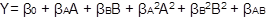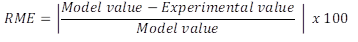INTRODUCTION
Cape gooseberry (Physalis peruviana L.) is characterized by its high nutritional value: vitamins A, B (thiamine, niacin, and riboflavin) and C, proteins and minerals such as phosphorus, iron, potassium and zinc (Olivares-Tenorio et al. 2016), antioxidant compounds such as tocopherols and carotenoids (Etzbach et al. 2018), and withanolides that provide health benefits: repellent, antibacterial, anti-inflammatory, antitumor, and antihepatotoxic activity (Sang-Ngern et al. 2016). The fruit has a weight percentage distribution of extracted pulp of 75.4 - 84.5%, seeds of 7.3 - 13.2%, and peel of 5.8 - 11.4% (Petkova et al. 2021). Seeds have nutritional value due to the content of essential fatty acids, natural antioxidants, and phytosterols such as campesterol, β-sitosterol, and stigmasterol, which provide antioxidant properties and reduce blood cholesterol levels. In addition, the presence of pectins as a contribution of dietary fiber (4.9 g 100 g-1) is highlighted in the peel and pulp of cape gooseberry (Petkova et al. 2021).
One way to preserve these potential healthy characteristics of cape gooseberry, in all its constituents, is through the spray-drying (SD) micro-encapsulation process. In this context, homogenized fruits are heterogeneous colloidal systems of two or more phases with the presence of insoluble particles, comprised of fragments of cellular tissues, and oily particles dispersed in an aqueous phase rich in soluble compounds such as sugars, acids, salts, pectins, phenolic compounds, among others (Dahdouh et al. 2016). These systems depend on particle-particle and particle-continuous phase physicochemical interactions, which define physicochemical stability based on the forces present: attractive or Van der Waals forces, repulsive or electrostatic, steric, hydration, or hydrophobic forces (Piorkowski & McClements, 2014).
In micro-encapsulation processes using SD, the physicochemical interactions of nutrition are closely related to chemical composition and more particularly to the presence of proteins, polysaccharides of the cell walls of fruits (pectins, cellulose, hemicelluloses), and drying additives such as maltodextrin (MD), gum arabic (GA), and others. These act as micro-encapsulants with high molecular weight and high glass transition temperature (Tg), which protect the active components and reduce the stickiness and hygroscopicity of the product obtained by SD (Islam Shishir & Chen, 2017). Additives such as MD and GA have functions that include volume and film formation properties, binding capacity, and reduction of the O2 permeability of the matrix (Lee et al. 2018). This compositional complexity modifies the rheology of the continuous phase of the colloidal system, due to the presence of a large number of hydroxyl (-OH) groups that increase affinity with H2O molecules, reduce kinetic movement, and improve molecular and interparticle interactions, contributing to increasing the stability of the colloidal system (Dahdouh et al. 2016). In SD micro-encapsulation processes, in addition to the design of a thermodynamically stable formulation (Islam Shishir & Chen, 2017), a maximum content of total solids (TS) and a viscosity (() adjusted to the SD design criteria, which allows effective spraying (Santos et al. 2017), is required.
The objective of this research was to optimize the colloidal system of cape gooseberry pulp, seed, and peel, together with GA and MD (SCU+GA+MD), allowing its effective use in the micro-encapsulation process.
MATERIALS AND METHODS
Colombia ecotype cape gooseberry fruits from eastern Antioquia were used. The fruit had a maturity degrees of 4, 5, and 6 according to NTC 4580 (ICONTEC, 1999). The micro-encapsulants for drying were GA (767 AA Master Gum FT) and MD (Ingredion MOR-REX 1720) with dextrose equivalent 18-20.
Preparation of cape gooseberry-based colloidal system formulations (SCCG+GA+MD). The cape gooseberry fruits were disinfected by immersion in a 1400 ppm solution of Citrosan® (0.25% v/v) (Diken International, Mexico) for 10 minutes. They were disintegrated in a homogenizer (Sammic TR-350), and the resulting dispersion was homogenized again in a JMF 80A colloidal mill (Wenzhou Qiangzhong Machinery Technology Co., Ltd.) with the clearance adjusted at the minimum and recirculation time of 3 min, thus obtaining the homogenized integral cape gooseberry (UH). Batches of 2000 g of SCU+GA+MD were prepared according to the treatments described in the experimental design (Table 1). UH, GA, and MD were mixed in a homogenizer (Silverson Machines Ltd. Model L5M-A, England) at 10,000 rpm for 10 min. A temperature-controlled bath at 25 ± 1°C was used.
