Introduction
In December 2019, a novel respiratory syndrome appeared in the city of Wuhan, China. Later, the pathogen was identified as a beta-coronavirus, which was named Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), and the disease associated with it was named Coronavirus disease 2019 (COVID-19)1. By the end of December 2021, more than 280 million confirmed cases and more than 5.4 million deaths have been identified worldwide. In Colombia, more than 5 million cases were diagnosed and more than 129'000 lives were taken by the end of December 20212.
The appearance of this pandemic has changed the medical practice of nowadays, raising multiple concerns in various scenarios of the medical field. Many questions have appeared when performing invasive procedures such as elective surgery in the out-patient setting3, mainly when it comes to determine if it is safe to these procedures, and if so, to determine the necessity of screening COVID-19 asymptomatic patients to avoid exposure and risk of infection to the medical team, as well as to decrease the risk of mortality and postoperative complications in the patient. Here we present our results of screening patients scheduled to undergo elective surgery with rt-PCR within 7 days prior surgery.
Methods
Here we present an observational, descriptive, retrospective study of a cohort of patients undergoing elective surgery from May 4th, 2020 to April 23th, 2021 at Clínica de Marly in Bogotá Colombia. Data collection was done under the supervision of the scientific committee Clínica de Marly. Informed consent was obtained from all individual participants included in the study prior to the invasive procedure. No identifying information is exposed in this study.
Demographic and clinical variables of patients and operative data were abstracted.
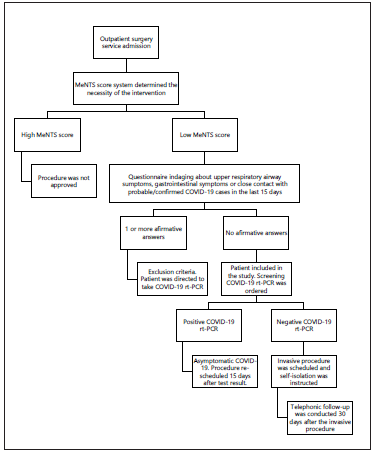
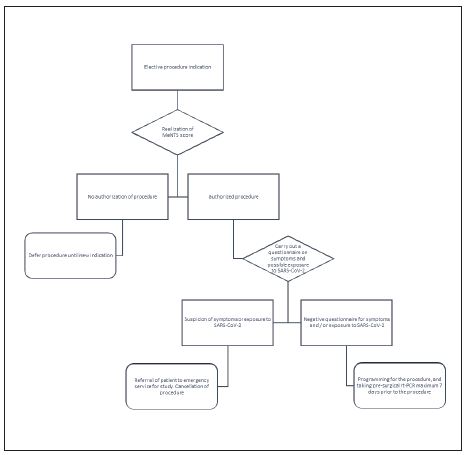
Prior to scheduling the procedure, any programmed intervention in the pandemic was supervised by a specialized committee, and the MeNTS score system was used in order to determine the necessity of the intervention4. If the procedure was approved, patients were asked to fill a questionnaire that inquired for the presence of upper respiratory airway symptoms (URAS), gastrointestinal (GI) symptoms or the contact with probable/confirmed COVID-19 cases in the last 15 days (table 1). If one of these questions was positive, the patient was addressed to attend to the emergency room for mandatory COVID-19 screening. Instead, if these questions were negative, a naso/oropharyngeal swab was taken to detect SARS-CoV-2 ARN with rtPCR within 7 days prior to surgery. Patients were instructed to self-isolate until the day of surgery in order to minimize the risk of COVID-19 infection (Fig. 2). The timing of SARS-CoV-2 diagnosis was recorded as preoperative.
Table 1 Characteristics of the population
| Characteristics | Patients n=1837 N. (%) |
|---|---|
| Gender | |
| Female | 904 (49.3%) |
| Male | 933 (50.7%) |
| Age (mean) | 49.5 |
| Intervention | |
| Surgery | 1655 (90%) |
| Gastro | 182 (9.9%) |
| Rt-pcr sars-cov-2 | |
| Positive | 104 (5.66%) |
| Negative | 1733 (94.33%) |
Patients that underwent emergency surgery or with positive symptoms/contact with probable/confirmed cases of COVID-19 were excluded from our study analysis.
