Introduction
Various studies analyzing lawsuits in anesthesiology and examining adverse events link failures in airway management to preventable death and permanent brain damage 1-2. Despite proposals suggesting that injuries and deaths related to airway management should be considered “never events” 3, a historical observational study spanning 20 years found that 31% of preventable errors leading to unexpected deaths were due to failures in airway management, particularly in the timing of advanced management (4). Analyses of lawsuits for perioperative pulmonary aspiration conclude that the second leading cause, accounting for 33% of cases, was the failure to properly perform a Rapid Sequence Induction and Intubation (RSII) 5.
RSII was first proposed in 1970. The classic description included pre-oxygenation for two minutes, the use of thiopental and succinylcholine, the application of cricoid pressure, a period of nonventilation with a face mask, and finally, intubation using an endotracheal tube with a cuff 6. Over the years, each of these components has been scrutinized and elaborated upon, leading to changes in the original description. This necessitates a synthesis of available evidence. A narrative review is conducted for this purpose.
Methodology
Information Sources and Search Strategy
A non-systematic search was carried out on Medline, Embase, and CENTRAL databases, using the MeSH terms Rapid Sequence Induction and Intubation, Intratracheal Intubation, Ketamine, Etomidate, Propofol, Midazolam, Succinylcholine, Rocuronium, Emergency Medical Services, Emergency Medicine, and Airway Management. This was complemented by a search for references and other sources considered relevant by the authors.
Definition
Rapid Sequence Induction and Intubation is a strategy based on the administration of full-dose neuromuscular relaxation and induction, aiming to minimize complications arising from bronchoaspiration, apnea, and autonomic response and to improve the conditions for advanced airway management. While the choice of the inducing agent and its dosage will depend on the patient’s clinical context, the use of full doses of drugs, especially of the neuromuscular relaxant, shortens the latency period of their effects and allows for quicker tracheal intubation.
Indications
RSII is indicated in scenarios involving the loss of airway protection or patency, the deterioration of ventilation or oxygenation, and cases where such losses are anticipated and there is a high risk of bronchoaspiration. Common indications include acute neurological disorders associated or not with trauma or poisoning, imminent ventilatory failure due to unresponsive obstructive disorders, and, in the perioperative setting, non-fasting patients requiring urgent surgical intervention 7.
Contraindications
The contraindications for RSII are relative. In patients with clear predictors of difficult airway (DA), the safest approach is awake orotracheal intubation, as conventional techniques are often not successful; thus, induction and relaxation are not recommended for these patients. If emergency action is required due to imminent ventilatory failure in patients with DA, a double setup is recommended. This strategy involves having, in addition to RSII, all the preparations on the table for maneuvers of an open translaryngeal technique like cricothyroidotomy 8-9. On the other hand, RSII is not indicated in the case of loss of ventilation with airway collapse, that is, in situations of no ventilation and no oxygenation, where immediate cricothyroidotomy should be performed due to the high risk of permanent brain damage and death 8,10. Finally, this strategy is not indicated in the scenario of crash intubation or crash airway, which applies to unresponsive patients with deteriorated or absent cardiopulmonary function (cardiopulmonary arrest) or nearing death, who cannot maintain ventilation and oxygenation themselves and are in an altered state of consciousness with loss of muscle contraction 11.
