Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Boletín de Investigaciones Marinas y Costeras - INVEMAR
Print version ISSN 0122-9761
Bol. Invest. Mar. Cost. vol.28 no.1 Santa Marta Jan./Dec. 1999
A COMMUNITY ANALYSIS OF THE SOFT BOTTOM MEGAFAUNA (CRUSTACEA, MOLLUSCA) FROM THE SOUTHWESTERN REGION OF SANTA MARTA, COLOMBIAN CARIBBEAN
ANÁLISIS DE COMUNIDAD DE LA MEGAFAUNA (CRUSTACEA; MOLLUSCA) DE FONDOS BLANDOS EN LA REGIÓN SUROCCIDENTAL DE SANTA MARTA, CARIBE COLOMBIANO.
Claudia P. Arango1 and Oscar D. Solano2.
1 Dept. of Zoology and Tropical Ecology, James Cook University, Townsville 4811, Qld Australia. E-mail: claudia.arango@jcu.edu.au (C.P.A.).
2 Instituto de Investigaciones Marinas y Costeras -INVEMAR, A.A 1016 Santa Marta, Colombia. E-mail: odsolano@invemar.org.co. (O.D.S.).
ABSTRACT
Soft bottom megabenthic communities have been poorly studied in the Caribbean Sea. In this study we describe the structure and species composition of a Crustacea-Mollusca megafaunal community based on beam trawl samples taken between 13 and 60 m depth at the southwestern region of Santa Marta, Colombian Caribbean. Classification and ordination analyses using abundance data of crustaceans and molluscs produced two main groups (A and C), which seem to be controlled by depth and sediment characteristics. Group A consisted of species collected at the deeper stations and high content of silts (between 30 and 60 m depth) and exhibited the highest density and biomass mean values. The decapod Chasmocarcinus cilindricus was found as the characteristic species for the group A. The bivalve Laevicardium pictum occurred as characteristic in the shallower cluster C (14 to 17 m) where the sediment was coarser. Trachypenaeus similis, Portunus spinicarpus, Lupella forceps and Penaeus duorarum were generalist species for both groups and were found as the most abundant species overall. There was not a clear evidence of temporal variation of the groups, however an increase in the abundance of dominant species at the shallower area during March may be related to the upwelling phenomenon known for the Santa Marta area.
KEY WORDS: Megafauna, soft bottoms, communities, Caribbean, Colombia.
RESUMEN
Las comunidades megabentónicas de fondos blandos han sido pobremente estudiadas en el mar Caribe. En este estudio describimos la estructura y la composición de especies de una comunidad de la megafauna de Crustacea-Mollusca basados en muestras de red de arrastre tomadas entre 13 y 60 m de profundidad en la región suroeste de Santa Marta, Caribe colombiano. Los análisis de clasificación y ordenación usando datos de abundancia de crustáceos y moluscos produjeron dos grupos principales (A y C) que parecen estar controlados por la profundidad y las características del sedimento. El grupo A consistió de especies colectadas en las estaciones más profundas y con alto contenido de sedimentos muy finos (entre 30 y 60 m) y exhibió los valores medios de densidad y biomasa más altos. El decápodo Chasmocarcinus cilindricus se encontró como la especie característica de este grupo. El bivalvo Laevicardium pictum apareció como característico del grupo más somero C (13 a 17 m) donde el sedimento fué más grueso. Trachypenaeus similis, Portunus spinicarpus, Lupella forceps y Penaeus duorarum fueron especies generalistas en ambas zonas y fueron las más abundantes entre todas. No hubo una evidencia clara de variación temporal de los grupos, sin embargo, un aumento en la abundancia de especies dominantes en el área somera durante marzo podría estar relacionado con el fenómeno de la surgencia conocido para el área de Santa Marta.
PALABRAS CLAVE: Megafauna, fondos blandos, comunidades, Caribe, Colombia.