Patients that underwent invasive procedures were followed up 30 days after the intervention in order to detect any adverse outcomes. If patients were positive for COVID-19, the procedure was delayed for at least 14 days and rescheduled once the patient was free of respiratory symptoms. The primary outcome was the incidence of COVID-19 in asymptomatic patients and without any contact with probable/ confirmed COVID-19 cases. Secondary outcomes were the incidence of complications 7 days after the surgery of recovered COVID-19 patients, and death by 30 days after the procedure.
Study definitions:
Asymptomatic patient for COVID-19 was defined as a patient without any classic symptoms and no contact with probable/ confirmed COVID-19 cases in the last 15 days.
Patients are considered as COVID-19 positive if there is viral RNA amplification in real time - reverse transcriptase polymerase chain reaction (rt-PCR).
Molecular screening for COVID-19:
Laboratory testing for SARS-CoV-2 was made by rt-PCR detection of viral RNA in oropharyngeal/nasopharyngeal swabs. rt-PCR was performed in different laboratories approved by the Ministry of Health of Colombia and the National Institute of Health of Colombia.
Statistical analysis:
Descriptive statistics were used to gather and summarize the data. Results are reported as means. Analysis was performed with Stata 14 software (StataCorp). Continuous variables were expressed in mean ± standard deviation (SD) or median (min-max), while categorical data were expressed in number and frequency
Results
Demographic data:
A total of 1837 patients were included. From this sample, mean age was 49.5 years (range, 0-98 yr, standard deviation: 18.74), 933 (50.7%) were male and 904 (49.3%) were female. All the patients included in this sample denied having COVID-19 symptoms or contact with probable or confirmed cases in the last 15 days (Tabla 1).
From the 1837 patients, 104 rt-PCRs tested positive for SARS-CoV-2, leading to an incidence of 5.66% of identified asymptomatic patients. Mean age was 48 years (range, 3-86 yr). 52 out of the 104 patients were female (50%) and 52 patients were male (50%)(Tabla 2).
Table 2 Asymptomatic participants
| Asymptomatic participants | Patients n=104 |
|---|---|
| Gender | |
| Female | 52 (50%) |
| Male | 52 (50%) |
| Not operated | 14 (13.4%) |
| Operated | 90 (86.6%) |
| Surgery | 80 (76.9%) |
| Gastro | 10 (9.6%) |
| Mean days intervention | 45 Days |
| Mode for intervention day | 14 |
| Range of intervention weeks | 2-27 Weeks |
| Complications at 7 days | 0 |
| Mortality at 30 days | 0 |
Follow-up results:
No matter if the patient was positive or negative for CO-VID-19, follow-up was made on the 30th day after the procedure looking for adverse outcomes by calling our patients.
Until April 23st, 2020, 1822 patients were taken to surgery and responded to our 30th day follow-up. From those 1822 patients, 29 patients reported non-respiratory complications, including 8 surgical site infections that didn't require new surgical treatment. 14 patients reported respiratory symptoms after surgery, but none of these patients reported further testing to rule out COVID-19. 1 patient passed away in our follow-up, but the cause of death wasn't attributed to the surgery nor respiratory complications. 1 patient reported respiratory symptoms with a positive COVID-19 test, although the patient stated in the follow-up close contact with a positive case of COVID-19.
From the 104 asymptomatic COVID-19 patients detected in our study, 90 patients were able to be taken to surgery. No mortality nor respiratory complications were evidenced in the 30th day follow-up in asymptomatic COVID-19 patients. 14 patients were not taken to surgery, 10 referred personal reasons like fear of hospitalization in the pandemic and vacations, 2 have been operated in other institutions and 2 lose the follow up (Tabla 2).
Discussion
The COVID-19 pandemic has imposed many challenges to medical practice, being one of the most defiant invasive interventions and elective surgery, defined as invasive procedures that have no immediate indication due to a life-threatening emergency5.
It has been stated that taking a COVID-19 patient to surgery could accelerate and exacerbate disease progression of COVID-19, and also it could increase the risk of ICU hospitalization and mortality6,7, development of renal injury and myocarditis8 in comparison to patients without prior SARS-CoV-2 infection, not to mention the increased risk of transmission of health care providers due to high exposure and high risk activities such as airway management9. A number of researchers have evaluated the ideal timing of surgical intervention following SARS-CoV-2 infection a multicenter international cohort study involving 24 countries and 1128 patients, evaluated mortality and pulmonary complications in the first 30 days after surgery in COVID-19 patients, revealing that 24.8% of patients died in the first 30 days and shockingly, more than half of the sample (51.2%) developed pulmonary complications7. Afterwards, in march 2021 the COVIDSurg Collaborative group published an international and multicentre study with 140.231 participants, in which 3.127 patients were diagnosed with COVID-19 prior to surgery, the outcome showed that mortality was increased in participants being subjected to surgery within the first six weeks of diagnosis, especifically 0-2 weeks OR 4.1, 3-4 weeks OR 3.9 and 5-6 weeks OR 3.6, compared to participants without COVID-19 with an OR of 1.5, nevertheless after the seven weeks participants appeared to have the same mortality risk as the control group. Therefore the final recommendation was to perform any surgical intervention at least seven weeks after the diagnosis of SARS-CoV-2 infection10.
