Lettuce (Lactuca sativa) is a green leaf vegetable cultivated all around the world; the plant was first cultivated by the ancient Egyptians who considered it sacred, which then rapidly spread to Greece, Rome, and the rest of Europe (Hart, 2005). The leaves are used in a great variety of foods, including soups, juices, salads, wraps, sandwiches, and processed meals (Kim et al., 2018). Lettuces are diverse in color, varying from green to purple; they also have different shapes, surfaces, margins, and leaf textures (Mou, 2008). According to Lebeda et al. (2007), at least seven different morphotypes of lettuce are cultivated, (1) Butterhead (var. capitata L. nidus tenerrima Helm) (Kopfsalat, Laitue pommé), (2) Crisphead (var. capitata L. nidus jäggeri Helm) (Iceberg type, Eissalat, Batavia), (3) Cos (var. longifolia Lam., var. romana Hort. in Bailey) (Römischer Salat, Laitue romaine), (4) Cutting (var. acephala Alef., syn. var. secalina Alef., syn. var. crispa L.) (Gathering lettuce, Loose-leaf, Picking lettuce, Schnittsalat, Laitue à couper), (5) Stalk (var. angustana Irish ex Bremer, syn. var. asparagina Bailey, syn. L. augustana Hort. in Vilm.) (Stem lettuce, Stengelsalat, Laitue-tige), (6) Latin (no scientific name), and (7) Oilseed lettuce (Křístková et al., 2008). Currently, the main world producers are China (13,659,250 t year-1), The United States (3,791,110 t year-1), India (1,097,102 t year-1), Spain (902,941 t year-1), and Italy (709,373 t year-1). Altogether, sum for 20,159,776 t year-1 (FAO, 2017; Shi et al., 2015).
Different agricultural systems have been used to cultivate lettuces. There are four widely used systems for lettuce cultivation, conventional and organic farming in open field, hydroponic cultivation in a controlled environment, and soil cultivation in greenhouses (Henz and Suinaga, 2009). These four systems differ in the environment, cultivated area, yield, and, more recently, in physiological properties, especially in lettuce (Souza et al., 2019). Hydroponic systems have many advantages compared to other culture systems such as absence of soil-borne pathogens, higher yields, superior quality, precise nutrition control, resistance to traditional agricultural practices (weeding, spray watering, and tilling), enhancement of early yield in crops planted during the cold season, reduction of fertilizer application, and elimination of nutrient leaching into the environment (FAO, 2013; Sharma et al., 2018). In terms of productivity, it varies from 3.9 kg m-2 year-1 with the conventional soil system to 41 kg m-2 year-1 with the hydroponic system (Barbosa et al., 2015), gaining popularity between farmers and producers for the high profits.
Lettuce is an excellent source of vitamins A and K as well as having a good amount of C, some B vitamins, and other phytonutrients (Bunning and Kendall, 2012). Several studies have shown benefits of the consumption of lettuces; this vegetable contains secondary metabolites such as phenolic compounds, fIavonols, phenolic acids, carotenoids, ascorbic acid, and folate, which can enhance health promotion (Sofo et al., 2016; López et al., 2014). In addition, red leaf lettuce accumulates a significant amount of anthocyanin pigments, which have antioxidant properties (Kong et al., 2003). These phenolic compounds produce antioxidant activity and provide free radical scavenging ability. Polyphenols can prevent cancer and cardiovascular diseases (Manach et al., 2004; Hooper and Cassidy, 2006). Adesso et al. (2016) reported that lettuce leaf extracts could reduce both the inflammatory and oxidative stress in murine monocyte-macrophage cells by decreasing reactive oxygen species and nitric oxide release. In this sense, quercetin seems to play an important role, and it is probably responsible for antioxidant and anti-inflammatory properties. The concentration of quercetin in lettuce is higher than most of the commonly eaten food. Nishimuro et al. (2015) found a concentration of quercetin of 30.6 mg 100g-1 of Fresh Weight (FW) in red leaf lettuce in Japan. Gan and Azrina (2016) reported a range for total flavonoid content between 2.28 and 21.96 mgQE 100g-1 FW. However, changes in the light might affect physiological and biochemical processes, metabolite profiles, and lettuce quality (Ntsoane et al., 2016). Sivakumar et al. (2017) showed that the accumulation of phytochemicals during lettuce production depended on many factors, such as light quality and quantity, types of varieties or cultivars, growing seasons, and metabolic factors. Other authors have suggested that color shade nets affect antioxidant properties in lettuce during the growing period, such as carotenoids, chlorophyll, phenols, and morphological properties (Ilić et al., 2017). Also, nutritional factors could affect antioxidants, Sofo et al. (2016) compared the total phenolic acids and flavonols in conventional and organic agronomical systems and found values significantly higher in conventional systems.
