Introduction
Rabeprazole (RPZ), a gastric proton pump inhibitor (PPI), inhibits H+/K+-ATPase activity in gastric parietal cells. Thereby reducing the overall acid secretion and providing an anti-secretory effect 1. It is used in peptic ulcers and gastro-esophageal reflux disease (GERD) as it can inhibit the overproduction of stomach acid 2. RPZ is also used in multiple endocrine adenomas and systemic mastocytosis 3, associated with other anti-Helicobacter pylori medications 4 (BNF, 2018) to eradicate the bacterium and treat hypersecretory conditions 2,3.
The US Food and Drug Administration (FDA) approved RPZ 2, although it has some common side effects, including constipation, feeling weak, and throat inflammation. Moreover, it can cause osteoporosis, low serum magnesium, Clostridium difficile infection, and pneumonia 2. On the other hand, its use during pregnancy and lactation is controversial 5. Drug toxicity refers to the harmful effects that a drug can have on the body 6. The median lethal dose (LD50) value of RPZ in rats is 2.4215 mol/kg 7. It seems RPZ is well tolerated in animals. Generally, potent toxic drugs are not approved for use on humans or animals. Thus, analysis of the safety profile of a particular drug is crucial. A study reported that RPZ (0.1 and 0.2 mM) has cytotoxic effects on several human gastric cancer cell lines 8. This suggests that RPZ has a cytotoxic profile. Drugs with cytotoxicity may cause health hazards. This is because these drugs are irritating and can also produce local harmful effects on specific organs (e.g., skin and eyes) 9.
Due to their health effects, as they contain a specific aroma and many important bioactive chemical compounds, including sulfur compounds and flavonoids 10, many species of the genus Allium are commonly used as foods and traditional medicines. Allium cepa is a vegetable that is the most widely cultivated species in the genus Allium. A. cepa has been extensively used to evaluate the toxicogenetic effects (e.g., toxicity, cytotoxicity, genotoxicity, and mutagenicity) of a wide variety of substances. It is more sensitive than others because the onion roots are sensitive to many toxic molecules. Thus, it plays an important role in biomonitoring 11. The A. cepa test model is rapid and precise, allows the assessment of several endpoints (e.g., chromosome aberrations, micronuclei formation, mitotic index), and helps us to evaluate the toxic effects of various substances for environmental monitoring 12,13.
Plants and their seeds, when exposed to a high concentration of toxic substances in soil or culture media, exhibit toxicity symptoms such as a decrease in height 14, inhibition of seed germination, decrease in tillering, reduction in root or shoot growth, decrease in fruit and grain yield 15,16 and even death 17. Cicer arietinum seeds, widely cultivated in Bengal, are a major food source in the region. The in vitro toxic effects of chemical substances can be studied by knowing the germination and seedling profiles of C. arietnum18.
Considering the facts mentioned above, the current study aimed to evaluate the toxic effects of rabeprazole sodium on A. cepa, A. sativum, and C. arietinum.
Methods
Collection of test systems
Medium-sized onions (A. cepa), garlic (A. sativum), and fresh brown C. arietinum were purchased from the local market in Gopalganj district, Bangladesh, and subjected to toxicity analysis.
Reagents and chemicals
Rabeprazole sodium (RPZ-Na) was obtained from Aristopharma Ltd., Bangladesh, while CuSO4 was purchased from Merck, India.
Preparation of test concentrations
Test concentrations were selected according to previous studies. Gu et al. 8 performed an in vitro cytotoxicity analysis of RPZ on several human gastric cancer cells at 0.1 and 0.2 nM. Yaşar et al. 19 carried out a study on human pylorus muscle cells at 0.001 to 1 mM RPZ. Therefore, this study evaluated the toxic effects of RPZ-Na on A. cepa, A. sativum, and C. arietinum test systems at five concentrations: 0.025, 0.05, 0.1, 0.2, and 0.4 mM. RPZ-Na was dissolved in distilled water.