General Overview and Steps
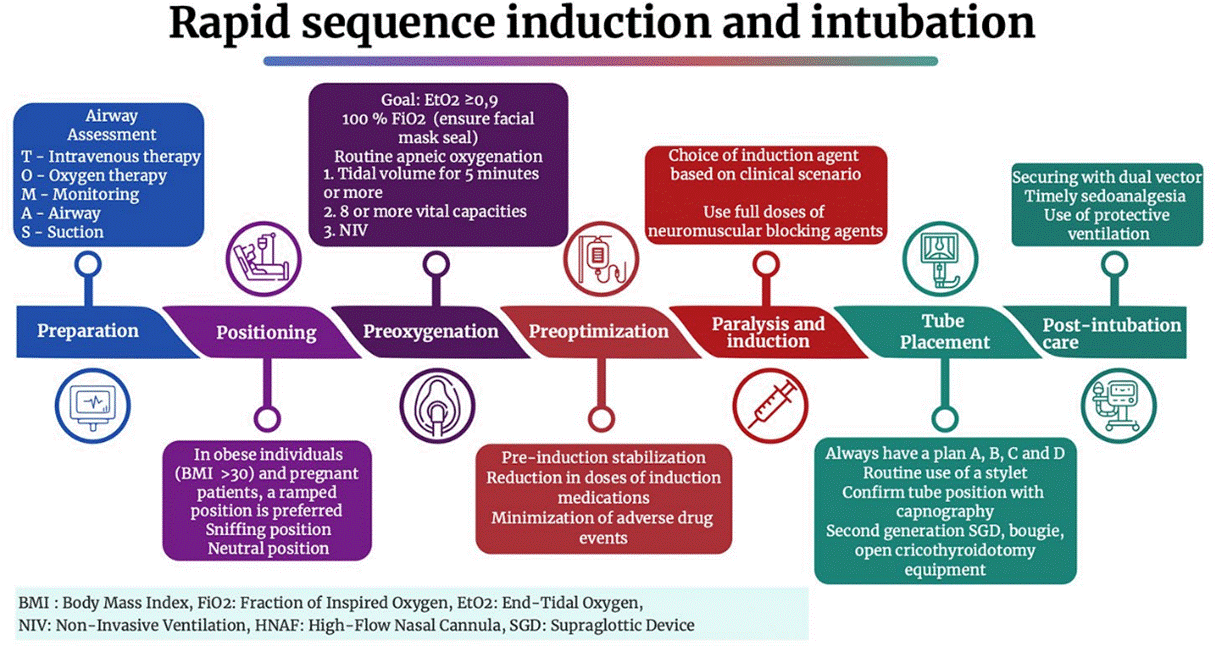
As a didactic strategy, the mnemonic of the “seven Ps” has been used, consisting of various points that help to remember the steps to follow in an RSII (see Figure 1).
Preparation
This step begins with knowledge acquisition, initially theoretical and later practical, through simulation and hands-on patient experience. It also involves identifying the needs for supplies and medications based on an understanding of the work service’s operation, even before interacting with the patient, to reduce complications and increase the likelihood of successful intubation on the first attempt. The equipment that should be prepared includes the following:
Intravenous Therapy: Verified permeable venous access and required medications.
Oxygen Therapy: Sources of oxygen, equipment for administering pre-oxygenation and positive pressure ventilation, for example, self-inflating bag with reservoir and oxygen connection, Ayre Rees or anesthesia machine with circuit and facial mask according to patient type.
Focused Monitoring for Identifying Complications: In a multicenter study across 29 countries involving 3659 patients undergoing emergency intubation, 45.2% experienced at least one major peri-intubation adverse event. The predominant event was cardiovascular instability, observed in 42.6% of all patients undergoing emergency intubation, followed by severe hypoxemia (9.3%) and cardiac arrest (3.1%) 12. Tachycardia was reported in 32.7% and bradycardia in 5.2% of patients 13. Additionally, a first-attempt intubation success rate of only 84.1% (95% CI 80.1 87.4) was reported, esophageal intubation in 3.5%, the requirement of three or more intubation attempts in 0.8%, and the need for cricothyroidotomy in 0.3% of patients 14. Given the frequency of complications, routine non-invasive blood pressure monitoring, continuous pulse oximetry, cardioscopy, and capnography for intubation verification are suggested.
Aspiration Equipment: Includes portable aspirators or medical gas network aspirators and the use of non-collapsible cannulas, such as the Yankauer cannula.
Airway Management Devices: Laryngoscope with tested batteries, orotracheal tubes of different sizes, oroand nasopharyngeal cannulas, and equipment used in alternative techniques (second-generation supraglottic device, videolaryngoscopy, intubation adjuvants like Frova® and open cricothyroidotomy equipment [i.e., No. 10 scalpel blade, 6 mm tube, and Bougie]).