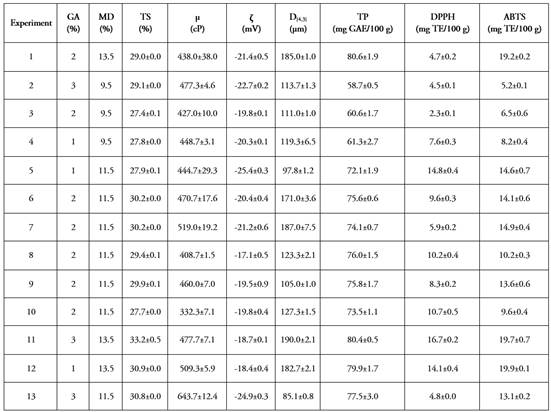
Table 1 Results of variables dependent on the colloidal system of cape gooseberry (pulp, peel, and seeds), gum arabic, and maltodextrin.
Characterization of SCU+GA+MD. TS: were determined by the difference 100 - Xw (%). X w : method 20.013 (AOAC, 2012). μ: methodology described by Wardy et al. (2014), rheometer (Brookfield DV-III Ultra, Brookfield Engineering Laboratories, Inc, USA), 25°C, RV3 spindle, 100 rpm. ζ: methodology described by Tamnak et al. (2016), Zetasizer Nano ZS90 (Malvern Instruments Ltd., Worcester, UK). D [4,3] : methodology described by Dahdouh et al. (2018), Mastersizer 3000 (Malvern Instrument Ltd, Worcestershire, UK), Hydro LV system, refractive index of SCU+GA+MD. (1.368), water refractive index (1.330), particle absorption index (0.45), and laser obscuration level (15). For TF and antioxidant capacity, a methanolic extraction was carried out according to the methodology described by De los Rios et al. (2021). TF (Folin-Ciocalteu method) and antioxidant activity (ABTS and DPPH) were carried out according to the methodology described by Gallón Bedoya et al. (2020). Gallic acid equivalent (GAE) calibration curves were performed between 0 to 300 µg GAE/mL (R2 = 0.989) and TF results were expressed as mg GAE 100 g-1 SCU+GA+MD. Trolox equivalent (TE) calibration curves for ABTS and DPPH were constructed from 50 to 250 µM (R2= 0.998) and 0.02-0.12 mg mL-1 (R2= 0.997), respectively, and values were expressed as mg TE 100 g-1 SCU+GA+MD. For the active components, the characterization of a fresh (unhomogenized) cape gooseberry control was included for a comparative analysis.
Experimental design, statistical, and data analysis. We worked with a face-centered central composite design (( = 1) based on the independent variables GA (1.0-3.0%) and MD (9.5-13.5%), and the dependent variables total solids (ST), viscosity (µ), zeta potential (ζ), particle size D[4,3], total phenols (TF), and antioxidant capacity (DPPH and ABTS methods) (13 experiments, Table 1). For statistical analysis, Statgraphics Centurion XVII.II software was used, and an analysis of variance (ANOVA) with a significance level of 5% (p<0.05) was performed.
The experimental optimization was carried out by setting desirable criteria, weights, and impacts to the dependent variables, according to the desirable characteristics in the final product. Data were adjusted to a second-order polynomial model (equation 1), where Y is the dependent variable, β0 is constant, βA and βB are the linear coefficients; βA 2 and βB 2 are the quadratic coefficients; and βAB is the coefficient of linear interaction. The validation of the models was carried out based on the relative mean error (RME) (equation 2) between the value of the variable predicted by the model and the experimental response to the optimal condition (3 replicates).
RESULTS AND DISCUSSION
Table 1 presents the mean values and standard deviations, and Figure 1 shows the response surface graphs of the variables dependent of SCU+GA+MD.
Total solids. The mean TS values ranged between 27.4-33.2%, with the ANOVA showing significant differences (p<0.05) regarding the MD. The response surface graph (Figure 1a) shows an increase in TS (yellow-orange zones) when the MD and GA in the SCU+GA+MD increase. The effect of MD is greater than that provided by GA, due to the greater contribution of kg of solids in the formulations. This situation is attributed to the fact that these high molecular weight ingredients have hydrophilic functional groups (aldehydes, OH, among others), that interact with water molecules through Van der Waals attractions and H2 bridges (Saavedra- Leos et al. 2018). The increased TS decreases the Xw content of SCU+GA+MD, which favors energy expenditure during the micro-encapsulation process (Islam Shishir & Chen, 2017).
