Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Boletín Científico. Centro de Museos. Museo de Historia Natural
Print version ISSN 0123-3068
Bol. Cient. Mus. Hist. Nat. Univ. Caldas vol.17 no.1 Manizales Jan./June 2013
FRAGMENTATION of a TROPICAL LOWLAND FOREST in a trans-boundary region: Colombia and Ecuador*
La fragmentación de un bosque tropical de tierras bajas en una región transfronteriza: Colombia y Ecuador
Andrés Viña1 & Jaime Estévez**2
* FR: 26-X-2011. FA: 26-X-2012.
1 Center for Systems Integration and Sustainability, Michigan State University 1405 S. Harrison Road Suite 115 Manly Miles Bldg. East Lansing, MI 48823-5243U.S.A.,vina@msu.edu
2 Universidad de Caldas, Departamento de Ciencias Biológicas, Calle 65 No. 26-10, Manizales-Colombia
Abstract
The conversion of tropical forest ecosystems to agro-pastoral ecosystems and other land uses continues unabated in Latin America. The present document constitutes a comparative study in which the degree of forest fragmentation through a 23-year period (between 1973 and 1996) was evaluated in two areas located within the same ecological region, but in two different countries (Colombia and Ecuador) which exhibit different patterns of human colonization. Landscape structure rates were used to evaluate the degree of forest fragmentation at both sides of the international border. Results show that the extent of forest fragmentation has been considerably higher on the Colombian side, suggesting that the natural ecosystems might be subject to higher degradation pressures, with potentially important detrimental effects on the abundance and distribution of the biota.
Key words: Colombia, Ecuador, fragmentation, tropical forest
Resumen
La conversión de los ecosistemas de bosque tropical a ecosistemas agro pastoriles y otros usos de la tierra continúa imparable en América Latina. El presente documento comprende un estudio comparativo en el cual se evaluó el grado de fragmentación del bosque durante un periodo de 23 años (entre 1973 y 1996) en dos áreas localizadas dentro de la misma región ecológica, pero en dos países (Colombia y Ecuador) que exhiben diferentes patrones de colonización antrópica. Se usaron índices de estructura del paisaje para evaluar el grado de fragmentación del bosque a ambos lados de la frontera internacional. Los resultados muestran que la extensión de la fragmentación del bosque ha sido considerablemente mayor en el lado colombiano, lo que sugiere que los ecosistemas naturales podrían estar sujetos a mayores presiones de degradación, con efectos perjudiciales potencialmente importantes sobre la abundancia y distribución de la biota.
Palabras clave: Colombia, Ecuador, fragmentación, bosque tropical
INTRODUCTION
The issue of biological diversity impoverishment is perhaps one that attracts most of the attention of all the biophysical changes associated with tropical deforestation. In a deforestation process, a continuous forest cover is usually replaced by a mosaic of forest fragments of different sizes and shapes, surrounded by a matrix of pastures, croplands and secondary forests of different ages. It has been pointed out that this fragmentation induces a regional "bleakness" of biological diversity, in terms of reductions in within-species genetic variability (Harris, 1984). It can also lead to local, regional and global extinction of species (Harris, 1984; Dobson & Gentry, 1991; Saunders et al., 1991) and homogenization of regional and continental biotas by the increase of "weedy" species (Noss, 1983).
Habitat fragmentation results in a reduction and disruption of a previously continuous population (Wilcox, 1980; Wilcove et al., 1986), increasing the likelihood of extinction of some species, particularly those with low population sizes (Simberloff, 1976; 1990; Saunders et al., 1991). Thus, the number of species present in a region is reduced. Fragmentation may also increase the local species richness, but by the substitution of species that are common for those that are rare (Noss, 1983), since some species simply may not occur in small fragmentary patches of habitat that are dominated by edge characteristics. Thus, the overall trend is a global reduction of species diversity. Nevertheless it is now being recognized that isolated forest fragments, selectively logged forests and second-growth forests have an important value in the conservation of biological diversity (Chazdon, 1999), as they represent an important component of the regeneration capacity (i. e. resilience) that characterizes many lowland tropical forests. This regeneration capacity is evidenced, among other things, by their gradual recovery after hurricanes, landslides (Walker et al., 1996) and conversion to pastures (Viña & Cavelier, 1999), and by the fact that tropical forests have been burned for centuries, as is shown by charcoal deposits in the sediments of lowland tropical forests of the Amazon regions of Colombia and Venezuela (Saldarriaga & West, 1986). Forest fires have been occurring at intervals tied to ENSO-induced drought cycles and other climatic extreme events (Nepstad et al., 1998; Soubies et al., 1998; Uhl, 1998; Cochrane et al., 1999; Nepstad et al., 1999). The regeneration capacity of tropical forests carries a hopeful message, in the sense that although human-impacted, many of the deforested areas could be managed in order to prevent further losses of species.