In Colombia, lettuce production is mainly carried out in open fields. However, new technologies like hydroponics in greenhouses have been incorporated in recent years. On the contrary, the cultivated area is about 3,714 ha, production is all year long due to the tropical conditions, and the most cultivated lettuce types are Iceberg, green leaf, and red leaf. The per capita consumption of lettuce in Colombia is estimated at 4.8 kg year-1 (MinSalud, 2012), which is low compared to 11.4 kg year-1 in the United States (USDA, 2016). However, Colombia exports fresh lettuces to The Netherland Antilles, Panama, The USA, and Canada (MADR, 2014). In addition, the country is planning to expand the cultivated area up to 5,414 ha, due to lettuce is considered a potential exportation product of the national horticultural plan that will be effective in 2020 (MADR, 2006).
According to the aforementioned, this study aimed to compare the antioxidant contents of green and red leaf lettuces produced in two hydroponic systems, Aeroponic and Run-to-Waste, in a greenhouse constructed with Guadua wood (Guadua spp) and covered with UV filter polyethylene film; against the natural antioxidant content of lettuces conventionally cultivated in open fields by traditional Colombian farmers.
MATERIALS AND METHODS
Location site
The experiment was performed at Universidad Catolica de Oriente - Rionegro (679868.04 m N; 459607.51 m E (WGS84 System)). Aeroponic and Run-to-Waste hydroponic systems were carried out in a 300 m2 greenhouse covered with plastic walls and a UV polyethylene filter roof film with 2% transmittance 290-340 nm as a sunroof. A 500 m2 open field next to the greenhouse was used for conventional cultivation during the experimentation.
Plant material and culture systems
Lettuce plants were obtained from certified seeds bought from a local dealer (Sakata.co Instituto Colombiano Agropecuario (ICA), under law 3168 of 2015) and corresponded with the green and red leaf lettuce varieties. The seeds had 85% of germination, did not contain impurities, and were germinated into trays of 138 cells with peat as substrate. After three weeks, 10 cm lettuce plantlets were transplanted into the hydroponic system and open field cultivation.
Aeroponic and run to waste systems
The aeroponic system consisted of a 100 m2 by 1 m tall wooden bed wrapped with black polyethylene film around the sides, and white polystyrene on the top. The top cover had holes every 20 cm filled with 2×8 cm (diameter and length) mesh pot nets. Under the cover, an irrigation system composed of two PVC pipelines of 2.54 cm diameter with mist nozzles every 40 cm, supplied the nutrients. The system was connected to an electric water pump (Pedrollo PKM60 of 0.5 HP), which provided the nutrients by mist to the lettuce roots. The irrigation program lasted 1 minute every 3 min, for a 10-hour daily period with recirculation.
The run-to-waste system consisted of 4-liters black plastic pots hung in a row by wires - 1 m above the fIoor. Each run-to-waste pot was filled with 4 kg of a substrate composed of rice husks, coconut chips, and river sand at a ratio of 45:45:10 and planted every 30 cm. The irrigation system was composed of a 1.25 cm diameter PVC pipe and irrigation drippers connected every 20 cm. The system was open with no recirculation and was connected to the same nutrient solution dispenser as that of the Aeroponic system. An electric water pump (0.5 HP Electric Line) supplied the nutritive solution to the system for 5 min every hour for 10 hours daily.