In this observational study, we found that 104 out of 1837 patients were identified as asymptomatic carriers of SARS-CoV-2 which reinforces the key role of molecular screening preceding any surgical intervention. Out of the total number of 104 patients, 90 were surgically intervened in a range of 2 to 27 weeks, within our protocol patients required at least a two-week recovery period to intervene, the predominant cause of further delay of the procedure was patient personal reasons such as holidays coming-up or fear of hospital stay in the midst of a pandemic. No complications were detected in the first 30 days after surgery in this group identified at follow-up. Nevertheless, our results are limited to our sample size.
Concerning the hematologic patients, we identified five bone marrow donors that were positive for SARS-CoV-2 rt-PCR. Malard et al reported that 52% of it's sample (18 patients) developed ARDS in the first 29 days after COVID-19 diagnosis, and from those 18 patients, 9 died11, which raises the concern of performing bone marrow biopsy in the pandemic. This being said, the European Society of Bone Marrow transplantation recommends having a negative rt-PCR before performing any transplant12.
To finalize, there is no clearness on viral shedding in the GI tract, although it has been shown viral shedding in stool specimens both in asymptomatic and symptomatic COVID-19 patients13, raising the question of possible transmission of COVID-19 when intervening the GI tract. Besides that, endoscopy has direct contact with the oropharyngeal mucosa, increasing the risk of inhalation of airborne droplets, making endoscopies a high risk procedure in the pandemic14. This is why various authors strongly suggest assessing COVID-19 status with both clinical and molecular testing15.
In Colombia, as of October 6th 2020, 862'158 patients have been diagnosed for COVID-19, including an astonishing 12.83% asymptomatic cases16, and according to our findings there is a 5.66% of asymptomatic participants, even so these statistics may not evidence reality due to the lack of diagnostic and surveillance capacity17.
As described before, there is a significant population of asymptomatic patients in Colombia without any contact with possible/confirmed COVID-19 patients, which raises the question of the necessity to perform screening tests in order to reduce exposure and risk of infection to health-care providers as well to reduce the risk of complications and mortality of patients. Performing rt-PCR for SARS-CoV-2 detection in non-urgent, elective surgeries and invasive procedures has been suggested by many organizations and authors such as Spanish Association of Surgeons, the Italian Society of Oncologic Surgeons, the American College of Surgeons, the European Society of Bone Marrow Transplantation and the Intercollegiate General Surgery Guidance on COVID-1 98,12,18-22. With accordance to our results, we also strongly suggest performing preoperative rt-PCR screening in asymptomatic patients undergoing invasive interventions, as well as consider that the minimum period required to reduce the possibility of complications and mortality rates after SARS-CoV-2 infection is two weeks, in contrast to the outcome of COVID Surg Collaborative group.
Limitations
Samples for rt-PCR for SARS-CoV-2 were processed and analyzed in multiple laboratories, even though they are approved by the Colombian Ministry of Health and National Health Institute, generating a possible lack of uniformity in the results.
Conclusions
We present our findings regarding the key role of molecular SARS-CoV-2 diagnosis prior to any surgical intervention, even in asymptomatic patients due to a 5.66% prevalence of positive rt-PCR participants without manifestations of COVID-19. These findings are extremely relevant in order to make the most responsible decisions in patient care. Asking for symptoms and close contact in the questionnaire does not replace rt-PCR screening, and safe screening will eventually decide if these invasive interventions can be postponed, or, if the benefit outweighs the risk. Also, We conclude that there needs to be further research regarding timing to invasive surgical intervention in patients with diagnosis of COVID-19, in order to consider reduce the waiting times of patients in need of a invasive procedure in context of asymptomatic SARS-CoV-2 infection, considering that, the minimum planned delay in our protocol was two weeks and we found no complications or mortality in the first 30 days afterwards.