Forest fragmentation through deforestation is a complex process that involves many variables; thus two forest landscapes are unlikely to show identical trajectories of change. Nevertheless, landscapes located in a given region may have similar patterns of fragmentation if they are subjected to the same kind of development or resource exploitation. An ideal study area to observe the impact on forest fragmentation of different deforestation processes is along international borders. In these areas, distinct rates and spatial patterns of forest fragmentation manifest themselves due to the particular historical events as well as colonization schemes experienced on both sides of the border. Thus, since each country adopts its own strategy to colonize the forested regions, based on its political and economical situations, the outcome of the deforestation process, in terms of habitat fragmentation, may be particular to each country. The result is different impacts on the biophysical environment manifested on the landscape as different spatial patterns of fragmentation, with subsequent distinctive effects on the biological characteristics of the region.
The main goal of this study was to make a comparison of the spatial patterns of forest fragmentation between two areas located in the same ecological region [Napo forest ecoregion sensu Prance (1977)], but in two different countries (Colombia and Ecuador). Each country on either side of this border has experienced distinct economic and political pressures that have resulted in distinct and contrasting colonization schemes and rates of deforestation (Viña et al., 2004).
METHODS
Study Area. The study area comprises 3,197.9 km2 of the Amazon basin (between 0°0'21" to 0°27'25" north latitude and 76°26'1" to 76°59'56" west longitude) and occupies portions of Colombia and Ecuador (44 % of the study area is located in the Department of Putumayo, Colombia, and 56 % in the province of Sucumbios, Ecuador). It includes parts of the San Miguel (international border), Putumayo and Aguarico river basins (Figure 1). The altitudinal range is between 250 and 500 m. At an altitude of 260 m (Puerto Asís weather station), mean annual rainfall and temperature are 3,528 mm and 25.3 °C, respectively. Even though precipitation is abundant year round, with all the months receiving more than 200 mm, two especially wet periods are distinguishable, one between April and June, and a second one in November. The temperature has only one maximum, which occurs during the low-sun months of the Northern Hemisphere. According to its temperature and precipitation regimes, the vegetation of the study area is classified as Lowland Tropical Rain Forest in the Holdridge life zone system (Holdridge, 1967).
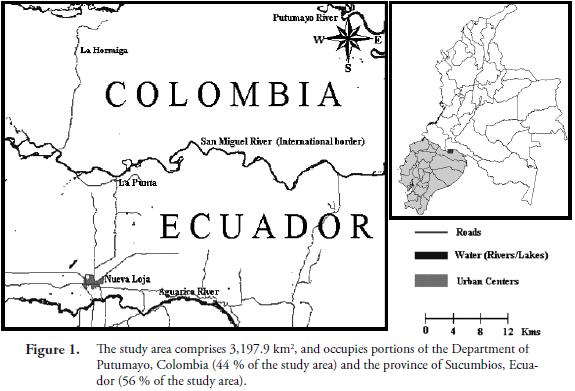
The study area has been little studied in biological terms but it is considered to have a high biological diversity (Hernández-Camacho, et al., 1992). For instance, it exhibits the highest diversity of monkey species known in Colombia (15 species), and some of these are subspecies endemic to the region (e.g. Callicebus cupreus discolor and Cebus albifrons yuracus; Hernández-Camacho, et al., 1992). Additionally the whole upper Amazon (which includes the study area) is considered to have high tree species richness (Gentry, 1992). Viña et al. (2004) provide a description of the history of human colonization and deforestation rates on the study area.