Toxic effects on root growth profile
A. cepa test
The outer layer(s) and the budding parenchyma of the central crown were carefully removed to promote the root growth of A. cepa. A small, circular incision was also made. Then, the bulbs were rinsed with tap water for 30 minutes. The root portion of each onion was soaked in distilled water in a plastic container (capacity: 15-20 mL) for the first 24 h at 25 ± 1 °C in the dark. A. cepa samples (five for each concentration) with fair root growth were reassigned to the NC (negative control, distilled water) and test samples containing the concentrations mentioned above of RPZ-Na for a further 72 h. After every 24 hours, the number and length of the 10 longest roots were counted for each bulb. The root length was measured. The toxicity of the RPZ-Na was determined by evaluating the root growth inhibition profile of the test sample or PC (positive control treated with 38 µM CuSO4) during inspection of the NC group 20. The half maximal inhibitory concentration (IC50) was also determined for the RPZ-Na at each exposure time by non-linear regression analysis using Graph Pad Prism software, as mentioned below.
A. sativum test
The outer cloves of A. sativum were collected. Both the inner and outer peels of each clove were carefully removed. Similarly, the budding parenchyma from the central crown of each clove was carefully removed by making a small spheroid incision to facilitate root growth. The cloves were washed with tap water for 5 min, and the root portion was soaked in the test sample or control plastic containers (capacity: 15-20 mL) up to 72 h at 28 ± 1 °C in the dark. After every 24 hours, the number and length of the 10 longest roots were counted and measured for each bulb. The root length was calculated similarly to the A. cepa plant test and measured to determine the toxic effects of the RPZ-Na and controls. Distilled water and 38 µM CuSO4 were used as NC and PC groups, respectively. The IC50 values were calculated as mentioned above.
Toxic effects on germination profile
C. arietinum test
The chickpea seeds (60 days old) were rinsed three times and soaked for 10 minutes in distilled water. Seed germination was tested on moist sanitary napkin tissue papers. For this purpose, two-layered seedling beds were prepared with small pieces of tissue paper on clean plastic cups (capacity: 15-20 mL). The first bed was wetted with the test sample (RPZ-Na at different concentrations, the same as the A. cepa test). Then, the seeds (five for each concentration or sample) were placed on the respective beds, maintaining optimal distance between them. Similarly, the second bed was prepared with small pieces of tissue paper and placed in the distributed seeds in each treatment. The cups were covered with plastic covers and kept in a 12-hour dark-light cycle for 72 hours at 28 ± 1 °C. Each treatment cup containing the bed was wetted with the respective sample or controls every 24 hours. Distilled water and CuSO4 (50 µM) were used as NC and PC, respectively. The shoot and root lengths were measured according to Bhattacharya et al. 18. The IC50 values were calculated as mentioned above.
Results
A. cepa test
Table 1 indicates that RPZ-Na concentration-dependently inhibited root growth (RG) in A. cepa. RPZ-Na at 0.4 mM exerted the highest RG inhibition at 72 h. Compared to 24 h, exposure time 72 h showed a time-dependent toxic effect on A. cepa roots. The standard CuSO4 exhibited higher toxicity in the test system than the RPZ-Na. RPZ-Na at 0.025 mM showed a negligible toxic effect on the test system compared to the other test concentrations of RPZ-Na. The IC50 values calculated at 24, 48, and 72 h were 0.20 ± 0.06, 0.22 ± 0.06, and 0.20 ± 0.08 mM, respectively.
