliar P concentration (Figs. 3A-C).
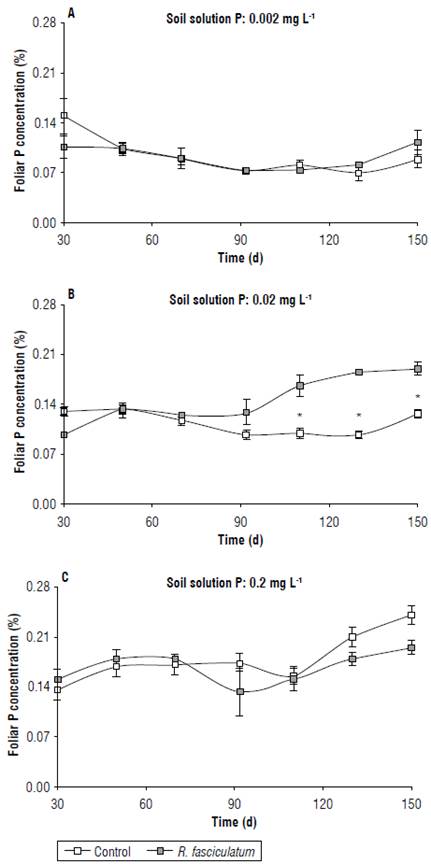
FIGURE 3 Foliar P concentration (%) in C. arabica cv. Colombia seedlings grown in a substrate either uninoculated or inoculated with R. fasciculatum and three levels of P in solution. The asterisks indicate significant (P<0.05) differences at the respective time between the respective means. Bars represent standard errors.
In addition, the P foliar content (µg/leaf disc) showed a similar behavior to the P concentration (%). At the 0.002 mg L-1 level, the AMF did not promote increase of foliar P content at anytime, the values ranged from 0.93 to 1.19 µg/ leaf disc. By contrast, at the 0.02 mg L-1, the inoculation with R. fasciculatum significantly increased this nutrient in the leaf tissues, particularly after 110 d of growth. For example, uninoculated plants had values among 1.11,1.08, and 1.22 µg/leaf disc at 110, 130, and 150 d, respectively, while the inoculated plants had 2.44, 2.38, and, 2.34 µg/leaf disc at the same sampling days. These results represent increases of foliar P content of 119%, 120%, and 92%, respectively (Figs. 4A-C).
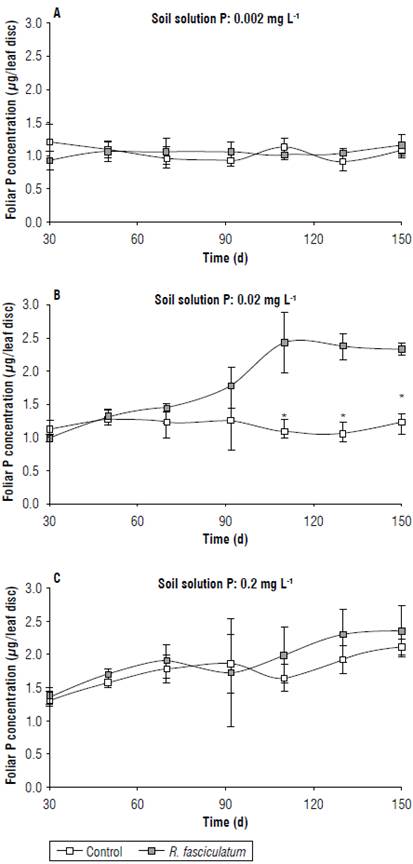
FIGURE 4 Foliar P content (jug/leaf disc) of coffee C. arabica cv. Colombia seedlings grown in a soil either uninoculated (control) or inoculated with the AMF R. fasciculatum at three levels of soil solution P The asterisks indicate significant (P<0.05) difference at the respective time between the respective means. Bars represent standard errors.