Multi-spectral Digital Data. For the purpose of evaluating the patterns of forest fragmentation in the study area over 23 years of human colonization and deforestation (from 1973 to 1996), three dates of imagery, obtained within this time frame, were analyzed. Landsat Thematic Mapper (TM) digital data for October 10, 1996 and for January 24, 1985, and Landsat Multi-Spectral Scanner (MSS) digital data for February 7, 1973 were purchased from the U. S. Geological Survey, EROS Data Center, Sioux Falls, South Dakota, U.S.A. These images correspond to the Landsat worldwide reference scene identified as Row 60 Path 9 and cover an area of approximately 31,450 km2 centered at 0° 0' 11" N and 76 ° 59' 0" W. For the purpose of establishing the location and areal extent of the forest vegetation in the study area at the three dates of imagery, each of the three multi-spectral data sets was classified into two categories: Forest Vegetation (including primary and mature secondary forests), and Other (including cropland/pastures, urban/barren, open water and clouds). Image processing was performed using the ISODATA unsupervised classification technique (Jensen, 1996; Erdas, 1997). Ground truth was obtained through field observations collected in the Ecuadorian portion of the study area during August 1997. A forest cover classification accuracy of 90% was obtained, thus the forest vegetation was correctly differentiated from other land cover classes in the imagery.
Analysis of Forest Fragmentation. A number of measures (indices) have been proposed in order to quantify landscape structure and its modification through time (TURNER, 1989). The relevance of these measures in the determination of the effects of fragmentation on biological diversity has not been firmly established, although they have been proven useful for establishing general trends, and as a monitoring approach (Meffe & Carroll, 1997). These indices determine the structure of a landscape by analyzing its composition and configuration (Turner, 1989). In the present study, composition refers to the presence and amount of forest fragments, without considering their spatial distribution. This is very important in many ecological processes and thus in the conservation of biological diversity, since the total amount of suitable habitats for any forest species influences its occurrence and abundance within the landscape. Configuration, on the other hand, refers to the spatial character of the forest fragments. This is also very important since the distribution of suitable habitats influences the colonization capacity of forest species, and thus their abundance and distribution within the landscape (Turner, 1989). Over sixty indices have been developed to quantify landscape structure (Dale & Pearson, 1997), but just a combination of some of these measures is required to provide a suitable description of the abundance and spatial pattern of natural habitats. Four general types of indices are useful: area of habitat, frequency distribution of patch sizes, measures of patch shape and length of the edges. Usually, calculating one index for each of these types provides an interpretable set of quantitative descriptors of landscape structure (Dale & Pearson, 1997).
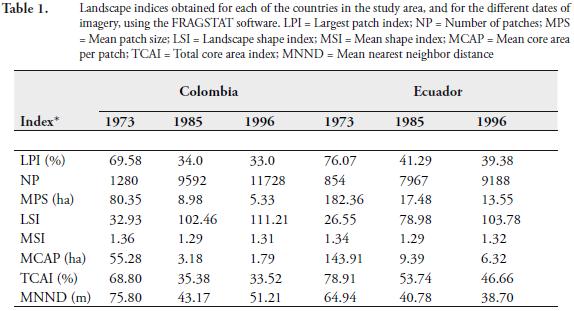
The structure of the forest patches on both sides of the international border and its modification through time (i.e. fragmentation patterns), were analyzed by determining the number of forest patches present, the average patch size, degree of isolation, and extent of the edge areas. These parameters were estimated for each date of imagery, on each side of the international border, using the FRAGSTATS software (McGarigal & Marks, 1995). The indices calculated are:
1. Largest patch index (LPI). Corresponds to the percentage of the landscape comprised by the largest forest patch.
2. Number of forest patches (NP). The number of patches can alter the stability of species interactions and also may affect the propagation of disturbances across the landscape. Also, a landscape with higher number of patches is heterogeneous at finer resolutions. Thus, it can be used as a heterogeneity index but with a limited interpretative value, since it conveys no information about area, distribution or density of forest patches. However, if the total area of the landscapes to be compared is identical, then this index has more value as an estimate of landscape heterogeneity (McGarigal & Marks, 1995).
3. Mean patch size (MPS). This index corresponds to the average size of the forest patches. A landscape with a smaller mean forest patch size than another might be considered more fragmented.
4. Landscape shape index (LSI). Based on Patton (1975), it corresponds to the sum of all of the forest patches boundaries (perimeter), divided by the square root of the total area, of forest cover, adjusted by a constant for a square standard (as opposed to a circle standard, since the images used are raster based, with square pixels). This square standard was used as the shape with minimum edge.