In both systems, the photoperiod was 12 hours of natural light. Luminosity (Lx) was measured inside and outside the greenhouse using the ZigBee devices that was developed by Gutierrez et al. (2019). Figure 1 shows the description of nutrient fIow in both systems.
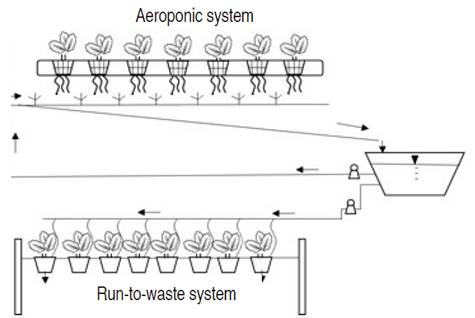
Figure 1 Aeroponic and Run-to-Waste systems. Both hydroponic systems were built in plastic and wood at the height of 1 m above theflfIoor; in the Run-to-waste system, pots were hung with wires. The nutrient solution was the same for both hydroponic systems. Arrows show the fIow direction.
A total of 60 green and red leaf lettuces were planted into the aeroponic (n=30) and run-to-waste system (n=30), distributed to 15 green leaf and 15 red leaf plants, respectively. Run-to-waste lettuces were planted in pots and repositioned every week to guarantee the same environmental conditions as the greenhouse. The aeroponic system did not allow plant rotation.
Nutrient solution
A Steiner salt solution modified with Molybdenum was used in both cases. Nutrient solution was prepared with the concentrations proposed by Stenier (1984), that consisted of Nitrogen (N) 168 mg L-1, Phosphorus (P) 31 mg L-1, Potassium (K) 273 mg L-1, Calcium (Ca) 180 mg L-1, Magnesium (Mg) 48 mg L-1, Sulphur (S) 336 mg L-1, Iron (Fe) 4 mg L-1, Copper (Cu) 0.02 mg L-1, Zinc (Zn) 0.11 mg L-1, Manganese (Mn) 0.62 mg L-1, Boron (B) 0.44 mg L-1. Additionally, Molybdenum (Mo) was added in a concentration of 0.2 mg L-1, according to Cooper nutrient solution (Cooper, 1988; Trejo-Tellez and Gomez-Merino, 2012). The pH of the nutrient solution was set in a range of 5.7 - 6.2, using HCl (3 M) or NaOH (3 M) according to the nutrient solution needs. The conductivity was set at 1.5±0.1 mS cm-1 and maintained during the entire experiment.
Conventional cultivation system
The soil was prepared according to traditional Colombian farmer plant cultivation. The conventional open field cultivation consisted of soil loosening using manual hoes, collecting and burying the organic coverage between the rows to fertilize naturally, and the addition of dolomitic lime at a concentration of 25 g per plant to adjust the soil’s pH to 5.8. One week later, inorganic fertilizing was done using commercial 15-15-15 N-P-K at a concentration of 15 g per plant. Another week later, 15 green leaf and 15 red leaf lettuce plantlets were transplanted in the rows separated 30 cm and irrigated every two days during the cultivation time. One more final fertilization was performed after 20 days of plantlets transplantation and consisted of 50 g per plant of the same commercial fertilizer, spread around each plant.
Sample Collection and preparation
Lettuce samples were taken after 45 and 90 days from both the hydroponic and conventional cultivation systems. Samples were collected when lettuces button leaves reached senescence and were composed of bulk of four (4) lettuces per sample, keeping both varieties (green and red). Due to the differences in harvesting time in lettuces, 45 days in hydroponic systems, and 90 days in the conventional system, 30 samples (that corresponded to all treatments) were taken after 45 days and 10 more samples from the conventional system after 90 days. Samples were refrigerated at 4 °C for posterior antioxidant analysis. In Table 1, a description of samples is shown.