Table 1 Toxic effects of rabeprazole sodium and controls on Allium cepa root meristems at different exposure time
| Treatments/ at different exposure time | Root length (mm) | Inhibition of root growth (%) | IC50 [CI; R2] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | ||||
| NC | 53.33 ± 2.91 | 70.33 ± 2.73 | 88.33 ± 2.19 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | - | - | - | |||
| PC | 08.02 ± 1.97* | 16.38 ± 2.17* | 20.39 ± 2.17* | 88.71 ± 1.97 | 76.71 ± 2.17 | 76.92 ± 2.17 | - | - | - | |||
| RPZ-Na (mM) | 0.025 | 52.51 ± 3.39 | 68.28 ± 2.08 | 87.33 ± 3.58 | 01.54 ± 3.39 | 01.13 ± 2.08 | 01.36 ± 3.58 | 0.20 ± 0.06 mM [0.14 - 0.31 mM; 0.96] | 0.22 ± 0.06 mM [0.15 - 0.33 mM; 0.96] | 0.20 ± 0.08 mM [0.13 - 0.31 mM; 0.95] | ||
| 0.05 | 46.23 ± 2.54* | 60.67 ± 3.24* | 75.56 ± 2.93* | 13.31 ± 2.54 | 13.88 ± 3.24 | 14.46 ± 2.93 | ||||||
| 0.1 | 36.13 ± 2.93* | 50.36 ± 2.13* | 57.33 ± 2.78* | 32.25 ± 2.93 | 28.39 ± 2.13 | 35.10 ± 2.78 | ||||||
| 0.2 | 28.67 ± 2.33* | 42.33 ± 3.08* | 51.12 ± 3.28* | 46.24 ± 2.33 | 39.81 ± 3.08 | 42.13 ± 3.28 | ||||||
| 0.4 | 19.33 ± 2.85* | 24.67 ± 2.67* | 28.67 ± 2.96* | 63.75 ± 2.85 | 64.92 ± 2.67 | 67.54 ± 2.96 | ||||||
Values are mean ± standard error of the mean (SEM) (n = 5); ANOVA followed by Tukey post hoc test, considering p< 0.05 with a confidence level of 95%; NC: negative control; PC: positive control (CuSO4; 38 µM); RPZ-Na: rabeprazole sodium; IC50: half maximal inhibitory concentration; CI: confidence of interval; R2: coefficient of determination at 95% confidence intervals.
Figure 1 shows the percentage increase in RG profile in the samples and control groups at 48 and 72 h compared to 24 h of exposure time. Both samples and controls did not increase in RG capacity at 72 h inspection of 48 h of exposure time; therefore, this was not shown in the figure. RPZ-Na increased the RG profile at 48 h at 0.025, 0.1, and 0.2 mM, while at 72 h at 0.025 and 0.2 mM. However, compared to 48 h, there was a decrease in the RG profile with these two concentrations of RPZ-Na. The standard CuSO4 also decreased the RG profile at 72 h more than at 48 h of exposure time. RPZ-Na at 0.05 mM and the highest test concentration, 0.4 mM, did not increase RG at 48 and 72 h compared to 24 h as well as 72 h compared to 48 h of exposure time.
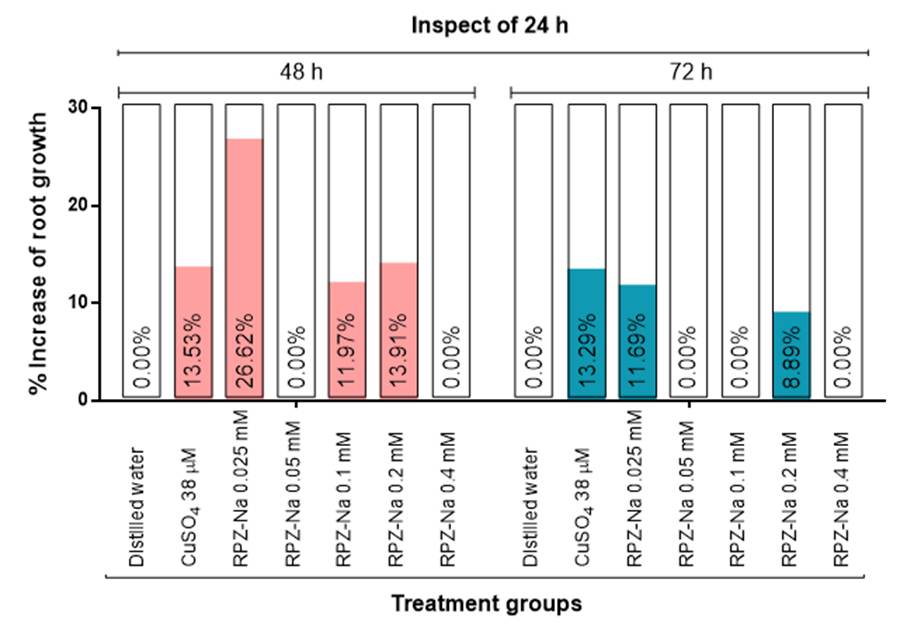
Figure 1 Adaption towards the toxic effects of the test sample and controls on Allium cepa compared to 24 h of exposure time [Values are percentage decrease in comparison to the NC (distilled water) group in toxic response in the same treatment group, compared to 24 h of exposure time. Negative values were omitted in the graph. NC: distilled water; PC: positive control (CuSO4)]