INTRODUCTION
The marine benthic communities that reside within and on the sedimentary bottoms constitute one of the richest species pools of the oceans, however knowledge on them is scarce and scattered mainly because of the logistic problems and lack of research effort (Snelgrove, 1999). After the 1940’s the importance of the dynamic processes occurring in these ecosystems of the continental shelf became during studies of commercial fisheries of the coastal countries of the North Sea (Gray et al., 1988; Alongi, 1989). Since then, considerable information has been gathered about the patterns of distribution and factors influencing benthic communities from temperate regions (Holme and McIntyre, 1984; Basford et al., 1989; Hostens and Hamerlynck, 1994). In the Caribbean Sea and specifically on the Colombian continental shelf, the soft bottom benthos has been scarcely studied at the community level. A few local investigations have been carried out on the ecological structure of the infauna (García and Sandoval, 1983; García et al., 1992; Guzmán and Díaz, 1993), but epifaunal organisms have only been studied for taxonomy (Acero et al., 1990; Puentes et al., 1990; Blanco, 1993). The present work is part of a broader research project designed to study the macrozoobenthic communities (infauna and epifauna) and physicochemical parameters of the area of Pozos Colorados and Bahia El Rodadero. This work focuses on describing the composition and the structure of an epibenthic megafaunal community of crustaceans and molluscs, the dominant invertebrate groups of the soft-bottom communities in terms of their abundance and species richness in tropical and temperate areas (Acero et al., 1990; Pires, 1992; Long et al., 1995; Gallardo et al., 1996). Our aim is to determine spatial and temporal patterns of the organisms, relating them to some measured environmental factors. In this region this is the first attempt to describe and analyse the distribution of epifaunal organisms from semi-quantitative data taken from standardised trawls. Additionally, the information presented here increases the knowledge of the fauna present at depths below 30m on the Colombian continental shelf. It is expected that the present work could be useful as a descriptive baseline of the megabenthic communities present at one of the most important and rapidly increasing touristic and industrial zones of the Colombian coast.
STUDY AREA
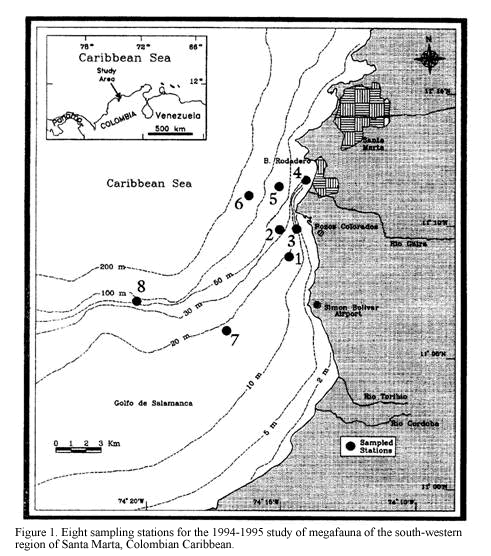
The study area, Pozos Colorados-Bahia El Rodadero (11o04’-11o13’N and 74o13’-74o18’W) is situated in the Gulf of Salamanca, south-west of Santa Marta on the continental shelf of the Colombian Caribbean (Figure 1). The area is characterized by turbid waters presenting an essentially sedimentary-origin bottom of fine and very fine sands with coral rubble patches. The sedimentary conditions are influenced by the cyclic discharges of inshore waters from the Ciénaga Grande of Santa Marta and nearby rivers, mainly from the Magdalena river, Colombia’s largest river (Blanco, 1988). The climate of this region is mainly influenced by the northeasterly trade winds, which determine an upwelling period of colder (mean 25oC, lowest 21oC) saline (mean 35, highest 37) and nutrient-rich waters during the dry season (December-April) when their frequency and intensity are greater. The rainy season (May-November) imposes elevated inputs of continental and estuarine waters, in association with increasing sea water temperature and lowered salinity (Ramírez, 1990; Blanco, 1988).
MATERIALS AND METHODS
Field methods
Megafauna (> 1 cm) sample collection was carried out onboard the R/V “Ancón", property of Invemar, provided with necessary equipment for trawling activities. An Agassiz 2 m beam trawl with 1 cm mesh size in the cod end was modified according to Rogers and Lockwood (1989). The modification consisted of a spiked tickler chain mat with
three chains 2.5 m long. This provides better quantitative data for repeatable estimates of community structure density and biomass by reducing inter-sample variation, compared with unmodified trawls (Kaiser et al., 1994, see details of modification in Rogers and Lockwood, 1989). The sampling cruises were performed in December of 1994 and March, June and September of 1995, trawling at eight stations located between 10 and 60 m depth (Figure 1). A single sample was taken at each station, trawling at 2 knots for approximately 7 minutes. Collected material was washed, sorted, fixed with 4% neutralised formaldehyde and stored in plastic bags for later identification and analysis. Sediment samples were taken at the same time at each station by means of a 0.5 m2 Van Veen grab. The repeated location of the sampling sites was possible by the availability of the GPS FURUNO with an error of 15 m. Temperature and salinity near the bottom were measured at each station employing a CTD sounder of continuous record.
Laboratory methods.
Crustaceans and molluscs were identified to species level or putative taxon. They were counted, damp dried and weighed to the nearest 0.01 g. Biomass was determined as wet weight on formalin for each individual and molluscs were weighed without shells. To determine sediment grain size, sediment samples were wet sieved using 2 - 0.063 mm mesh size sieves according to Wentworth scale (Buchanan, 1984; McManus, 1988). Organic matter content (% OM) was measured by ignition at 500oC. Other sediment variables such as organic carbon (%OC), organic nitrogen (%ON) and organic phosphorus (ug/g OP), were determined and the employed methods are described elsewhere (Invemar, 1997).