Leaf Extract Preparation
Samples were rinsed with water. Later, 1±0.1 g of leaves were placed in an extracting solution consisting of 25 mL of acidulated methanol (HCl 1%). Then, they were mixed individually with a homogenizer T 25 digital ULTRA-TURRAX®. The extracts were filtered through 0.20 µm filters and immediately stored at -20 °C.
Antioxidant Activity
FRAP (Ferric Reducing Ability of Plasma) analysis. The reduction ability was measured using the methods of Benzie and Strain (1996). Aliquots of 50 µL were mixed with 50 µL of acetate buffer pH 3.6, and 900 µL of FRAP solution (FeCl3, TPTZ (Tripyridyl-s-triazine) in HCl 40 mM, and the buffer at 1:1:10 proportion). The increase of absorbance was measured at 590 nm in a spectrophotometer (PG instruments T80). The FRAP values were expressed as milligrams of Ascorbic Acid Equivalent per 100 g of lettuce (mgAAE 100g-1).
DPPH analysis. The ability of leaf extracts to scavenge the DPPH (2,2-diphenyl-1-picrylhydrazyl) radical was measured using the methods of Brand-Williams et al. (1995). Aliquots (10 µL) of leaf extract were added to 990 µL of the DPPH standard solution. The absorbance was determined at 517 nm after 30 minutes. The results were expressed as millimoles of Trolox Equivalent per 100 g of lettuce (mmolTE 100g-1).
ABTS (2,2’-azino-bis (3-ethylbenzothiazoline-6- sulphonic acid)) analysis. Radical scavenger activity against the stable radical ABTS was measured according to Mesa-Vanegas et al. (2015). The reaction was composed of 10 µL of sample and 990 µL of standard ABTS. The absorbance was determined at 720 nm after 30 minutes. The results were expressed as millimoles of Trolox Equivalents per 100g of lettuce (mmolTE 100g-1).
Total phenols analysis. Lettuce leaf extracts (50 µL) were mixed with 125 µL of Folin-Ciocalteu reagent, 425 µL of H2O and 400 µL of CaCO3 (7.1%). The absorbance was measured at 760 nm after 30 minutes in the dark. The results were expressed as milligrams of Gallic Acid Equivalents per 100 g of lettuce (GAE 100g-1). This procedure is described by Singleton and Rossi (1965).
Total Anthocyanins analysis. For anthocyanin measurement, a differential pH method was used. The absorbance was measured at 530 nm and 700 nm with buffers of pH 1 and 4.5. The expression A=[(A530-A700) pH 1.0- (A530-A700) pH 4.5] was used for calculating the total anthocyanins. The results were expressed as milligrams of cyanidin-3-glucoside per 100 g of lettuce (mgC3G 100g-1) (Zambrano- Moreno et al., 2015).
Statistical Analysis
A two way-ANOVA factorial design was performed to test the effect of three levels of cultivation (conventional, run to waste and aeroponics), two levels of lettuce plants (green and red) and their interaction with antioxidant activities (DPPH, ABTS, FRAP, total phenols, and total Anthocyanins analysis). Data normality was tested using the Shapiro-Wilk test (P>0.05) and homoscedasticity with the Levene test (P>0.05). Once these assumptions were verified, an analysis of variance (ANOVA) and Tukey test using Honest Significant Difference (HSD) was used to establish the statistical differences between cultures and plants. The confidence level used for ANOVA analysis was 95%.
RESULTS AND DISCUSSION
FRAP analysis
The results of antioxidant activity measured by FRAP (reduced capacity) showed the highest values in red lettuce compared to green lettuce. Two-way ANOVA determined the effects of different cultivation systems and plants on FRAP. In this sense, the cultivation factor alone and interaction cultivation:plant did not differentiate in FRAP (mgAAE 100g-1) (P=0.35, 0.37). The plant factor showed differences between the green and red lettuces (P=0.00). All red lettuces showed higher FRAP concentration compared to the green ones. The red lettuce at 90 days of conventional culture harvesting exhibited the highest FRAP value (655.3±82.6 mgAAE 100g-1).