Initial Patient Evaluation: Must be performed in all cases, both for the clinical state that leads to RSII indication and for airway evaluation.
Special situations requiring immediate consideration for a Difficult Airway (DA) include anatomical abnormalities (craniofacial malformations, tumors), post-radiation therapy changes in the head and neck, oral opening <2 cm, cervical immobility, history of DA, and the presence of two or more minor predictors 15.
In a quick physical examination, other clinical predictors that are associated with DA to varying extents should be sought: Class 3 upper lip bite test (positive LR 14, 95% CI 8.9 22), reduced hyomental distance (positive LR 6.4, 95% CI 4.1 10), subjective retrognathia or mandible <9 cm (positive LR 6, 95% CI 3.1 11), limited mandibular protrusion (positive LR 5.5, 95% CI 2.1 15), reduced cervical mobility (positive LR 4.2, 95% CI 1.9 9.5), reduced sternomental distance (positive LR 4.1, 95% CI 2.7 6.1), modified Mallampati ≥III (positive LR 4.1, 95% CI 3 5.6), and reduced thyromental distance (positive LR 3.3, 95% CI 2.4 4.4) 16. None of these offer sufficient diagnostic accuracy to rule out or confirm the presence of a DA as a sole predictor, so they should be applied collectively.
Position
This can be considered a preliminary step to preoxygenation, as it will aid in enhancing it as well as in patient ventilation (Figure 2).
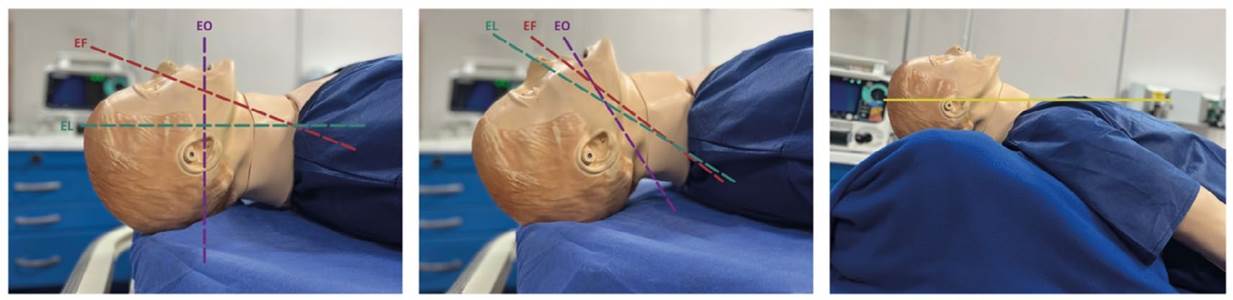
From left to right, neutral, sniffing, and ramp positions. EO: oral axis (purple), EF: pharyngeal axis (red), EL: laryngeal axis (green). Yellow line: The ramp position aims to align the axis of the external auditory canal with the sternum in the horizontal plane. Source: Authors. Photographs taken in the Simulation Laboratory of the Faculty of Medicine at the University of Antioquia.
Figure 2 Positions
Sniffing Position: Traditionally recommended as the optimal position for direct laryngoscopy in most patients, it theoretically allows alignment of the three axes (oral, pharyngeal, and laryngeal, as indicated in Figure 2) and improves glottic visualization 17. A better score on the intubation difficulty scale has been demonstrated (RR 1.28, 95% CI 1.15 1.42, p < 0.0001), so it remains relevant 18.
Ramp Position: Compared to the sniffing position in adult general populations, no statistically significant differences were found in terms of first-attempt success, intubation attempts, or glottic vision; thus, it is not conventionally recommended for all patients 19. However, when compared to the supine position, it clearly improves ventilation in obese patients in terms of exhaled tidal volume (mean ± SD 9.3 ± 2.7 vs. 7.6 ± 2.4 ml/kg; p <0.001). Furthermore, several clinical trials indicate that the ramp position in morbidly obese patients improves glottic visualization compared to the sniffing position 20-21. In critically ill patients, this position has been associated with a higher likelihood of a Cormack-Lehane score of 1-2 (OR 2.05, 95% CI 1.26 3.32, p = 0.004) and a lower likelihood of a score of 3-4 (OR 0.49, 95% CI 0.3 0.79, p = 0.004) 19.