A. sativum test
Table 2 shows that RPZ-Na concentration-dependently inhibited root growth (RG) of A. sativum. RPZ-Na at 0.4 mM exerted the highest RG inhibition at 24 h. However, at 72 h, it also showed almost similar (64.29 ± 4.12%) inhibition of the RG profile. The standard CuSO4 exerted a more toxic effect on the test system than the RPZ-Na. RPZ-Na at 0.025 mM showed a negligible toxic effect on the test system compared to the other test concentrations of RPZ-Na. RPZ-Na at all concentrations decreased the %inhibition of RG at 48 h compared to 24 h of exposure time, which was then increased at 72 h compared to 48 h of exposure time. Both the standard and RPZ-Na inhibited the RG profile in a time-dependent manner from 24 to 72 and 48 to 72 h exposure time, respectively. The IC50 values calculated at 24, 48, and 72 h were 0.21 ± 0.06, 0.36 ± 0.08, and 0.21 ± 0.07 mM, respectively.
Table 2 Toxic effects of rabeprazole sodium and controls on Allium sativum root meristems at different exposure time
| Treatments | Root length (cm) | %Inhibition of root growth | IC50 [CI; R2] | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | ||||||
| NC | 14.20 ± 4.73 | 44.80 ± 2.78 | 84.00 ± 4.08 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | - | - | - | |||||
| PC | 3.78 ± 1.08* | 16.20 ± 2.22* | 26.43 ± 2.78* | 73.38 ± 1.08* | 63.84 ± 2.22* | 68.54 ± 2.78* | - | - | - | |||||
| RPZ-Na (mM) | 0.025 | 13.43 ± 3.76 | 43.20 ± 3.07 | 80.20 ± 2.93 | 05.42 ± 3.76* | 03.57 ± 3.07* | 04.52 ± 2.93* | 0.21 ± 0.06 mM [0.13 - 0.28; 0.96] | 0.36 ± 0.08 mM [0.17 - 0.38; 0.91] | 0.21 ± 0.07 mM [0.12 - 0.29; 0.95] | ||||
| 0.05 | 11.97 ± 1.73* | 36.40 ± 2.58* | 67.60 ± 4.13* | 18.75 ± 2.58* | 15.70 ± 1.73* | 19.52 ± 4.13* | ||||||||
| 0.1 | 9.87 ± 2.96* | 35.00 ± 1.83* | 55.00 ± 3.19* | 30.49 ± 2.96* | 26.88 ± 1.83* | 34.52 ± 3.19* | ||||||||
| 0.2 | 7.20 ± 2.58* | 32.40 ± 4.02* | 43.20 ± 2.93* | 49.30 ± 2.58* | 41.68 ± 4.02* | 48.57 ± 2.93* | ||||||||
| 0.4 | 4.98 ± 2.79* | 19.20 ± 3.93* | 30.00 ± 4.12* | 64.93 ± 2.79* | 57.14 ± 3.93* | 64.29 ± 4.12* | ||||||||
Values are mean ± standard error of the mean (SEM) (n = 5); ANOVA followed by Tukey post hoc test, considering p< 0.05 with a confidence level of 95%; *p< 0.05 when compared to the NC (negative control) group; PC: positive control (CuSO4; 38 µM); RPZ-Na: rabeprazole sodium; IC50: half maximal inhibitory concentration; CI: confidence of interval; R2: coefficient of determination at 95% confidence intervals.