Data analysis.
Abundance data was standardised according to the trawled area as number of individuals/ha (although the efficiency of the trawl is unknown) and then transformed with log (x+1). Rare species were eliminated by using only those with an abundance higher than 3% of the total abundance in at least one of the sampling stations (Field et al., 1982). Data analyses were performed using the Bray-Curtis similarity index and UPGMA for clustering and Non-metrical Multidimensional Scaling (NMDS) ordination routines in order to identify spatial and temporal patterns of epifaunal abundance. Environmental data were correlated with the biotic matrix using the Spearman correlation coefficient as proposed by Clarke and Ainsworth (1993). Based on relative abundances an inverse analysis was performed following the Kaandorp technique to identify exclusive, characteristic and generalist species of the groupings (Kaandorp, 1986). Each sample is named by the first letter of the correspondent month followed by the number of the station (e.g. D1: sample from December, station 1 and so on; M= March; J= June and S= September).
RESULTS
Physical and environmental factors
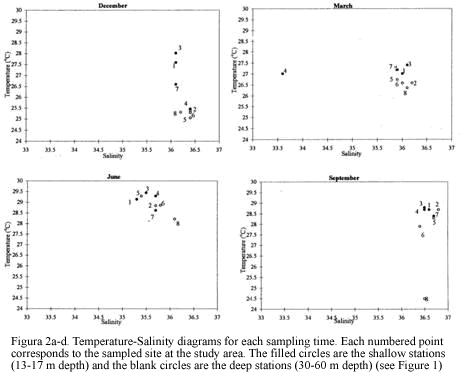
Eight stations were trawled ranging in depth from 13 to 60 m. The sampled area per station ranged between 936 m2 and 1429 m2. There were variations throughout the year for the bottom salinity values, fluctuating between 30.1 in September to 36.4 in December (Figure 2). Temperature near the bottom at 13-30 m depth was higher than for the deeper sites for all sampling periods. The high salinity measured in December samples and the low temperatures in March (Figure 2) support the notion of an upwelling influence during the dry season known to occur during the period from December to March (Blanco, 1988).
Sediments
Percentage values of grain types and phi values (f) at each station are shown in (Tabla 1). According to the Wentworth scale, silts and fine sands are dominant in the area, but stations 1, 2 and 7 presented higher contents of coarser grain > of 2mm, corresponding to more calcareous material and rubble at these sites. Stations 5 and 6 located at El Rodadero showed higher contents of silt and the highest values of (%OM) and (%OC), the rest of the stations did not present well-defined patterns during the study (Tabla 1). %OM, %OC, %ON and ug/g OP, showed slight increases in March. These data are detailed elsewhere (Invemar, 1997).
Megafauna
A total of 6495 individuals of crustaceans and molluscs were caught during the study, comprising 85 species of Crustacea and 74
species of Mollusca (see Appendix). Trawl catches were dominated by Portunidae (Portunus spinicarpus and Lupella forceps) and Penaeidae (Trachypenaeus similis and Penaeus duorarum) constituting 44% of all the crustaceans collected, whilst gastropod species Calyptraea centralis represented 35% of the molluscan fauna caught.
Multivariate analysis
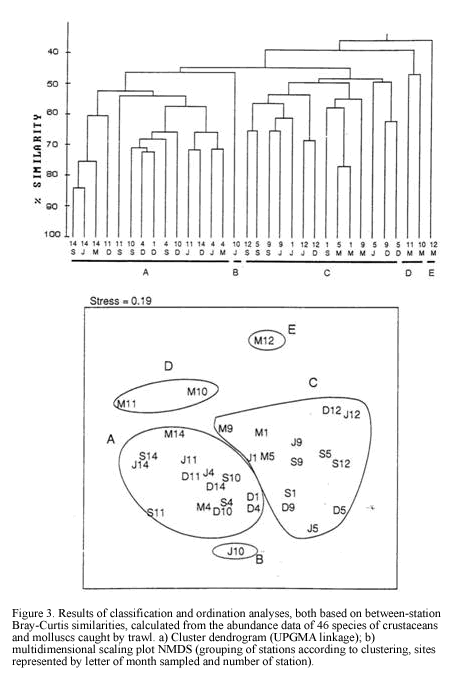
Dendrogram of Figure 3 shows the numeric classification of 32 samples (8 for each sampling period) yielding 5 groups at a similarity level of 48%. Both classification and ordination analyses (Figure 3) show two main groups A and C, considered as deep (30-60 m) and shallow (<20 m) assemblages respectively, and isolated clusters B, D and E.