Neutral Position: Supported in patients with RSII in cervical trauma scenarios 22. It has been described that if video laryngoscopy is chosen as the technique, the neutral position may not have significant differences compared to the sniffing position in terms of intubation difficulty (p = 0.384). Although the neutral position was associated with less glottic opening, it was also not related to laryngoscopy time, intubation time, or first-attempt success rate 23.
Preoxygenation
This is a technique of prior oxygen administration aimed at increasing oxygen reserves and extending safe apnea time 24. It is indicated in all patients, especially those who are critically ill, urgent, pediatric, obese, and pregnant, given physiological conditions that reduce safe apnea time (functional residual capacity reduction, increased oxygen consumption, anemia, acidosis, cardiopulmonary disease) 25.
The goal is to achieve an expired oxygen level of 0.9 or higher 26. Various techniques exist to achieve this: tidal volume ventilation (TVV) for 3 to 5 minutes with fresh gas flow (FGF) >5 L/min or 8 deep breaths for one minute with FGF of 10 L/min 25. Technical considerations include proper mask attachment and the use of 100% inspired oxygen fraction (FiO2). Transnasal Humidified RapidInsufflation Ventilatory Exchange (THRIVE) has also been suggested, which involves administering 100% FiO2 through a high-flow nasal cannula (HFNC) for 3 minutes at 60 L/min, appearing to be as effective as TVV for 3 minutes at 10 L/min 27.
The original RSII protocol advised against the use of positive pressure ventilation (PPV) for fear of gastric inflation leading to pulmonary aspiration; however, this hypothesis has been debated. Those refuting it argue that providing ventilation just before attempting intubation is essential to avoid desaturation, especially in those with low functional residual capacity or high oxygen consumption, who may not optimally benefit from preoxygenation 28. A network meta-analysis published in 2019 found that, in patients with acute respiratory failure, non-invasive mechanical ventilation with a face mask is associated with fewer adverse events (OR 0.43, 95% CI 0.21 0.87) and less desaturation (MD 5.53, 95% CI 2.71 8.34), as is the use of a high-flow nasal cannula 29.
Apneic oxygenation can complement preoxygenation and is described through various methods: nasopharyngeal cannula, THRIVE, or simple nasal cannula (NODESAT [nasal oxygen during efforts securing a tube]). In the latter, oxygen is administered through a cannula at 3 L/min before conventional preoxygenation and induction, then maintained at 5-15 L/min 30. A recent clinical trial found no differences between these techniques in obese patients (safe apnea of 601 (268-900) vs. 537 (399-808) seconds, p = 0.698 in the HFNC group), suggesting that simple nasal cannulas, being more readily available, are likely to be more cost-effective 31.
Preoptimization
Previously, the term “premedication” was used for this step. Physiological disorders reduce the patient’s tolerance to repeated or prolonged attempts at laryngoscopy, and as a result, hypoxemia and hemodynamic deterioration are common complications. In this regard, various strategies have been proposed to optimize the patient’s physiological state before and during anesthetic induction. This is done with three main objectives: to reduce the dose of induction drugs, to decrease adverse drug events, and to preoptimize hemodynamic status (see Table 1).