5. Mean shape index (MSI). Also based on Patton (1975), it corresponds to the average shape index of all the forest patches comprised in the landscape.
6. Mean core area per patch (MCAP). It corresponds to the average core area per forest patch.
7. Total core area (TCA). It equals the percentage of the total forest cover that is core area, based on an assumed value of the edge width. For this study, the value of the edge width was assumed to be 120 m, following the suggestion of Harris (1984) in which the edge width corresponds to three times the height of the tallest trees.
8. Mean nearest neighbor distance (MNND). It corresponds to the average distance from each forest patch to its nearest neighboring patch.
RESULTS AND DISCUSSION
Although remotely sensed data and landscape indices derived from them provide a means to describe landscape patterns, it should be kept in mind that no single set of measurements can adequately describe landscape patterns for all species or ecological processes. Thus, the significance of a particular landscape pattern should be evaluated from the point of view of individual species and ecological processes (Dale & Pearson, 1997).
Figure 2 shows the maps of forest cover (i.e. forest patches) in the study area, for each date of imagery, obtained through the classification algorithm used. Table 1 shows the value of the landscape indices calculated for each of the forest cover maps, separated for each country studied, using FRAGSTATS. Some of these indices showed a statistically significant (p<0.05) correlation among them. But although correlated, these indices measure different aspects of the statistical distribution of the forest patches. For instance, LPI, MPS and MNND were inversely related with NP, suggesting that there is a strong relation between the size of the forest patches and the distance among them, to the number of patches present in the landscape. Similarly, the core area indices (i.e. MCAP and TCA) were related to both the patch size index (i.e. MPS) and the patch shape indices (i.e. MSI and LSI).
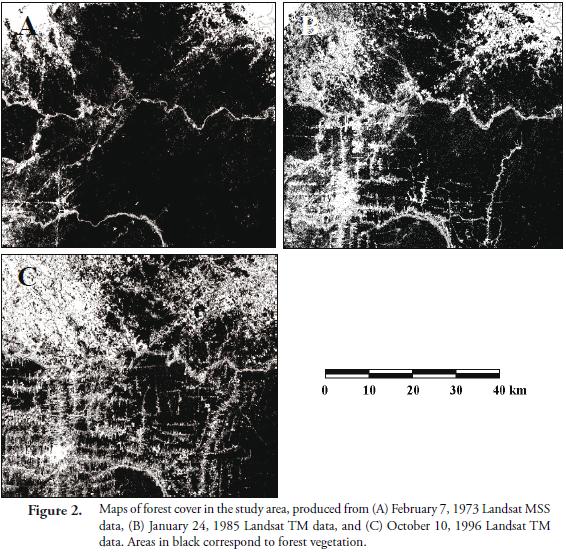
Number of forest patches, patch size and patch isolation. In the study area, the number of forest patches increased an order of magnitude from 1973 to 1996 on both sides of the international border (Figure 2; Table 1). This increment was associated to a reduction (also one order of magnitude) in the average patch size, as well as to a reduction in the average distance among forest patches, particularly in the Ecuadorian side (Figure 2; Table 1). A landscape with a smaller mean forest patch size than another might be considered more fragmented (McGarigal & Marks, 1995). Thus, since the areas located on both sides of the international border have a comparable area (each comprised around half of the whole study area), it can be established that the forests in the Colombian side appeared more fragmented than the forests in the Ecuadorian side during 1973, but the fragmentation became similar in both areas during 1996, after the deforestation process (Table 1). The landscape patch index (LPI), which corresponds to the percentage of the landscape comprised by the largest forest patch (McGarigal & Marks, 1995), showed this same pattern (Table 1).
The total number of patches present in a landscape can alter the stability of species interactions and also may affect the propagation of disturbances across the landscape. Also, a landscape with higher number of patches is more heterogeneous, in terms of the number of habitats present (McGarigal & Marks, 1995). Thus, the most conspicuous effect of the increment in the number of patches over the biota present in the study area would be a modification of its distribution and abundance, such that very different species combinations would be found in the different forest patches present.