The percentage increase in RG profile in the test sample and control groups at 48 and 72 h inspection of 24 h exposure time in the A. sativum test system (Figure 2). RPZ-Na increased the RG profile at 48 h at all the test concentrations, while at 72 h it was 0.025, 0.2, and 0.4 mM. RPZ-Na showed the highest increase in RG at 0.025 mM (34.13%). However, compared to the 48 h, there is a decrease in the RG profile at these three concentrations of RPZ-Na. The standard CuSO4 also decreased the RG profile more at 72 h than at 48 h compared to 24 h of exposure time. RPZ-Na at 0.05 and 0.1 mM did not increase the RG profile at 72 h compared to 24 and 48 h of exposure time. The sample and controls did not increase RG capacity at 72 h compared to 48 h of exposure time. Therefore, this was not shown in the figure.
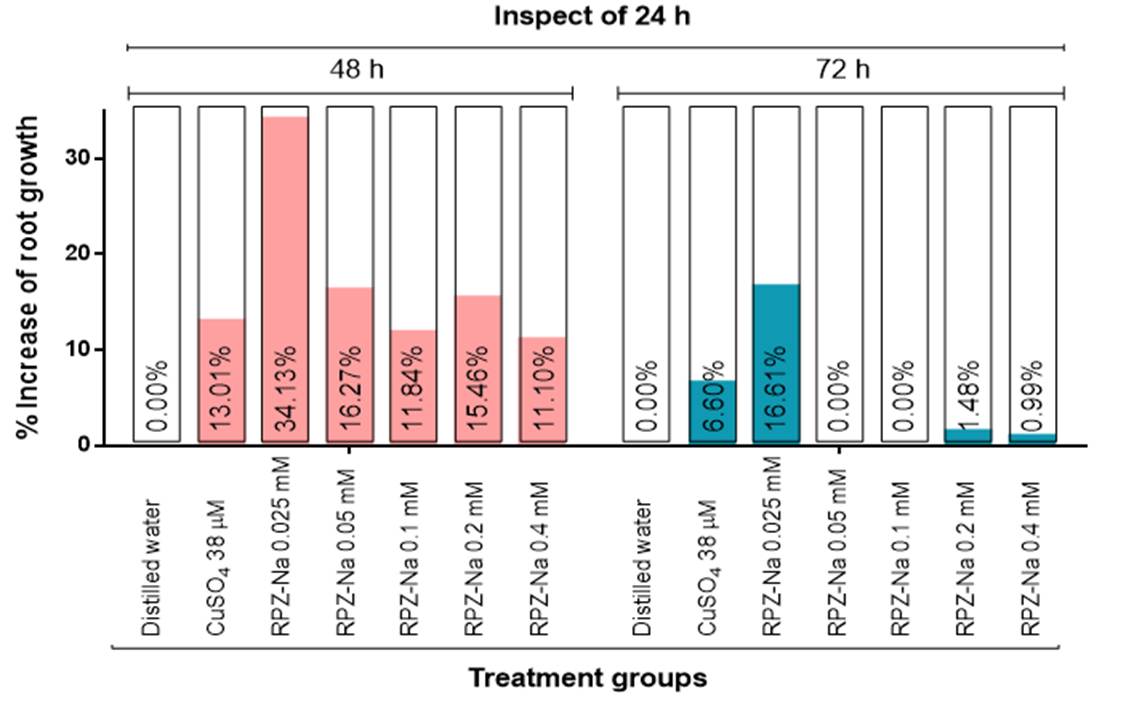
Figure 2 Adaption towards the toxic effects of the test sample and controls on Allium sativum compared to 24 h of exposure time [Values are percentage decrease in toxic response in the same group of treatment, compared to 24 h of exposure time. Negative values are omitted in the graph. NC: distilled water; PC: positive control (CuSO4)]
C. arietinum test
Both CuSO4 and RPZ-Na decreased the shoot and root lengths in C. arietinum. However, their effects were more prominent on the root growth than the shoot growth profile. RPZ-Na significantly (p< 0.05) concentration-dependently decreased the shoot and root lengths in C. arietinum. At 0.4 mM, RPZ-Na showed the highest inhibition of shoot (57.10 ± 0.58) and root (62.25 ± 0.88) lengths. The standard CuSO4 decreased the shoot and root lengths by 73.99 ± 3.08 and 82.73 ± 2.19%, respectively. The IC50 values calculated for the %inhibition of the shoot and root lengths were 0.36 ± 0.12 and 0.13 ± 0.11 mM, respectively (Table 3).