Viscosity. ANOVA did not show significant differences in µ (p>0.05) of SCU+GA+MD regarding the independent variables nor with their linear or quadratic interactions. Its mean values range between 332.3 and 643.7 Cp. This rheology characterizes SCU+GA+MD as a fluid system that would contribute to forming smaller droplets in the SD process (Islam Shishir & Chen, 2017). The fluctuations found can be attributed to changes in the maturation of cape gooseberry, which modifies the content of the pectic, cellulosic, and hemicellulosic components of the cell walls (Guevara Collazos et al. 2019). The response surface graph (Figure 1b) shows a trend of increasing µ when GA levels were higher, given that its structure has long branches with a voluminous arrangement able to form bonds, through H2 bridges, with water molecules, producing an increase in hydrodynamic volume, which induces changes in the deformation resistance to the matrix (Tuan Azlan et al. 2020). In turn, protein (0.3 to 1.9 g 100 g-1 U) and seed oil (1.8 to 2.0%) (Petkova et al. 2021), could be interacting with GA, which has emulsifying properties, being absorbed onto oil droplets through its protein residues (Tuan Azlan et al. 2020).
Zeta Potential (ζ). Zeta (ζ) is a parameter associated with the repulsive forces present in colloidal systems. ANOVA did not show significant differences in ζ (p<0.05) concerning the independent variables or their interactions, with mean values fluctuating between -17.1 and -25.4 mV. The SCU+GA+MD is characterized by the fact that the Stern layer (1st electrical layer) has a negative charge at the interface of the particles (insoluble material and fatty component), provided by the anions dissociated from the aqueous phase of the cape gooseberry and by the non-hydrolyzed pectin (Cano-Sarmiento et al. 2018). Meanwhile, the 2nd electrical layer (+) is mainly due to the dissociated cations from the cape gooseberry (Ca, P, Fe) (Petkova et al. 2021).
This double electrical layer contributes to the stability of adjacent particles due to the electrostatic repulsion forces generated between them (Matusiak & Grządka, 2017). The overall stability of SCU+GA+MD depends on the balance between attractive Van der Waals forces and electrostatic or repulsive forces, and other types: steric, hydration, hydrophobic, and phase separation forces (Wan et al. 2018; Zhu et al. 2020). The response surface graph of ζ (Figure 1c) shows a curvilinear behavior, where the highest negative electrical potential (> (( () (>) is reached when the SCU+GA+MD formulation has concentrations of MD between 9.5 and 12.5% and GA between 2.6 and 3.0%. This behavior could be attributed to the interaction of GA with fat particles and insoluble material, where the increase in GA provides a greater surface charge (-) in the proximity of the interfaces due to the higher content of tails (non-polar compounds) or polymeric segments of the polysaccharide. The emulsifying properties of GA could allow the absorption at the interface of SCU+GA+MD oil droplets through their protein residues (Babbar et al. 2015). In adsorbed macromolecules, the polysaccharide segments are located towards the aqueous phase, due to the presence of carboxylic groups. Meanwhile, the polypeptide chains remain mainly linked like a train to the interface of the oil particles from the seeds. In this way, there is a cooperation that reduces the free energy around the oil droplets and favors the stability of SCU+GA+MD (Tuan Azlan et al. 2020). Authors such as Cano-Sarmiento et al. (2018) recommend that ζ values in colloidal systems should be on the order of ±30 mV, to guarantee good physicochemical stability. However, other authors such as Gallón Bedoya et al. (2020) suggest a similar effect due to the synergy of ζ (values < (±30 ( mV) and with the ( of the continuous phase of the colloidal system. The results of ( found are comparable with those reported by Wan et al. (2018) for carrot juice fermented with probiotics, and Gallón Bedoya et al. (2020), for a suspension based on cape gooseberry, strawberry, and blackberry.
Particle size. The particle size of food colloidal systems from fruits are indicator associated with phase separation (Dahdouh et al. 2018). In SCU+GA+MD a homogeneous dispersion of the particles was observed, with mean values of D[4,3] between 85.1 and 190.0 μm and significant statistical differences (p<0.05) concerning the MD content. This variability is mainly attributed to the combined effect of the applied homogenization conditions and the independent variables considered (MD and GA). In this sense, the TS content of each formulation will be directly related to the particle sizes of SCU+GA+MD. The TS provided by UH as the main raw material is represented by soluble solids (SS) (sugars, acids, soluble fibers, salts, and others) (Mokhtar et al. 2018), by the insoluble solids provided mainly by the insoluble fiber, and by the fat content coming from the peel (3.43 g 100 g-1bs). The UH had a total fiber content of 185 g 100 g-1bs, where the insoluble fiber represents approximately 87.6% of the total fiber, corresponding to 162.06 g 100 g-1bs (Ozturk et al. 2017).