The immediate effect of a fragmentation process is an addition of species to the recently formed forest patches, most of which would be species that were already present in the landscape. But the species that were specific to the areas of forest that were subjected to a land cover transformation may vanish. The end result is that each patch will gain species, but the region as a whole will become less diverse (Andrén, 1994). A reduction in the average size of the forest patches present in a landscape will also induce a reduction in the number of species present at the patch level, since the diversity in a patch depends on its area (Andrén, 1994). Since both sides of the international border were subjected to a similar increase in the number of forest fragments present, as well as to a similar reduction in the average size of forest patches, then similar effects over the biota would be expected.
On the other hand, a less isolated patch may have increased in biological diversity (compared to a more isolated patch of similar size), due to the invasion from surrounding habitats (Ås, 1999). Thus, low dispersal rates (isolation) are often tied to population and species diversity declination in fragmented habitats (Schumaker, 1996). The increase in the number of patches associated with the reduction in the average patch size observed on both sides of the international border, may have more negative effects in the Colombian side, due to the higher average distance observed among forest patches (Table 1), which may have a negative effect in the dispersal success of forest species, thus generating lower invasion rates. Nevertheless this effect needs to be proven for different kinds of species, since the influence of forest fragmentation acts in a species-specific manner, and actually contradictory results may be obtained. Such is the case for tree species, in tropical forests of the Jacaré-Pepira region, Southeast Brazil, where fragment size and forest patch isolation were not linked to species diversity (Metzger, 1997).
Extent of Edge Areas and Edge Effects. The outer boundary of a patch (ecotonal area) or edge is a gradient from more survivable to less survivable conditions (Merriam & Wegner, 1992). This boundary constitutes an area that is species specific, and relevant only to what is measured. The total amount of edge is important to many ecological phenomena, since a different species composition and abundance is found in the edge. Forest edges result from differences in the physical conditions (such as wind and light intensity and quality, among many others) at the ecotonal areas that alter the microclimate and thus the biological entities within it. The proportion of forest patch that is affected by this edge depends upon the patch shape and orientation and also upon the land cover types adjacent to the forest fragment (McGarigal & Marks, 1995).
Generally, a larger patch will have a larger edge area in absolute terms, which probably will be translated into more species than the smaller edge area from a smaller patch. In a landscape with a given amount of habitat, however, fragmentation into small patches will lead to an increase of the total amount of edge area and a larger relative patch edge area (Harris, 1984). The relative proportion of edge-thriving species will therefore increase with fragmentation, probably with a negative impact on the species restricted to the interior (i.e. core) areas. If small patches are more species-rich than expected, and this increase in species number has been caused by species that are specialized on edge habitats, then small patches could increase the regional diversity, depending on whether edge habitat was common or rare before fragmentation (Andrén, 1994).
In this study, core area metrics were used to determine and compare the amount of edge areas at both sides of the international border. The core area is defined as the area within a forest patch beyond sufficient edge distance or buffer width from the edge, thus edge constitutes the inverse of the core area. For some ecological processes or organisms affected by edge, it seems likely that the core area would better characterize a patch than the total area, but it is difficult to establish the edge width for a forest fragment, since it is usually specific to the relationship established between the forest patch and the adjacent land cover type effects (McGarigal & Marks, 1995). For practical purposes, the distance from the core area to the boundary of a forest patch was uniformly established to be 120 m. This distance corresponds to 3 times the height of the canopy (ca. 30 m), as was suggested by Harris (1984). Based on this distance, two core indices were calculated (MCAP and TCA; Table 1). The MCAP was almost three times higher in the Ecuadorian side than in the Colombian side, during 1973, but the TCA was similar on both sides of the international border, with values 10 % higher in the Ecuadorian side. This pattern was highly reduced during the deforestation process that followed 1973, probably inducing a significant impoverishment in the biological diversity that needs to be determined and quantified through detailed biological inventories.
The shape of the patch, and its interaction with patch size, may also influence the extent of the edge effects (McGarigal & Marks, 1995). Thus, shape indices (LSI and MSI) were also calculated in order to establish differences in the presence of ecotonal areas, and their variation through time, between the two sides of the international border.
In general terms, the higher the LSI, the higher the amount of edge areas at the landscape scale (McGarigal & Marks, 1995). Both sides of the international border showed lower LSI values during 1973 and higher values during 1985 and 1996, respectively (Table 1). On both sides, the increment during the 1985-1996 period was not as pronounced as during the 1973-1985 period. Thus, the amount of edge drastically increased on both sides of the international border as a result of deforestation and forest fragmentation, particularly during the 1973-1985 period. The Colombian side showed higher LSI values than the Ecuadorian side (Table 1). This suggests that the overall amount of edge was higher in the Colombian side than in the Ecuadorian side, during the three dates of imagery.