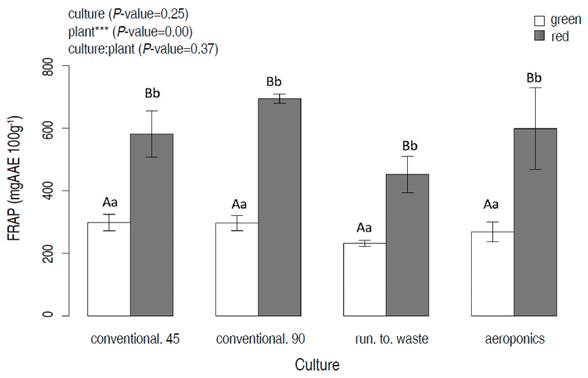
Figure 2 FRAP of two lettuce plants (green and red lettuces), as a function of cultivation systems (conventional at 45 and 90 days, run to waste and aeroponics). Different lowercase letters indicate significant differences between cultivation systems in the same plant; different capital letters indicate significant differences between plants at the same value of cultivar (P<0.05; Tukey HSD).
The results suggest that reductive capacity was not affected by system cultivation; however, they were affected by plant type. FRAP analysis indicated that red lettuce had more reductive capacity compared with green lettuce in all the cultivation systems. These results were compared with other studies, showing similar results in FRAP analysis (Gan and Azrina, 2016; Ozgen and Sekerci, 2011; Mampholo et al., 2016). So far, there are not studies that make this comparison with hydroponics.
DPPH analysis
The DPPH technique showed significant statistical differences between plants, cultivation systems, and the interaction plant:culture. Green lettuce presented the highest value in conventional cultivation at 45 days (20.7±5.6 mmolTE 100g-1). Conventional cultivation showed higher DPPH values than the hydroponic systems for both green and red lettuce varieties (Figure 3).
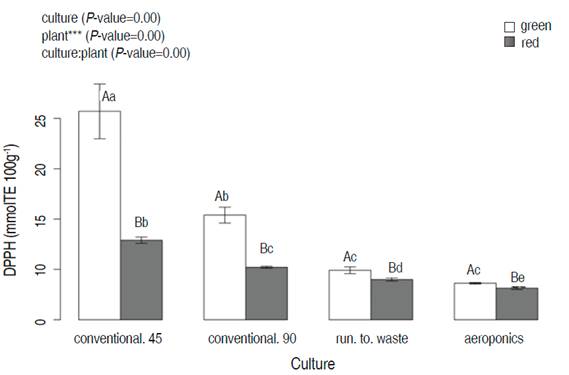
Figure 3 The DPPH of two lettuce plants (green and red after 45 days of harvesting time) as a function of different cultivation systems (conventional, run to waste, and aeroponics). Different lowercase letters indicate significant differences between cultivation systems in the same plant; different capital letters indicate significant differences between plants at the same value of cultivar (P<0.05; Tukey HSD).
At 90 days, the antioxidant activity decreased in both green and red plants (green 10.0±1.8 and red 7.9±0.7 mmolTE 100g-1). This change might be explained by environmental conditions that infIuenced the phenolic content and antioxidant activity (Liu et al., 2007).
For the DPPH technique, Pellegrini et al. (2003) reported a concentration of DPPH of 0.133 mmolTE 100g-1 FW for green lettuce, and Chon et al. (2012) showed 59.87 µmTE g-1 of Dry Weight (DW) in Seoul red lettuce cultivar. These values are lower than reported in our results.
ABTS analysis
In the case of ABTS technique, cultivation systems did not show significant differences between means. On the other hand, the red and green lettuces presented significant differences in all cultivation systems evaluated when means were compared for the Tukey test (P=0) (Figure 4). Red lettuces exhibited the greatest value at 45 days in conventional cultivation (17.8±6.9 mmolTE 100g-1).