The response surface graph (Figure 1d) illustrates the decrease in D[4,3] with the reduction of MD, which is consistent because lower ST in the SCU+GA+MD contributes to a higher shear effect on the particles. This results in better disintegration of the cell membranes during the homogenization time due to lower resistance in the process, leading to an increase in the surface area of the particles and greater particle-particle and particle-aqueous phase interaction. In this context, the OH groups of glucose in MD and protein residues of GA, contained in the aqueous phase, have greater interaction through H2 bridges, favoring the stability of SCU+GA+MD (Lee et al. 2018).
Gallón Bedoya et al. (2020) report that in strawberry, blackberry, and cape gooseberry suspensions, smaller particle sizes occur when the total solids of cape gooseberry are low. Greater contribution of solids is represented by fruit seeds with high mechanical resistance and a pectin matrix in their peel. De los Rios et al. (2021), reported similar behavior in blackberry suspensions. These authors reported that decreased blackberry solids and hydrocolloids (GA) resulted in a decreased particle size, which was associated with higher μ. Consequently, it can be assumed that particle sizes between 40-100 μm consist of individual cells and cell fragments, while those between 100-125 μm may be small groups of cells, and values above 250 μm may be tissue remnants (Moelants et al. 2014).
Total phenols and antioxidant capacity. The TFs in the SCU+GA+MD showed mean values between 58.7-80.6 mg GAE 100 g-1. A significant effect (p>0.05) of the MD variables and the quadratic interaction of MD occurred. The TF behavior in the response surface graph (Figure 1e) shows a curvilinear trend, where the highest contents in SCU+GA+MD are reached with MD concentrations between 12.5-13.5% and throughout the GA range. The variation in TF content in SCU+GA+MD is mainly attributed to two scenarios: 1) to the variation in STs provided by the cape gooseberry in each formulation, which are determined according to the balance of matter and according to the MD and GA concentrations established by the experimental design and, 2) to the variation in TF of fresh cape gooseberry (control) (84.1±5.7 mg GAE 100 g-1 ( 4.7±0.3 mg GAE g-1STU, variability coefficient = 6.8%). Given this situation, the variability coefficients of TF were determined for the 13 experiments, based on the contributions of mg GAE g-1 STUchuva (0.4 - 4.5%), which corresponded in some experiments to degradation up to 20%. Meanwhile, in other experiments, there was no degradation, probably due to a higher TF content in the UH than in the control.
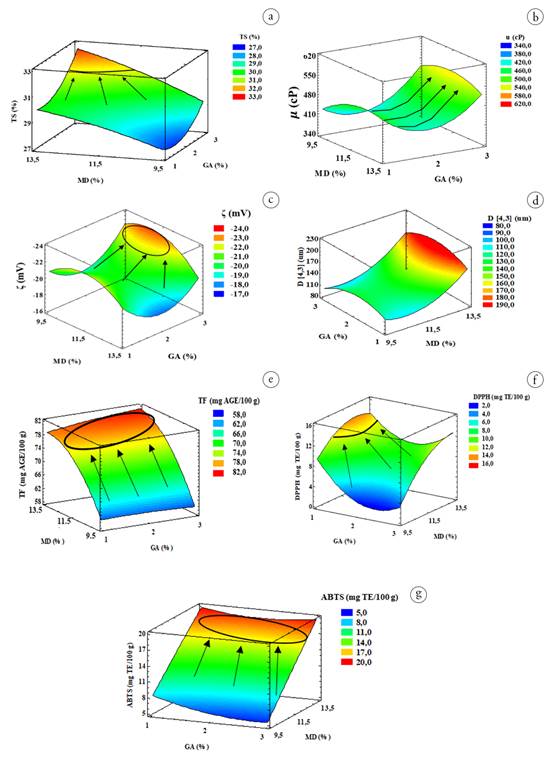
Figure 1 Response surface graphs of variables dependent on the colloidal system of cape gooseberry (pulp, peel, and seeds), gum arabic, and maltodextrin. a) TS (Total solids); b) μ (viscosity); c) ζ (zeta potential); d) D[4,3] (particle size); e) TF (total phenols); f) DPPH (2,2-Diphenyl-1-picrylhydrazyl); g) ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)).
The shear homogenization process leads to the degradation of the active components due to the reheating experienced by the matrix. In addition, there is also a greater residual activity of enzymes such as polyphenoloxidase and peroxidase in UH, since TFs are their substrates (Vega-Gálvez et al. 2014; Etzbach et al. 2019). In turn, the breakage of cell membranes improves the extraction of TFs and their interaction with MD and GA (Vega-Gálvez et al. 2014; Zhu, 2018).