The group or assemblage (A) was characterised by the brachyuran Goneplacidae Chasmocarcinus cilindricus, the most frequent exclusive species
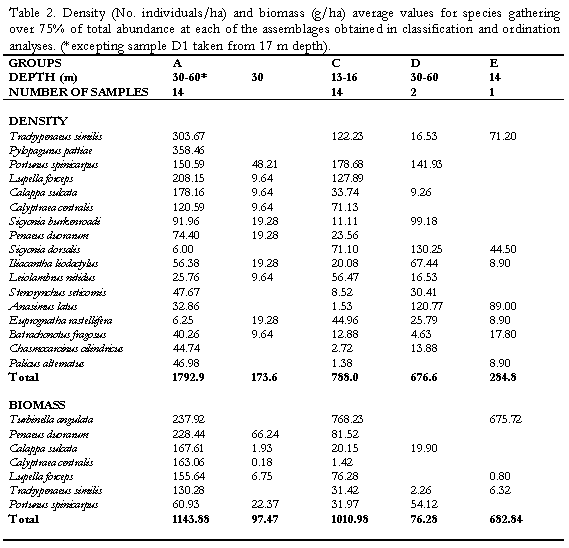
in this assemblage (Figura 4). Raninoides lamarcki, Aequipecten lineolaris, Nuculana cestrota and Microcardium tinctum were abundant but showed a frequency below 70% within the group (Figura 4). The penaeid Trachypenaeus similis exhibited the highest abundance values (Tabla 2) and a frequency of 100% within the assemblage, though it was a generalist species Group A consisted of samples from the deeper stations mainly at 30 and 60 m depth. Physically, it showed a dominance of fine sediments with high percentages of silts and phi values between 3.3 and 4.35 and low percentages of coarser (>2 mm) sediment (Table 1). The assemblage C grouped the samples from depths between 13 and 16 m (excepting M7), and was characterized by the bivalve Laevicardium pictum, with a within-group frequency above 70%. Less frequent exclusive species of the assemblage were the gastropod species Clathrodillia minor, Chicoreus brevifrons and Antillophos chazaliei (Figure 4). Two portunid species, P. spinicarpus and L. forceps were the most abundant species in the assemblage C. Total biomass from this assemblage was lower than from the deeper one (A) as well as biomass of the dominant species, the pink shrimp P. duorarum (81 g/ha), P. spinicarpus and T. similis, accounting with values below 31 g/ha. According to the grain size analysis, group C contains a lower percentage of silt (<0.063 mm) with respect to the deeper
assemblage. Highest values for coarser sediments (2mm) were found at station 7 (Table 1), the southernmost site of the study.
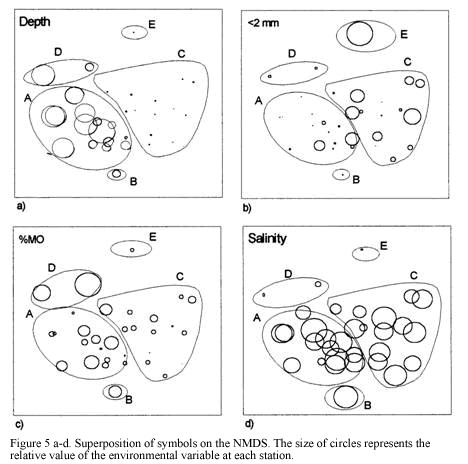
There is a clear segregation of sample J5 (cluster B) from the main grouping A. It was a 30 m depth site at El Rodadero showing a very low abundance of dominant species such as L. forceps (9.6 ind/ha) and T. similis. Itshowed a lower percentage of coarse sediment as well as organic matter content, compared to values obtained for the same site at other sampling months, as observed in the ordination of symbols representing environmental variables (Figure 5c). Cluster D consisted of two samples
taken in March at 30 and 60 m depth at El Rodadero. Portunus spinicarpus was the most abundant species for that cluster, followed by the spider crab Anasimus latus. It did not show any characteristic species and the segregation of this cluster out of the main groupings could be attributed to the absence of the dominant species such as L. forceps and P. duorarum, and the low density of T. similis. Sample M7 was also segregated forming cluster E. It is the most isolated sample in the dendrogram appearing at its first branching level showing a similarity level below 40%. The low values of species abundance, its higher content of coarse sediments (coral fragments, cobbles and shells) and a very low percentage of organic matter content are some the reasons believed to explain the segregation of this sample (Table 1). It did not present exclusive species and A. latus appeared as the most abundant for this sampling site.
It is concluded that as most of the species seem to be generalist according with the inverse analysis (Figure 4). The segregation of clusters could be due not to differences in species composition, but determined by spatial and temporal variations in abundance values of the dominant species. Four samples taken in March were isolated from the main assemblages suggesting special conditions prevailing during that time that caused the absence or low abundance of the dominant penaeids and portunids in these samples.