On the contrary, the MSI, did not show a significant change with time or between both sides of the international border (Table 1). Thus, at the patch scale, both sides of the international border exhibit a similar amount of edge (on average) that did not show the differences established by the different colonization schemes observed in the region.
CONCLUSIONS
A distinct spatial pattern of forest fragmentation was observed for each of the different countries comprised in the study area, as a result of two different colonization schemes. By these means, two different cultural landscapes were produced in a once continuous natural landscape. These two cultural landscapes are different in terms of the heterogeneity contained within them, but the biological implications of this outcome are far from simple to establish. However, it seems that more fragmentation is occurring in the Colombian side, thus the detrimental effects of habitat fragmentation (i.e. species extinction) may be stronger on that side of the border. Thus, it is necessary to develop a comparative study in which the abundance and distribution of individual species are compared between these two different cultural landscapes, in order to determine the actual effects of different colonization schemes on the biological diversity of the region.
ACKNOWLEDGEMENTS
D. C. Rundquist provided helpful comments on an earlier version of the manuscript. Partial funding for the study was provided by the Colombian institute for the advancement of science and technology – Francisco José de Caldas (COLCIENCIAS) and by the Conservation and Survey Division at the University of Nebraska-Lincoln.
BIBLIOGRAPHY
Andrén, H., 1992.- Corvid density and nest predation in relation to forest fragmentation: a landscape perspective. Ecology 73:794-804. [ Links ]
Ås, S., 1999. Invasion of matrix species in small habitat patches. Conservation Ecology [online] 3(1):1. Available from the Internet. URL: http://www.consecol.org/vol3/iss1/art1. [ Links ]
Chazdon, R.L., 1999.- Tropical forests: Log them or leave them? Science 281: 1295. [ Links ]
Cochrane, M.A., Alencar, A. Schulze, M.D. Souza, C.M. Nepstad, D.C. Lefebvre, P. & Davidson, E.A., 1999.- Positive feedbacks in the fire dynamic of closed canopy tropical forests. Science 284:1832-1835. [ Links ]
Dale, V.H. & Pearson, S.M., 1997.- Quantifying habitat fragmentation due to land use change in Amazonia. In: Laurance, W.F. & Bierregaard, R.O. (Eds.) Tropical forest remnants. Ecology, management and conservation of fragmented communities. Pp. 400-409. The University of Chicago Press. [ Links ]
Dodson, C.H. & Gentry, A.H., 1991.- Biological extinction in western Ecuador. Annals of the Missouri Botanical Garden 78: 273-295. [ Links ]
ERDAS., 1997.- ERDAS Field Guide. Atlanta, Georgia: ERDAS, Inc. [ Links ]
Gentry, A.H., 1992.- Tropical forest biodiversity: Distributional patterns and their conservational significance. Oikos, 63: 19-28. [ Links ]
Harris, L.D., 1984.- The fragmented forest. Island biogeography theory and the preservation of biotic diversity. The University of Chicago Press. Chicago and London. [ Links ]
HERNÁNDEZ-CAMACHO, J. HURTADO-GUERRA, A. ORTIZ-QUIJANO, R. & WALSCHBURGER, T., 1992.- Unidades biogeográficas de Colombia. In: Halffter, G. (Ed.). La diversidad biológica de Iberoamérica. Instituto de Ecologia, A.C., Mexico. [ Links ]
HOLDRIDGE, L.R., 1967.- Life zone ecology. Tropical science center. San José, Costa Rica. [ Links ]
JENSEN, J.R., 1996.- Introductory digital image processing. A remote sensing perspective. Second Edition. Prentice Hall. [ Links ]