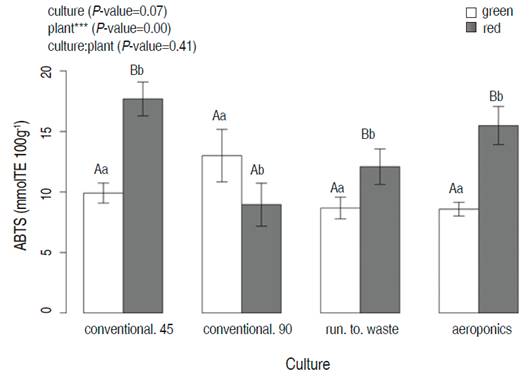
Figure 4 The ABTS for green and red lettuces after 45 days harvesting time as a function of three cultivation systems (conventional, run to waste, and aeroponics). Different lowercase letters indicate significant differences between cultivation systems at the same plant; the capital letters indicate significant differences between plants at the same value of cultivation systems (P<0.05; Tukey HSD)
The antioxidant activity measured by ABTS decreased in red lettuce between 45 and 90 days. The ABTS results of this study were higher than the ones found by Mampholo et al. (2016), who reported 2.73 mgTE 100g-1 FW (0.011 mmolTE 100g-1 FW) for red lettuce.
Moreover, the DPPH values were lower than the ABTS. This could be explained by the stereochemical differences of both radicals. On the one hand, the ABTS is a cationic radical that is reduced by antioxidants that might transfer electrons. The ABTS is a fIat molecule of easy access for antioxidants. On the other hand, the DPPH has an active site between two voluminous groups perpendicular to each other, which allows only a certain type of small antioxidant, or those with more exposed active sites (Huang et al., 2005).
Total phenols analysis
Like FRAP, total phenols showed differences between means for green and red lettuces. However, the culture factor did not present significant differences (P=0.13). The interaction between culture and plant showed significant differences. The higher value was exhibited by red lettuce in conventional cultivation at 45 days (680.2±69.3 mgGAE 100g-1).
Total Phenols were higher than the reported by Gan and Azrina (2016), who found the total phenolic content of 30.39 mgGAE 100g-1 FW of green lettuce and 76.05 mgGAE 100g-1 FW of red lettuce. Also, Sofo et al. (2016) reported a value of 199 mgGAE 100g-1 FW for the green cultivar “Maravilla de Verano.” Another author reported higher values for lollo rosso lettuce (490 mgGAE g-1) in tropical conditions (Vargas-Arcila et al., 2017).
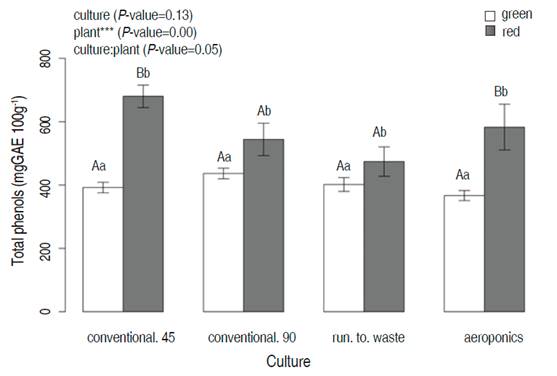
Figure 5 Total phenols of green and red lettuce after 45 days harvesting time as a function of different cultivation systems (conventional, run to waste, and aeroponics). Different lowercase letters indicate significant differences between cultivation systems in the same plant; different capital letters indicate significant differences between plants at the same value of cultivar (P<0.05; Tukey HSD)
Total Anthocyanins analysis
Anthocyanins were analyzed only in red lettuce (RA, RR, RC45, and RC90), due to green lettuce does not present any content of these metabolites. The cultivation systems exhibited significant statistical differences for the Tukey test (P=0.00) (Figure 6). The conventional cultivation system presented the highest anthocyanins concentration at 45 days with a mean of 126.2±6.9 mgC3G 100g-1 FW, followed by the conventional system at 90 days (90.5 mgC3G 100g-1). A decrease in anthocyanin could be observed between 45 and 90 days. The aeroponic system with 87.5 mgC3G 100g-1 FW; and Run-to-Waste system with 60.1 mgC3G 100g-1 FW.