Biotic and environmental relationship
The technique proposed by Clarke & Aisnworth (1993) was employed to explore the relationships between environmental factors measured (tempertaure, salinity, sediment properties, etc, for details see Invemar, 1997) and the biological data (density of species) collected. The combination of depth, phi (f), salinity, organic matter content (%OM), and organic phosphorus (%OP), reached the highest Spearman ranked correlation coefficient (pw=0.45) with the abundance data of the megafauna. Depth, salinity, %OM and f exhibited significant differences (Kruskal-Wallis p<0.05 for salinity and % OM; Mann-Whitney p<0.05 for f) but %OP was not significantly different between assemblages. Only depth showed a clear graphical correlation when values where superimposed on the NMDS (Figure 5a). Although the coarser sediment content was not included in the correlation (for showing intercorrelation to f), the symbolic representation of its values on the NMDS plot suggested that it could be an important factor segregating the groups. Coarse sediments are slightly higher at most of the stations of the shallower group C (Figure 5b).
Temporal variation
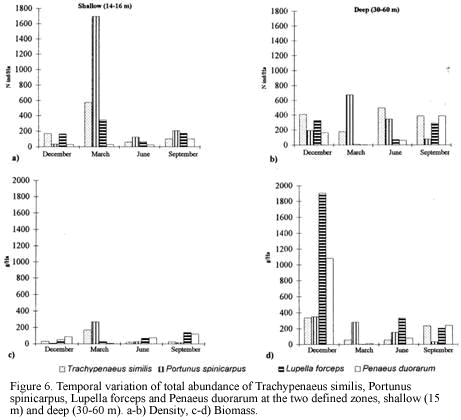
Classification and ordination analyses did not reflect a clear pattern of temporal variation of the soft-bottom community. However, it can be noticed that samples collected in March tended to be located away from the others on the NMDS (Figure 3). Seasonal patterns were then examined according to abundance fluctuations of dominant species through time (Figure 6). Portunus spinicarpus increased its abundance in March (dry season) at shallow and deep areas, Trachypenaeus similis also showed an increased abundance in March but only on the shallower samples (Figure 6), whilst Peneaus duorarum and Lupella forceps revealed high values on deeper sites only in the December and September samplings. Their abundance seemed to increase during the rainfall season from September to early December and decrease during the dry (March) and transition (June) periods (Figure 6b,Figure 6d). A distinction could be made between sediment variables for March and those of other sampling times, mainly at El Rodadero sites.
DISCUSSION
The characterization of the community presented here constitutes an initial approach to the structure and the spatial and temporal distribution patterns of the two most abundant and diverse major taxa (Crustacea and Mollusca) of the soft bottom megafauna in the region of Santa Marta (Invemar, 1997). The two assemblages obtained from the multivariate analysis seem not to be clearly defined by a clear boundary between them. The clusters do not differ significantly in terms of the species composition (36 out of 46 shared), so they are assumed as groupings of a continuum community rather than two distinct biological entities. These two groups are outlined by a bathymetric distribution of the abundance of species and slight differences in the sediment properties.
The segregation shown in the classification and ordination analyses is apparently due to important differences in the abundance of dominant generalist species of the community (Portunus spinicarpus, Penaeus duorarum, Lupella forceps and Trachypenaeus similis) between the shallow (<20m) and deep (30-60 m) areas. The low abundance of these dominant species in the isolated clusters B, D and E (obtained at a level of similarity of 48%), is considered an evidence to interpret the abundance of the penaeids and portunids as the main biological force driving the separation of the groups. The presence of few exclusive species for each of the clusters A and C could also partly explain the segregation, however, only the small goneplacid crab Chasmocarcinus cilindricus inhabiting the deep zone and the bivalve Laevicardium pictum occurring above 20 m depth are frequent enough (>70%) to be considered characteristic species of the clusters. Physically, the depth appears to be the determining factor of the structure of the assemblages (Figure 5a), interpreted as the variation in the abundance of characteristic species from shallow (cluster C, <20 m) to deep (cluster A, 30-60 m) zones.
Similar studies carried out on larger areas of the continental shelf at subtropical (Hoese, 1973; Pires, 1992; Felix-Pico and Garcia-Dominguez, 1993) and cold regions (Basford et al., 1989, 1990; Duineveld et al., 1991) have shown that there are bathymetric limits that determine the settlement of different types of communities, in terms of the structure and species composition. In the present work is possible that the relatively small depth range sampled (approx.50 m depth) partly explains the two clusters obtained that represent a variation of a single community rather than different communities which are likely to be delimited at a deeper boundary (>60 m depth). A similar consideration is made in relation to the sediment type and its organic content, which has some influence over the distribution of species on a given area (Basford et al., 1989). Slight differences in the content of coarser sediment were found between the shallow and deep assemblages (Figure 5b), however, the observed dominance of fine sediments in the whole area is another factor related to the community continuum observed.