Table 1 Preoptimization Strategies, Doses, and Indications
| Strategy, Dose and Presentation | Indications | Observations |
| Dose reduction of Induction Drugs | ||
| Lidocaine 0.5-1.5 mg/kg Available in 1% and 2%. Vials of 10, 20, and 50 ml. | Reduce pain associated with propofol administration 32,33, hemodynamic changes due to medication use 34, intubation 35-37, and reflexes induced by airway management. | Controversial effects due to heterogeneity in doses, administration routes, and outcomes. |
| Midazolam 0.01- 0.03 mg/kg Available in 5 mg/5 ml and 15 mg/3 ml. | Reduce required doses of induction agents. Pretreatment with midazolam significantly reduces propofol induction dose and time, as well as adverse effects such as hypotension, apnea, and injection pain 38. | In elderly patients, caution should be exercised when using pre-treatment with midazolam and remifentanil. Although these agents reduce the ED95 of propofol, they increase hemodynamic instability during induction 39. |
| Fentanyl 2-3 μg/kg Available in 50 μg/ml, vials of 10 and 20 ml. | To attenuate the hemodynamic response accompanying laryngoscopy and tracheal intubation, fentanyl is effective and comparable to other opioids 40. It is also widely available across various levels of healthcare. | Fentanyl has been associated with an increased risk of post-intubation hypotension (Odds Ratio 1.87, 95% Confidence Interval 1.05-3.34, p = 0.03) 41, a phenomenon also observed with propofol, midazolam, and ketamine 42. This risk might be mitigated if ketamine is the chosen induction agent 43. |
| Dexmedetomidine 0.5 μg/kg Available in 100 μg/ml, vials of 2, 4, and 10 ml. | To attenuate the hemodynamic response to laryngoscopy and intubation with efficacy similar to that of fentanyl 44. | Variable availability across different levels of healthcare. |
| Reduction of Adverse Drug Reactions | ||
| Atropine 0.02 mg/kg in children or 0.5 mg/dose in adults. Available in 1 mg/ml, 1 ml vial. | Prevention and treatment of bradycardia associated with intubation in pediatrics. Also associated with reduced secretions. | Its use is controversial. A systematic Cochrane review is currently underway with the aim of identifying and evaluating all clinical trials that compare pediatric endotracheal intubation with and without premedication with atropine 45. |
| Rocuronium with precurarization indication 10% of relaxation dose: 0.03-0.04 mg/kg 46,47. Available in 10 mg/ml, 5 ml vials. | To prevent muscle fasciculations caused by the use of succinylcholine, both visually and through the reporting of myalgias 46,47, as well as the elevation of creatine kinase levels 48. This thereby avoids increased oxygen consumption (reducing safe apnea time) and prevents the rise in intraocular and intragastric pressure. | Although the incidence of fasciculations is similar when comparing rocuronium with vecuronium, the former significantly reduces the intensity of postoperative myalgias, making it the recommended non-depolarizing muscle relaxant 49. Other medications, such as pregabalin at a dose of 300 mg, have been shown to decrease the incidence of fasciculations and myalgias associated with succinylcholine, although further studies are needed to confirm this finding 50. |
| Betablockers Esmolol 1.5 mg/kg followed by 0.1 mg/kg/min. Available in 10 mg/ml, 10 ml vials | To reduce the incidence of tachycardia and control heart rate immediately following tracheal intubation 35. | It may reduce the risk of arrhythmia and myocardial ischemia, although the evidence is not conclusive 51. |
| Preoptimization of Hemodynamic Status | ||
| Goal-directed resuscitation before intubation | To improve postoperative outcomes in patients with intermediate or high risk. It appears to be associated with reduced hospital stay, complications, and even mortality 52. It offers clear benefits in patients with sepsis. | Its routine use for all perioperative patients is not yet widely recommended 52. |
| Crystalloids | To prevent intubation-related hypotension, as it is independently associated with adverse outcomes such as mortality, prolonged hospital stay, and target organ injury. Patients who respond well to and tolerate fluids are indicated to receive fluid resuscitation prior to intubation, or at least during the intubation attempt 53. | Recommendation based on expert consensus. |
| Vasopressors Noradrenaline Push dose or titrated infusion Available in 1 mg/ml, 4 mL vials. | To prevent and treat hypotension associated with induction-intubation. When possible, vasopressor infusions should be initiated prior to intubation in patients who do not respond to volume resuscitation 53. | There is evidence supporting the use of norepinephrine as a first-line vasopressor agent for patients with septic shock 54. Early administration has even been associated with reduced mortality 55. |
Source: own elaboration
The use of combined strategies (preoxygenation, the presence of two operators, RSI, cricoid pressure, capnography, protective ventilation, fluid loading, early preparation and administration of sedation, and the use of vasopressors) has been shown to decrease life-threatening events (21% vs. 34%, p = 0.03) and other complications (9% vs. 21%, p = 0.01) 56.