MCGARIGAL, K. & MARKS, B.J., 1995.- FRAGSTATS: Spatial pattern analysis program for quantifying landscape structure. General Technical Report PNW-GTR-351. United States Deparment of Agriculture. Pacific Northwest Research Station. [ Links ]
MEFFE, G.K. & CARROLL, C.R., 1997.- Principles of Conservation Biology. 2nd.Edition. Sunderland, Mass. Sinauer [ Links ]
MERRIAM G. & WEGNER, J., 1992.- Local extinctions, habitat fragmentation and ecotones. Chapter 9 in: Hansen, A.J. & DiCastri, F. (Eds.). Landscape boundaries. Springer-Verlag. [ Links ]
METZGER, J.P., 1997.- Relationships between landscape structure and tree species diversity in tropical forests of South-east Brazil. Landscape and Urban Planning 37: 29-35. [ Links ]
NEPSTAD, D.C. MOREIRA, A. VERISSIMO, A. LEFEBVRE, P. SCHLESINGER, P. POTTER, C. NOBRE, C. SETZER, A. KRUG, T. BARROS, A.C. ALENCAR, A. & PEREIRA, J.R., 1998.- Forest fire prediction and prevention in the Brazilian Amazon. Conservation Biology 12:951-953. [ Links ]
NEPSTAD, D.C. VARISSIMO, A. ALENCAR, A. NOBRE, C. LIMA, E. LEFEBVRE, P. SCHLESINGER, P. POTTER, C. MOUTINHO, P. MENDOZA, E. COCHRANE, M. & BROOKS, V., 1999.- Large-scale impoverishment of Amazonian forests by logging and fire. Nature 398: 505-508. [ Links ]
NOSS, R., 1983.- A regional landscape approach to maintain diversity. BioScience 33 (11): 700-706. [ Links ]
PATTON, D.R., 1975.- A diversity index for quantifying habitat edge. Wildlife Society Bulletin 3: 171-173. [ Links ]
PRANCE, G.T., 1977.- The phytogeographical support for the theory of Pleistocene forest refuges in the Amazon basin, based on evidence from distribution patterns in Caryocaraceae, Chrysobalanaceae, Dychapetalaceae and Lecythidaceae. Acta Amazonica 3: 5-8. [ Links ]
SALDARRIAGA, J.G. & WEST, D.C., 1986.- Holocene fires in the northern Amazon basin. Quaternary Research 26: 358-366. [ Links ]
SAUNDERS, D.A. HOBBS, R.J. & MARGULES, C.R., 1991.- Biological consequences of ecosystem fragmentation: A review. Conservation Biology 5: 18-32. [ Links ]
SCHUMAKER, N.H., 1996.- Using landscape indices to predict habitat connectivity. Ecology 77:1210-1225. [ Links ]
SIMBERLOFF, D., 1976.- Species turnover and equilibrium island biogeography. Science 194:572-578. [ Links ]
SIMBERLOFF, D., 1990.- Species-area relationships, fragmentation, and extinction in tropical forests. In: Proceedings of the international conference on tropical biodiversity "In Harmony with Nature". Kuala Lumpur, Malaysia. [ Links ]
SOUBIES, F. SUGUIO, K. & VOLKMER-RIBEIRO, C., 1998.- Amazonia rainforest fires: A lacustrine record of 7000 years. Ambio 27: 139-142. [ Links ]
TURNER, M.G., 1989.- Landscape ecology: The effect of pattern and process. Annual Review of Ecology and Systematics 20: 171-197. [ Links ]
VIÑA, A. & CAVELIER, J., 1999.- Deforestation rates (1938-1988) of tropical lowland forests on the Andean foothills of Colombia. Biotropica 31: 31-36. [ Links ]
VIÑA, A. ECHAVARRIA, F.R. & RUNDQUIST, D.C., 2004.- Satellite change detection analysis of deforestation rates and patterns along the Colombia-Ecuador border. AMBIO 33: 118-125. [ Links ]
WALKER, L.R. SILVER, W.L. WILLIG, M.R. & ZIMMERMAN, J.K., (Eds.). 1996.- Long Term Responses of Caribbean Ecosystems to Disturbance. Biotropica 28 (4a). Special Issue. [ Links ]
WILCOVE, D.S. MCLELLAN, C.H. & DOBSON, A.P., 1986.- Habitat fragmentation in the temperate zone. In: Soule, M.E. (Ed.). Conservation Biology. Sinauer Associates, Inc. Publishers. Massachusetts. [ Links ]
WILCOX, B.A., 1980.- Insular Ecology and Conservation. In: Soule, M.E. & Wilcox, B.A. (Eds.). Conservation Biology. Sinauer Associates, Inc. Publishers. Sunderland, Massachussetts. [ Links ]