The spatial fluctuation of density and biomass of the species is an issue that should be considered in further studies. From the present work it can be inferred that the mobility range and the life history traits related to migrations within the life cycle and feeding habits of each species are some of the factors that could be influencing the abundance variations in a depth gradient.
There is not a clear temporal pattern observed in the structure of the community for the year of study according to the multivariate analysis. This result could be assumed as an indication of the stability of the community in a annual term, however this assumption is quite uncertain and unlikely when the density and biomass of the dominant species are plotted (Figure 6a). Considerable fluctuations were observed for all of the dominant species in the samples taken in March. This result can be related to the slight segregation of the samples from March in the upper section of the NMDS plot (Figure 3). This trend, together with the isolation of the clusters D and E could be linked to abiotic factors related to the upwelling conditions prevailing during the previous months of this sampling. Nevertheless, temporal variations of the community are not easily discernible from the analysis, possibly due to misinterpretations of mixed spatial and temporal variability. Likewise, temporal patterns of distribution at smaller scales (monthly or weekly) or spatial differences among samples due to patchy features of the substratum could mask seasonal variations at greater scales (Morrisey et al., 1992). Individually analyzed, dominant species showed an abundance increase or decrease in the dry season samples (March) supporting the results of Puentes et al. (1990) suggesting that there is an effect of the upwelling conditions and low input of inshore waters during this season over the crustacean communities. In this study P. spinicarpus was widely dominant in March suggesting that this portunid can successfully dislodge other species which are otherwise very abundant, such as P. duorarum and L. forceps which almost disappeared during this sampling month and returned during full wet season (Figure 6). The portunid P. spinicarpus was found by Acero et al. (1990) as the second most frequent for the Santa Marta area. Remarkably it is also the dominant megafaunal species at Ubatuba, Brazil (Pires, 1992) and it is also common on the Georgia coast of the USA (Hoese, 1973).
The dominance of these four decapod species is highly valuable information when assessing the efficiency of the sampling gear (Kaiser et al., 1994) or the design of a replicated sampling program (Underwood, 1993). B are necessary to improve the reliability of this sort of study and enhance its application in the assessment of environmental impacts. While the semi-quantitative nature of the present work gives an approximation of the abundance of megafaunal species caught by the trawl, we believe it constitutes a valid baseline for further investigations in the area.
In number and species richness decapod crustaceans (85 species) dominated the mobile megafauna in Pozos Colorados. In general decapods are highly diverse and have shown to be a significant component of the soft bottom megafaunal communities around the world (Ubatuba Ba\y, Brazil, Pires, 1992; Gulf of Carpentaria, Australia, Long et al., 1995; Central Chile, Gallardo et al., 1996). Unfortunately, the lack of information on the trawl efficiency for the capture of invertebrates does not allow us to make reliable comparisons. Numbers of organisms and species could significantly vary according to the gear employed and the conditions of the sampling at different localities or sampling times (Gibson et al., 1993). The use of diverse sampling methods is suggested to carry out more accurate studies of mobile megafauna, different types of gears and the inclusion of photographic and video techniques in situ has been also recommended (Sibuet and Segonzac, 1985; Kaiser et al., 1994).
Depth and the grain size of the sediments are determining factors controlling the structure and composition of soft-bottom communities (Wilde et al., 1986; Ward and Rainer, 1988; Gallardo et al., 1996; Basford et al., 1989; Duineveld et al., 1991; Karakassis and Eleftheriou 1997). In the present study it is concluded that for the bathymetric range studied (50 m depth) in the southwestern area of Santa Marta, these two factors could be related to the fluctuation of abundance of the generalist species of the community; however, there were no remarkable changes in the composition of species between the bathymetric groupings. A clearer relationship between biological data and environmental factors is expected to occur if the bathymetric range of sampling is increased. The replicated sampling of abiotic parameters, water-mass movements and inputs of nearby rivers could all help to elucidate clear patterns of distribution, abundance and diversity of organisms over the continental shelf. Particularly interesting is the uncertain relationship between upwelling events and the fluctuations of species abundance, and the attributes of the communities in terms of stability. Are spatial and temporal patterns maintained year-to-year?, Are they maintained over a larger study area?. Some of these subjects have been approached for many temperate communities (Basford, 1989, 1990 Duineveld et al., 1991, and others) and few tropical ones (Alongi, 1989; Turner et al., 1995; Gibson et al., 1993), however for the benthic communities from Colombia they remain unknown.
ACKNOWLEDGEMENTS
We are indebted to the Empresa Colombiana de Petróleos ECOPETROL for the financial support of the project. Many thanks to the personnel of Invemar and the R/V “Ancón" crew for their co-operation and help. Thanks to Dr. John Collins and the anonymous reviewers for their comments on the manuscript. This work was part of the B.Sc. thesis submitted by C. P. Arango to the Department of Biology, Universidad Javeriana, Bogotá, Colombia .