Paralysis and Induction
Neuromuscular Relaxant
Neuromuscular relaxants (NMR) are a critical pillar for the success of intubation. They have been shown to increase the incidence of first-attempt success in critically ill patients (OR 2.37, 95% CI 1.36-4.88). Similar results have been found in the subgroup of patients intubated with videolaryngoscopy (OR 2.50, 95% CI 1.43-4.37, p<0.001) 57. Avoiding the use of NMR significantly increases difficult laryngoscopy (RR 13.27, 95% CI 8.19-21.49; p<0.00001) and airway injuries (RR 1.37, 95% CI 1.09-1.74; p=0.008) and increases the risk of tracheostomy among emergency patients (OR 2.59, 95% CI 1.06-6.34; p=0.04) 59.
Two medications are supported by evidence for use in the RSI scenario: succinylcholine, a depolarizing NMR used at doses of 1-2 mg/kg with an onset of action of 30-60 seconds and an approximate duration of 10 minutes; and rocuronium, a non-depolarizing NMR used at a dose of 1.2 mg/kg with a similar onset and a prolonged duration of about 160 minutes. In a Cochrane meta-analysis, succinylcholine was found to generally provide better intubation conditions than rocuronium (RR 0.86, 95% CI 0.80-0.92; n=2690), but when compared with a 1.2 mg/kg dose of rocuronium, no statistical difference was found in intubation conditions. Succinylcholine was considered clinically superior due to its shorter duration of action 60.
It is crucial to consider the contraindications and adverse effects of both drugs. Succinylcholine is contraindicated in patients at risk of hyperkalemia, prolonged bed rest, rhabdomyolysis, burns after 24-48 hours, muscular dystrophy, or a family history of malignant hyperthermia 61. While rocuronium does not have these contraindications and appears to have a safer clinical profile 62, it is one of the drugs most commonly associated with anaphylaxis in anesthesia 63.
Induction Agent
The choice of the induction agent is a point of great contention in the literature; however, safety during laryngoscopy is ensured with adequate preparation in the preceding steps. As previously mentioned, full doses are indicated to ensure appropriate latency times.
The ideal induction agent should have a rapid onset of action, maintain optimal hemodynamics, and avoid secondary damage due to adverse effects 64. All these factors, in addition to the availability and experience of the operator, must be considered when choosing the induction agent (see Table 2).
Table 2: Choice of Induction Agents for Rapid Sequence Intubation (RSI)
| Induction Agent | Pharmacology 65 | Indications and advantages | Contraindications and observations |
| Ketamine | Mechanism of Action: NMDA antagonist Dosage: 1.5-2 mg/kg Presentation: 50 mg/ml in 10 ml vial Onset: <60 seconds Duration: 10-20 minutes | Stable hemodynamic profile, as it induces the release of catecholamines, which increases or maintains blood pressure. It has a potent analgesic effect, potentially obviating the need for associated fentanyl use 43. Suitable for use in patients with polytrauma and traumatic brain injury (TBI), as it elevates intracranial pressure (ICP) and mean arterial pressure (MAP), while maintaining cerebral perfusion pressure (CPP) 66. It induces bronchodilation, making it applicable for use in asthma patients. | May induce hypotension in patients with catecholamine depletion due to prolonged shock 67. Contraindicated at certain dosages in placental abruption. Should be used with caution in hypertensive crises, tachyarrhythmias, and coronary artery disease 65. |
| Propofol | Mechanism of Action: Positive allosteric modulator of GABAA receptors Dosage: 1-3 mg/kg Formulation: 10 mg/ml, available in 10, 20, and 50 ml vials Onset: 15-45 seconds Duration: 3-5 minutes | The required dose for achieving adequate anesthetic depth may vary based on the patient's clinical status. It has bronchodilatory, neuroprotective, and anticonvulsant effects, making it indicated for asthma, traumatic brain injury (TBI), and status epilepticus. | It has a vasodilatory effect that leads to a higher incidence of post-intubation hypotension 68. Additionally, it can cause myocardial depression. Caution is advised in cases of hemodynamic instability. |