Table 3 Toxic effects on the germination profile of Cicer arietinum seedlings by the rabeprazole sodium and controls at 72 h
| Treatments | SL (mm) | %ISL | RL(mm) | %IRL | IC50 [CI; R2] | ||||
|---|---|---|---|---|---|---|---|---|---|
| ISL | IRL | ||||||||
| NC | 33.33 ± 4.26 | 0.00 ± 0.00 | 83.00 ± 3.22 | 0.00 ± 0.00 | - | - | |||
| PC (standard) | 8.67 ± 3.08 | 73.99 ± 3.08 | 14.33 ± 2.19 | 82.73 ± 2.19 | - | - | |||
| RPZ-Na (mM) | 0.025 | 28.00 ± 1.53* | 15.10 ± 1.53 | 66.00 ± 4.58* | 20.48 ± 4.58 | 0.36 ± 0.12 mM [0.07 - 0.39; 0.85] | 0.13 ± 0.11 mM [0.06 - 0.26; 0.87] | ||
| 0.05 | 21.67 ± 2.85* | 34.98 ± 2.85 | 52.67 ± 1.23* | 36.54 ± 1.23 | |||||
| 0.1 | 19.33 ± 0.88* | 42.00 ± 0.88 | 46.54 ± 2.03* | 43.93 ± 2.03 | |||||
| 0.2 | 17.33 ± 0.88* | 48.01 ± 0.88 | 39.67 ± 3.48 * | 52.20 ± 3.48 | |||||
| 0.4 | 14.00 ± 0.58* | 57.10 ± 0.58 | 31.33 ± 0.88* | 62.25 ± 0.88 | |||||
Values are mean ± standard error of the mean (SEM) (n = 5); ANOVA followed by Tukey post hoc test, considering p< 0.05 with a confidence level of 95%; NC: negative control; PC: positive control (CuSO4; 50 µM); RPZ-Na: rabeprazole sodium; SL: shoot length; RL: root length; ISL: inhibition of shoot length; IRL: inhibition of root length; IC50: half maximal inhibitory concentration; CI: confidence of interval; R2: coefficient of determination at 95% confidence intervals.
Discussion
Exploration of drugs or chemicals toxicity towards living organisms is an important step before the industrial and commercial phases of any new drugs and/or chemicals. In vivo models consisting of experimental animals and/or their derived cells and tissues are expensive and time-consuming for toxicity assessment. Furthermore, ethical considerations about the handling and sacrifice of animals are other important issues.
Besides A. cepa, other species such as V. faba, T. paludosa, P. sativum, H. vulgare, and C. capillaris are also used for toxicological analyses. However, the A. cepa test is popularly used to determine toxicity in the laboratory due to its storage facility and availability 20. It is a rapid, precise, and cost-effective plant-based eukaryotic test model. It is well correlated with the higher eukaryotic test models 13. A. sativum might also be an economical test model, as it requires only garlic cloves for this assay. Each bulb of garlic produces multiple cloves. Both of these test systems do not require aseptic techniques. Therefore, these assays may complement the 3-(4,5-dimethylthiazol-2- yl)-2,5-diphenyltetrazolium bromide (MTT) assay in toxicology. Thus, the findings from this kind of toxicological study can be applied to animal-based toxicity studies 20. For example, the popularly used PPI omeprazole (OME) was seen to exert concentration-dependent (10, 20, and 40 µg/mL) toxic effects on the A. cepa test system 21. In another study, Braga et al. 22 treated Swiss mice with 10, 20, and 40 mg/kg for 14 days and found that OME dose-dependently exerted toxic effects on the stomach, bone marrow, and peripheral blood lymphocytes. Similarly, a clinical study reports that 152 patients using OME at 20, 30, and 40 mg/kg doses for a long time experienced serious toxic effects on stomach cells 23. The root length is an important parameter in A. cepa and A. sativum, while the shoot length is in the C. arietinum test system. These reflect the toxicity of any toxic substance capable of serving as a receptive external signal for steady internal cellular events 18,21. The toxic substances can accumulate in the roots, resulting in chromosomal aberrations (e.g., C-mitosis, chromosomal bridges, chromosomal tack, and micronuclei formation), inhibiting root growth in A. cepa24. The accumulation of toxicants in the root meristems substantially impairs the microtubule arrangements, thus inhibiting the root growth profiles of A. cepa and A. sativum. Both toxic and cytotoxic effects of a toxic substance are related to the elongation of the cell cycle in the differentiation phase 25, apical meristematic activity 26, and inhibition of protein synthesis in A. cepa meristems 27.