LITERATURE CITED
1 Acero, A.; N.H. Campos and J.M. Diaz. 1990. Tendencias en la distribución local de la fauna bentónica y demersal: un análisis basado en colectas de moluscos, crustáceos y peces en fondos sedimentarios. 304-333 p. In: J.M. Díaz.(ed.). Estudio ecológico integrado de la zona costera de Santa Marta y Parque Nacional Natural Tayrona. Final report. Invemar, Santa Marta. [ Links ]
2 Alongi, D.M. 1989. Ecology of tropical soft-bottom benthos: a review with emphasis on emerging concepts. Rev. Biol. Trop., 37: 85-100. [ Links ]
3 Basford, D.; A. Eleftheriou and D. Rafaelli. 1989. The epifauna of the northern North Sea. J. mar. biol. Ass. U.K., 69:387-407. [ Links ]
4 __________; A. Eleftheriou and D. Rafaelli. 1990 The infauna and epifauna of the northern North Sea. Neth. J. Sea Res., 25: 165-173. [ Links ]
5 Blanco, J.A. 1988. Las variaciones ambientales estacionales en las aguas costeras y su importancia para la pesca en la región de Santa Marta, Caribe Colombiano. Tesis M Sc. Univ. Nal. de Colombia, Bogotá, 59 p. [ Links ]
6 __________. 1993. Reconocimiento piloto de fondos, ambiente, fauna asociada y recursos pesqueros en aguas costeras del departamento del Magdalena. Final report, Invemar, Santa Marta, 223 p. [ Links ]
7 Buchanan, J.B. 1984. Sediment Analysis. 41-65p. In: N. Holme and A. McIntyre (eds.). Methods for the study of marine benthos. Second edition. I.B.P. Handbook No 16, London. [ Links ]
8 Clarke, K.R. and M. Ainsworth. 1993. A method of linking multivarate community structureto environmental variables. Mar. Ecol. Prog. Ser., 92: 205-219. [ Links ]
9 Duineveld, G.C.A.; A. Künitzer; U. Niermann; P.A.W. De Wilde and J.S. Gray. 1991. The macrobenthos of the North Sea. Neth. J. Sea Res., 28: 53-65. [ Links ]
10 Felix-Pico, E.F. and F. Garcia-Domingiuez. 1993. Macrobentos sublitoral de Bahia Magdalena, B.C.S. 389-410 p. In: Biodiversidad Marina y Costera de México. S. I. Salazar-Vallejo y N. E. González (eds.). Com. Nal. Biodiversidad and CIQRO, México. [ Links ]
11 Field, J.G.; K.R. Clarke and R.M. Warwick. 1982. A practical strategy for analysing multispecies distribution patterns. Mar. Ecol. Prog. Ser., 8: 37-52. [ Links ]
12 Gallardo, V.A.; R. Roa; F.D. Carrasco; J.I. Cañete; S. Enriquez-Briones and M. Baltazar. 1996. Bathymetric and seasonal patterns in the sublittoral megafauna off central Chile. J. mar. biol. Ass. U.K., 76: 311-326. [ Links ]
13 Garcia, C. and J.H. Sandoval. 1983. Comunidades macrozoobentónicas de fondos blandos en la plataforma continental de Ciénaga, Caribe colombiano. Tesis Biol. Mar., Univ. Jorge Tadeo Lozano, Bogotá, 84 p. [ Links ]
14 __________; J. Sandoval and H. Salzwedel. 1992. Caracterización puntual de las comunidades macrozoobénticas en la plataforma continental de Ciénaga, Caribe colombiano. Mem. VIII Sem. Cienc. Tec. Mar, Santa Marta, 2: 591-599. [ Links ]
15 Gibson, R.N.; A.D. Ansell and L. Robb. 1993. Seasonal and annual variations in abundance and species composition of fish and macrocrustacean communities on a Scottish sandy beach. Mar. Ecol. Prog.Ser., 98: 89-105. [ Links ]
16 Gray, J.S.; M. Aschan; M.R. Carr; K.R. Clarke; R.H. Green; T.H. Pearson; R. Rosenberg and R.M. Warwick. 1988. Analysis of community attributes of the benthic macrofauna of Frierfjord/ Langesundfjord and in a mesocosm experiment. Mar. Ecol. Prog. Ser., 46: 151-165. [ Links ]
17 Guzmán, A. I. and J.M. Díaz. 1993. Distribución espacial de la taxocenosis Annelida-Mollusca en la plataforma continental del golfo de Salamanca, Caribe colombiano. An. Inst. Invest. Mar. Punta Betín, 22: 45-49. [ Links ]
18 Hoese, H.D. 1973. A trawl study of nearshore fishes and invertebrates of the Georgia coast. Contr. Mar. Sci., 17: 63-98. [ Links ]
19 Holme, N.A. and A.D. McIntyre. 1984. Methods for the study of marine benthos. I.B.P. handbook No 16. Blackwell Scientific Publications, London, 387 p. [ Links ]