| Etomidate | Mechanism of Action: Positive allosteric modulator of GABA_A receptors Dosage: 0.3-0.6 mg/kg Formulation: 2 mg/ml, available in 10 ml vials Onset: 15-45 seconds Duration: 3-12 minutes | This induction agent has a lower incidence of post-intubation hypotension compared to ketamine 69. It does not produce vasodilation or negative inotropy, making it a potential drug of choice in cases of hemodynamic instability and cardiac diseases. | It can cause long-term adrenal suppression, and its use in a single dose is controversial in septic patients 70. Compared to ketamine, it may increase 7-day mortality in critically ill patients 71. It does not possess analgesic properties. |
| Thiopental | Mechanism of Action: Positive allosteric modulator of GABA_A receptors Dosage: 3-5 mg/kg Formulation: Vial for reconstitution containing 0.5-1 g Onset: 5-30 seconds Duration: 5-10 minutes | Thiopental was the induction agent of choice when the technique was first described. It has neuroprotective effects. | It has an unfavorable hemodynamic profile, including the risk of hypotension, negative inotropy 72, and histamine release. It is contraindicated in patients with porphyria and should be used cautiously in patients with asthma and local necrosis. |
| Midazolam | Mechanism of Action: Benzodiazepine, Positive allosteric modulator of GABA_A receptors Dosage: 0.1-0.3 mg/kg Formulation: 1 mg/ml (5 ml ampoules), 5 mg/ml (3 ml ampoules) Onset: 30-90 seconds Duration: 15-45 minutes | Midazolam has amnestic, anticonvulsant, and induction-sparing properties. It is indicated when other induction agents are not available. | It does not perform well as a standalone induction agent. It is commonly associated with hypotension and cardiorespiratory depression 65. |
*NMDA: N-Methyl-D-Aspartate Glutamate Receptor
†TBI: Traumatic Brain Injury
‡ICP: Intracranial Pressure
§MAP: Mean Arterial Pressure
||CPP: Cerebral Perfusion Pressure
GABAA: Gamma-Aminobutyric Acid A Receptor
Source: Authors' own compilation
The potential of using a combination of lower doses of different induction agents to potentiate their effects and minimize adverse events exists. For instance, the combination of ketamine and propofol, known as “ketofol,” has been used. However, its utility has not shown a significant difference in post-intubation hemodynamic stability when compared to etomidate in critically ill patients 73.
Tube Positioning
After achieving the appropriate anesthetic depth and paralysis, the next step is to position the tube through the glottis. There are multiple strategies available for this phase 9,74,75, and it is crucial to optimize the first attempt as much as possible. Studies suggest that the risk of an adverse event during emergency tracheal intubation increases significantly with the number of attempts (with an incidence of 14.2%, 47.2%, 63.6%, and 70.6% for one, two, three, and four attempts, respectively; adjusted OR for 2 or more attempts is 7.52, 95% CI 5.86 9.63) 76. It is also essential to have multiple fallback options available, including rescue devices like second-generation laryngeal masks.
The choice between videolaryngoscopy or direct laryngoscopy as a first-line option is a point of contention. Several factors, including the operator’s familiarity with each choice, influence this decision. Recent meta-analyses from the Cochrane collaboration suggest that first-line videolaryngoscopy decreases the likelihood of failed intubation (RR 0.41, 95% CI 0.26 to 0.65) and hypoxia (RR 0.72, 95% CI 0.52 to 0.99). Moreover, hyperangulated videolaryngoscopy blades significantly decrease failed intubation in anticipated difficult airway scenarios (RR 0.29, 95% CI 0.17 to 0.48) 77.
Recent studies have assessed the use of flexible stylets and bougies, concluding that both improve the percentage of first-attempt intubations in emergency and critical care scenarios and should be considered for routine use 78-80.