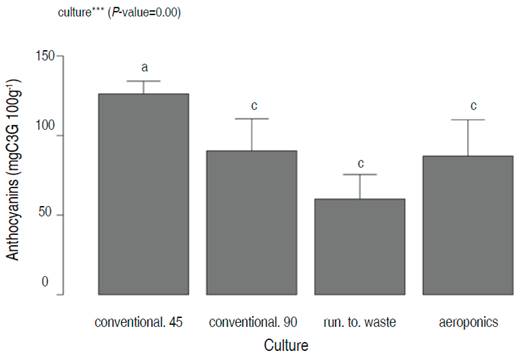
Figure 6 The anthocyanin analysis for red lettuce after 45 days of harvesting time. Different letters indicate statistical differences between cultivation systems (P=0.00, Tukey HSD).
The difference in anthocyanin concentration between 45 and 90 days might be due to the anthocyanin converting into chloroplast and light conditions such as quality and intensity affecting chloroplast-located level metabolites in plants (Chen et al., 2018). Other authors reported that light intensity infIuenced antioxidant contents at a higher light intensity, higher amounts of antioxidants (Zhou et al., 2009; Gazula et al., 2007).
Ozgen and Sekerci (2011) reported 0.628 mgC3G 100g-1 FW for similar lettuce varieties. These results were lower than the Anthocyanins of the samples determined in this study.
The correlation between antioxidant techniques
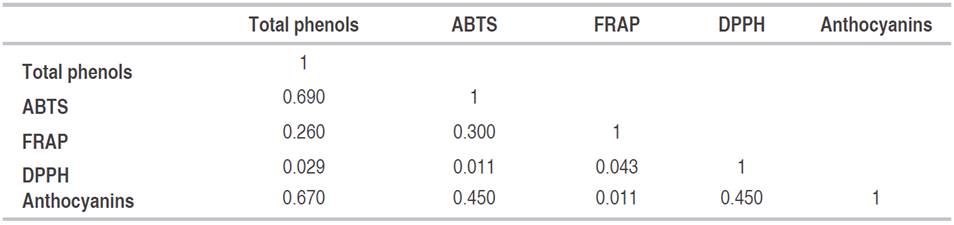
A correlation was performed with the results shown above. Antioxidant activity responded positively to phenolic compounds. The highest correlation obtained was between total phenols and ABTS of all treatment with a value of 0.69 (Table 2). Some authors have reported high correlations between total phenols and antioxidant capacity and concluded that the higher the concentration of antioxidants, the lower the amount of remaining DPPH, and the higher the free radical scavenging activity (Paixão et al., 2007; Kim et al., 2018).
The high correlation between ABTS and total phenols could be explained by the presence of compounds such as p-coumaric acid, caffeic acid, quercetin, and 2,4 dihydroxybenzoic acid reported in lettuce by Tiveron et al. (2012). These phenol compounds show high antioxidant activity. Recently, Kim et al. (2018) found that red leaf cultivars were rich in carotenoids, cyanidin, polyunsaturated fatty acids (PUFAs, primarily in the form of α-linolenic and linoleic acid) total phenolic contents (TPC), and antioxidant potential.
Additionally, in this study, some correlations were weak and positive in contrast with other authors (Mello and Quadros, 2014), who showed negative correlations between phenols and antioxidant activity in extracts from Camellis Sinensis. This could be explained due to some secondary metabolites do not exhibit antioxidant activity at harvest time. Different authors explained that during senescence, antioxidants in plant leaves might vary according to different traits and maturity genes related to lateness and fIowering (Dissanayaka et al., 2016; Li et al., 2017).