The antioxidant capacity measured by the DPPH and ABTS methods showed statistically significant differences (p<0.05) concerning MD, where its mean values ranged between 2.3-16.7 and 5.2-19.9 mg TE 100-1g SCU+GA+MD, respectively. The response surface graphs of both variables (Figures 1 f-g) show the highest contents with formulations whose composition is high in MD (12.5-13.5%) and low in GA (1.0-1.4%). In general, the antioxidant activity behaviors of SCU+GA+MD depend on the variability of the initial contents in the UH and the degrading effect conferred by the homogenization process applied. However, it can be inferred that there is a protective effect of MD and GA (encapsulants) at the previous concentrations on the active compounds present in the aqueous and lipid phases of SCU+GA+MD (Taheri & Jafari, 2019; Santos Araujo et al. 2020), and even exert a protective effect against shear forces and degrading enzymatic processes that occur when breaking the cellular structure (Gallón Bedoya et al. 2020).
The research led to the development of a formulation of SCU+GA+MD that fully utilized the fruit´s structure (pulp, seed, and peel), positioning it as a sustainable technological alternative. The SCU+GA+MD developed proposes technological alternatives for utilization, which may contribute to value generation in the cape gooseberry agricultural chain. The results obtained allow SCU+GA+MD to be used in the SD micro-encapsulation process. The independent variable with the greatest impact on SCU+GA+MD was MD. However, GA plays an important role in repulsive forces between particles, providing better physical and chemical stability at higher concentrations.
Mathematical modeling and experimental optimization. Table 2 shows the coefficients of mathematical models for the dependent variables of SCU+GA+MD and their respective R2. It is observed that most of the R2 values for the regression coefficients were not high (38.0-97.3). However, the lack of fit test did not show significant statistical differences (p>0.05) in most of the dependent variables, therefore, they are considered adequate to describe the behavior of the experimental data. Nevertheless, this did not occur for the DPPH, probably due to the different factors that may be affecting SCU+GA+MD: harvesting, maturation, mixing, homogenization, and formulation. On the other hand, all dependent variables showed a random distribution of the residuals. This ensures that the data can be parameterized according to a normal distribution and reaffirms that the models are suitable for describing the behavior of the results.
Table 2 Polynomial regression coefficients for the colloidal system surface model of cape gooseberry (pulp, peel, and seeds), gum arabic, and maltodextrin.
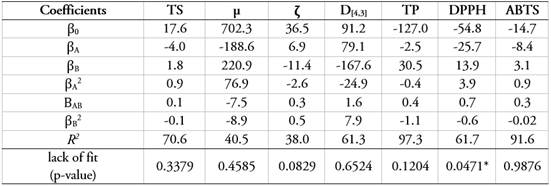
Table 3 shows the experimental optimization of multiple responses for SCCG+GA+MD, where different criteria were established to identify the most physicochemically stable formulation and the one with the greatest antioxidant activity: maximize TS, μ, TP, DPPH, ABTS; and minimize: ζ and D[4,3]. In addition, weights and impacts were defined in the experimental optimization, and the experimental validation of SCU+GA+MD was carried out through the calculation of the experimental mean error (RME), comparing the results of the dependent variables according to the model with those obtained experimentally under the optimal condition. The optimization showed a desirability of 72.0%. The independent variables were MD of 12,3% and GA of 330%. Other researchers have optimized colloidal systems based on the desirability, reporting the following results: 69.1% in a blackberry suspension with probiotics (Marín-Arango et al. 2019) and 74.3% in banana juice (Handique et al. 2019). It is observed that the majority of the dependent variables (TS, µ, ζ, DPPH, and ABTS) showed RME values less than 12.9%, which is acceptable and allows for validating the mathematical models. Variables D[4,3] and TP showed values greater than 20%, which is the consequence of the different factors mentioned above. However, it is highlighted that the experimental values contribute to better physicochemical stability and antioxidant activity, being better than those obtained by their mathematical models.
Table 3 Experimental optimization of multiple responses for the colloidal system of cape gooseberry (pulp, peel, and seeds), gum arabic, and maltodextrin.
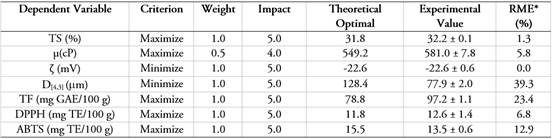
*RME: Relative Mean Error.
Experimental optimization enabled the determination of the most appropriate formulation for SCU+GA+MD. Additionally, the experimental validation ensured an acceptable prediction of the dependent variables.