20 Hostens, K. and O. Hamerlynck. 1994 The mobile epifauna of the soft bottoms in the subtidal Oosterschelde estuary: structure, function, and impact of the storm-surge barrier. Hydrobiologia, 282/283: 479 - 496. [ Links ]
21 Invemar, 1997. Monitoreo de parámetros fisicoquímicos y comunidades macrozoobentónicas en el área de influencia de la monoboya y linea submarina, Pozos Colorados, Santa Marta. Final report Invemar-Ecopetrol, Santa Marta, 123 p. [ Links ]
22 Kaandorp, J. A. 1986. Rocky substrate communities of the infralittoral fringe of the Boulonnais coast, NW France: A quantitative survey. Mar. Biol., 92: 255-265. [ Links ]
23 Kaiser, M.J.; S.I. Rogers and D.T. McCandless. 1994. Improving quantitative surveys of epibenthic communities using a modified 2 m beam trawl. Mar. Ecol. Prog. Ser., 106: 131-138. [ Links ]
24 Long B.G.; I.R. Poiner and T.J. Wassenberg. 1995. Distribution, biomass and community structure of megabenthos of the Gulf of Carpentaria, Australia. Mar. Ecol. Prog. Ser., 129: 127-139. [ Links ]
25 McManus, J. 1988. Grain size determination and interpretation. 63-85 p.In: M. Tucker (ed.). Techniques in sedimentology. Blackwell Scientific Publications, Oxford. [ Links ]
26 Morrisey, D.J.; A.J. Underwood; L. Howitt and J.S. Stark. 1992. Temporal variation in soft-sediment benthos. J. Exp. Mar. Biol. Ecol., 164: 233-245. [ Links ]
27 Pires A.M. 1992. Structure and dynamics of benthic megafauna on the continental shelf offshore of Ubatuba, southeastern Brazil. Mar. Ecol. Prog. Ser., 86: 63-76. [ Links ]
28 Puentes, L.; N.H. Campos and R. Reyes. 1990. Decápodos de fondos blandos hallados en el área comprendida entre Pozos Colorados y la Bahía de Taganga, Caribe colombiano. Bol. Ecotropica, 23: 31-41. [ Links ]
29 Ramirez, G. 1990. Evaluación de parámetros hidrográficos y su relación con la surgencia en aguas costeras:19-76 p. In: J.M. Díaz (ed.). Estudio ecológico integrado de la zona costera de Santa Marta y el Parque Nacional Natural Tayrona. Final report, Invemar, Santa Marta. [ Links ]
30 Rogers, S.I. and S.J. Lockwood. 1989. Observations on the capture efficiency of a two-metre beam trawl for juvenile flatfish. Neth. J. Sea Res., 23: 347-352. [ Links ]
31 Sibuet, M. and M. Segonzac. 1985. Abondance et répartition de l'épifaune mégabenthique. 143-156 p. In: L. Laubier and C. Monniot (eds.). Peuplements profonds du Golfe de Gascogne, Ifremer. [ Links ]
32 Turner, S.J.; S.F. Thrush; R.D. Pridmore; J.E. Hewitt; V.J. Cummings and M. Maskery. 1995. Are soft-sediment communities stable? An example from a windy harbour. Mar. Ecol. Prog. Ser., 120: 219-230. [ Links ]
33 Underwood, A.J. 1993. The mechanics of spatially replicated sampling programmes to detect environmental impacts in a variable world. 18: 99-116 [ Links ]
34 Ward, T.J. and S.F. Rainer. 1988. Decapod crustaceans of the North West shelf, a tropical continental shelf of north-western Australia. Aust. J. Mar. Freshwater Res., 39: 751-765. [ Links ]
35 Wilde, P.A.W.J. De; E.M. Berghuis and A. Kok. 1986. Biomass and activity of benthic fauna on the Fladen Ground (Northern North Sea). Neth. J. Sea Res., 20: 313-323. [ Links ]
DATE RECIEVED: 28/04/1998 DATE ACCEPTED: 01/09/1999
APPENDIX
Phylogenetic list of the species collected by a 2-m beam trawl at the south-western region of Santa Marta, Colombian Caribbean during 1994-1995. The months of the sampling are represented by D= December, M= March, J= June and S= September. The numbers indicate the station(s) where the species was collected (see Figure 1). The depth range of the stations is: 1, 3, 4, 7 = 13-17 m and 2, 5, 6, 8 = 30-60 m. Species collected only in some trawls that could not be replicated in time and were not included in the multivariate analysis are listed here as they are part of the community studied. The month and depth of the collection are indicated for each of these species.