The results suggest that the use of R. fasciculatum is adequate to promote plant growth and P uptake for coffee seedlings cv. Colombia. The results agree with those of Jaramillo and Osorio (2009), which studied the mycorrhizal and effectiveness dependency of coffee seedlings of C. arabica cv. Colombia and Caturra at the same soil P levels used in this study. Cuervo (2017), working with a sterile Bw horizon (Andisol) and with the same AMF and soil P levels, found for the coffee cultivars Castillo® Naranjal, Castillo® Tambo, and Tabi® comparable results in SDW and foliar P uptake. In that case, the SDW increase with AMF inoculation was 10 times higher in respect to the uninoculated plants. Earlier works of Habte and Biitenbender (1999) in an Oxisol soil from Hawaii with coffee seedlings variety Typica showed positive response in plant growth when Glomus aggregatum was used.
The lack of response at the lowest level of soluble P is because this concentration is too low for uptake by both plant and AMF. In other words, the low soil P availability was a limiting factor to the mycorrhizal dependency (Osorio & Habte, 2014). Since the plant cannot grow properly under such condition, it cannot share carbon with the mycorrhizal fungus. Mycorrhizal plants invest nearly 20 to 30% of the fixed photosynthate carbon compounds in order to satisfy the nutritional requirements of the fungus (Eskandari et al., 2017), and specifically in coffee plants increasing photosynthetic rate (Cruz et al, 2020). This carbon allocates to the extra radical fungal hyphae, which operate as an extension of the plant root system (Lebrón et al, 2012) and acquire P from surrounding soil areas beyond the plant roots zone (López-Arredondo et al, 2014).
An opposite scenario occurred at the highest soil P availability level, where the AMF inoculation seems to be unnecessary, because there is enough P for plant growth and the magnitude of the response is lower (Harrison, 1999). In fact, several authors showed that AMF inoculation at that high level of soil P can produce negative effects due to an imbalance associated to an expensive metabolic cost required to support a micro-symbiont system with carbon, which does not improve the plant performance (Roth & Paszkowski, 2017; Wang et al, 2017). For instance, Jaramillo and Osorio (2009) indicated negative effects in coffee seedlings cultivars Caturra and Colombia using Glomus fistulosum inoculum when the soil P availability was 0.2 mg L-1.
In order to contextualize the P values in the soil solution, González (2018) found that many soils in the Colombian coffee region according to the magnitude of P fixation in soils present values between P0.1 and P0.2 in soil solution, corresponding to P-Bray II levels between 10 and 30 mg kg-1.
As shown by Sadeghian and Ospina (2021), coffee plants require between 1.0 and 2.0 g of P205/plant to satisfy the requirements during the nursery stage. This amount generates, according to the soil order, P levels in the soil from 200 to 490 mg kg-1 P-Bray II. In consequence, high doses of fertilizers generate soil salinity, with a negative impact on the AMF colonization (Rashad et al., 2021) and, hence, a lower effect on plant growth.
It is also likely that at 0.2 mg L-1 the availability of other essential nutrients (e.g., Zn) can decrease (Bhattacharya & Bagyaraj, 2002; Ozdemir et al., 2010; Sewnet & Tuju, 2013; Zhang et al., 2017), thus, affecting the functioning, formation, and multiplication of AM fungi in the rhizosphere (Dutt et al., 2013; Sewnet & Tuju, 2014).
In summary, this study clearly demonstrated a positive effect of AMF R. fasciculatum inoculation on SDW and foliar phosphorus (P) content of coffee seedlings only if the P level in the soil solution is 0.02 mg L-1. At 0.002 mg L-1 of P, the AMF inoculation proceeds only if P fertilizers were added to reach an optimal P level for the mycorrhizal association. On the other hand, if the P level in the soil solution is 0.02 mg L-1, the AMF inoculation can be used without any P fertilization. By contrast, when P concentration in soil solution is as high as 0.2 mg L-1, the AMF inoculation seems to be unnecessary.