Copper (Cu) accumulates in root test systems (e.g., A. cepa, A. sativum) and can inhibit the root growth profile because of chromosomal aberrations (e.g., C-mitosis, the chromosomal bridges, the chromosomal tack, and the micronuclei formation) 24. Generally, Cu accumulated at the root meristems impairs the microtubule arrangements in these test systems. Therefore, the toxic effects on A. cepa and A. sativum may be due to an inhibition in root growth, possibly through elongation of the cell cycle during cell differentiation 25, apical meristematic activity (Webster and Macleod 1996), and inhibition of cellular protein synthesis 27.
Cu accumulated more easily at the root rather than at the shoot in Oenothera glazioviana. While CuSO4 at 50 μM in a 72-hour exposure time inhibited shoot and root growth with a considerable increase in lipid peroxidation level in the roots of 28-day-old seedlings 28. In our study, CuSO4 at 50 μM also significantly (p< 0.05) reduced the shoot length of C. arietinum seedlings compared to the RPZ-Na and NC groups. However, PC and RPZ-Na significantly reduced the root length of C. arietinum more than the shoot length.
The standard CuSO4 and the test sample RPZ-Na were found to increase the RG profile in A. cepa and A. sativum test systems at 48 and 72 h compared to 24 h of exposure time. However, this effect of the standard and test samples was not seen at the 72-hour inspection after 48 hours of exposure time. At 72 h, the % increase in RG profile was reduced compared to the 48-h inspection of the 24-h exposure time. It may be due to their damage-preventive capacity, probably by distressing the adaptive response pathways and/or cellular damage-repairing capacity in these test systems. A. cepa shows an adaptive response at low concentrations in the Al+3-induced genotoxicity assay 29. This may be due to its genomic protection capacity at low concentrations, regardless of exposure time 30. Therefore, in this study, the test systems A. cepa and A. sativum may show such adaptive responses at 48 and 72 h compared to 24 h of exposure time. However, at 72 hours compared to 48 hours of exposure time, the damaging events in these test systems may continue.
Finally, the test systems used in this study showed similar responses toward the standard and test samples, especially when compared to the effects on their RG profiles. The coefficient of determination (R2) values ranged from 0.85 to 0.96 at 95% confidence intervals, suggesting the toxicity study in these eukaryotic test systems is significant. The test sample and the standard used in this study inhibited the root length of the cloves of A. sativum and C. arietinum seedlings. These two test systems showed similar responses to the widely used test model, A. cepa. Therefore, A. sativum and C. arietinum test models can be incorporated into the toxicogenetic analysis of various substances in different areas of toxicological research.
Conclusions
RPZ-Na reduced the average root length on the plant-based eukaryotic test models in a concentration- and exposure-time-dependent manner. It may be due to inhibitory effects on the root meristems of A. cepa and A. sativum. Moreover, RPZ-Na also decreased the concentration- and time-dependently inhibited root and shoot lengths of C. arietinum. Together, RPZ-Na exerted more toxic effects on the test systems at 0.1 to 0.4 mM. These findings highlight the potential risks of RPZ-Na to plant growth and development, particularly at higher concentrations.