Several strategies exist for confirming the correct positioning of the endotracheal tube. The ASA’s Difficult Airway Algorithm recommends the use of capnography and, in cases of uncertainty, resorting to other options like flexible bronchoscopy, ultrasound, or X-ray 9,81.
Post-intubation
After the placement and confirmation of the endotracheal tube, taking appropriate measures to ensure the patient’s stability is advised. This includes proper tube fixation, the use of sedoanalgesia, continuation of hemodynamic resuscitation, and safe ventilation practices.
Securing the endotracheal tube is essential to avoid accidental extubation, a catastrophic complication for patients requiring secure airway management. Fixation can be achieved through commercial devices or adhesive tapes. Regardless of the method used, two vectors of force should be ensured for added safety 82 (see Figure 3).
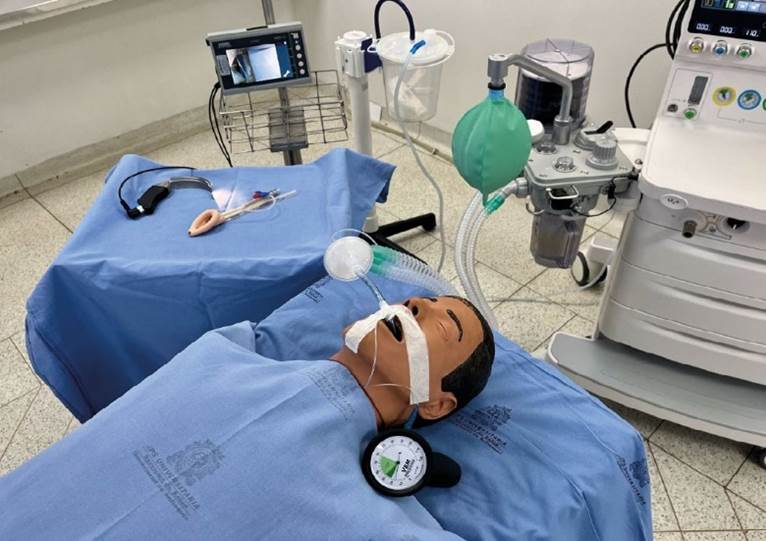
Source: own elaboration
Figure 3 Endotracheal tube fixation with double vector. Photograph taken at Clínica León XIII
Infusion or intermittent administration of sedoanalgesia is often necessary, as intubation is an uncomfortable and painful procedure that can induce anxiety and agitation in the patient 83. It is advisable to use the minimum effective dose to control symptoms. A protocol emphasizing analgesia can reduce the overuse of hypnotic agents 84. Early administration is crucial to avoid conscious paralysis, an event that can lead to post-traumatic stress 85-86.
The transition from physiological to positive pressure ventilation has significant hemodynamic implications due to decreased venous return. Moreover, the effects of the drugs used can either potentiate or worsen the hemodynamic status. Up to 24% of patients experience post-intubation hypotension, which is associated with increased mortality, and up to 3% experience cardiac arrest 12. Thus, it is vital to closely monitor and continue goal-directed resuscitation measures if needed to avoid hypoperfusion.
During transport, it is recommended to use a mechanical ventilator according to availability. Ideally, protective ventilation parameters should be used, which are associated with lower morbidity and mortality, even in patients without adult respiratory distress syndrome (ARDS) 87. Early initiation of these strategies is associated with better adherence later on 88. This strategy includes using a tidal volume of 6-8 ml/kg of ideal body weight and routine use of positive end-expiratory pressure (PEEP) between 5 and 8 cmH2O, with adjustments to the respiratory rate and FiO2 based on oxygen saturation and CO2 arterial pressure.
Conclusions
Recent advances in Rapid Sequence Induction and Intubation (RSII) highlight the importance of preparation, ramp positioning in special patients, routine preoxygenation coupled with apneic oxygenation, preoptimization before tracheal intubation, neuromuscular relaxation as a treatment cornerstone, rescue techniques for failed intubation, and post-intubation care.
It is imperative for physicians in emergency services, critical care, and anesthesiology to master each of these key points in proper RSII to reduce morbidity and mortality.











 text in
text in