The DPPH and Anthocyanin analysis showed significant statistical differences for green leaf and red leaf lettuces grown in greenhouses in hydroponic systems, against lettuces grown in conventional open field cultivation systems. On the contrary, FRAP, Total Phenols, and ABTS did not show differences between the cultivation systems, but between lettuce cultivars. FRAP, Total Phenols, and ABTS analysis showed higher concentrations for red leaf lettuce than for green leaf lettuce. The DPPH was the only analysis where green leaf lettuce exhibited higher potential antioxidants than red lettuces. As for the anthocyanin analysis, green leaf lettuces did not have any presence of those metabolites, but it was found high amounts of anthocyanins in red leaf lettuce, even higher than reported by different authors as stated above. In addition, it was found a higher amount of anthocyanins in red leaf lettuce samples from 45 days than 90 days, indicating that this compound could become degraded with time. Further research should focus on anthocyanins kinetics and evaluate more cultivation systems to determine the optimal harvesting time for red leaf lettuces to obtain functional foods.
Different authors have reported concentrations of phenolic compounds higher in red leaf than green leaf cultivars (Nicolle et al., 2004). In iceberg and romaine lettuces (green leaf types), the main phenolics are caffeic acid derivatives. Llorach et al. (2008) showed red oak leaf and lollo rosso had higher concentrations of caffeic acid derivatives, while anthocyanins can only be found in red leaf types. The red leaf types had a higher concentration of fIavones and higher antioxidant activity than green leaf cultivars (Llorach et al., 2008).
Light infIuence in antioxidant activity
According to the results above, lettuces grown in hydroponic systems in greenhouses had lower antioxidant concentrations for DPPH and anthocyanins than those conventionally cultivated in open fields by Colombian farmers. This indicates that greenhouses covered with UV filter polyethylene film have a detrimental effect on antioxidant content in lettuces even in tropical areas with high radiation all year long, like Colombia. The luminosity inside the greenhouse registered an average value of 200 Luxes, while outside it registered a value of 4,000 Luxes for a 10-hour daily photoperiod.
Hipol and Sese (2014) revealed that the activities of the enzymatic antioxidants superoxide dismutase (SOD) and catalase (CAT) increased according to light intensity. Some authors have studied the infIuence of plastic covers on vegetable and fruit production. Holeman et al. (2017) reported differences in cherry tomato yield in greenhouses with different plastic covers. Plastic anti- UV film and thermo-refIective shading screen showed lower fruit quantity and average fruit yield than diffusive plastic film. Alsadon et al. (2016) tested three kinds of plastic films to cover cucumber cultivation greenhouses with different transmittances (P1-46.75, P2-70.47, and P3- 56.43%). The authors found that growth indices for plants in P1 were the highest values for vegetative growth, fruit, and yield traits as compared with P2 and P3. Cemek et al. (2006) found higher yields in aubergine plants when culture under greenhouses with double layers of polyethylene (D-Poly) rooves, than UV+PE, IR+PE, and PE films. Ordidge et al. (2010), who showed that in red lettuce Lollo Rosso, total phenolics, anthocyanin, luteolin, and quercetin levels were all raised by changing from a UV blocking film to a low UV transparency film to a high UV transparency film.
CONCLUSIONS
UV filter polyethylene film used in greenhouse construction has a detrimental effect on antioxidant production in lettuces. This is confirmed by the DPPH and anthocyanin analyses, which indicate the high antioxidant capacity in lettuces cultivated in the conventional system used by Colombian farmers (after 45 days). The phenol contents did not show differences in the cultivation systems used, suggesting that UV filter might not affect this technique. This is confirmed by the ABTS results obtained in this experiment. Finally, red leaf lettuces have more antioxidant contents no matter the cultivation system used, and probably are more healthy food than green leaf lettuces.