INTRODUCTION
Traditional probiotics refer to a probiotic having viable and active cells. However, it is not enough that the cells are viable, they must exert health benefits to the consumer by lowering gut pH, producing vitamins, enzymes and antimicrobial compounds, balancing and rebuilding the intestinal microbiota after infections in the digestive tract, reducing the serum cholesterol level, regulating the immune system, exhibiting antioxidant activity, reducing poor lactose adsorption, and producing substances such as bacteriocins, hydrogen peroxide, butyric acid, lactic acid and acetic acid to mention a few (Zendeboodi et al., 2020). The term true probiotic is synonymous with traditional probiotic, considering all these properties (Guidelines for the Evaluation of Probiotics in Food, 2002). Castañeda-Guillot (2021) defined and compared the characteristics of traditional probiotics and new probiotics, describing mainly the use of traditional probiotics and their metabolites as binders, flavorings and preservatives for the food industry, which can be isolated from different substrates from the gastrointestinal tract of different animals from different ecosystems. Unlike traditional probiotics, new probiotics are characterized because their origin is primarily from the human gastrointestinal tract, and their use is mostly focused on the treatment of specific diseases, acting as biotherapeutics.
The objective of this review is to know the main characteristics of the microorganisms used as traditional probiotics and new probiotics.
Traditional probiotics
The word probiotic derives from the Greek pro (in favor) and biotic (life), and this term has undergone various changes over the years. The International Scientific Association for Probiotics and Prebiotics (ISAPP) defined them as "live microorganisms and their metabolites that when administered in an adequate manner and in adequate amounts provide a health benefit to the host" (Isolauri et al., 2001; Prats Capote, 2007). Finally, two more elements were added which state that probiotics have the capacity to stimulate bacterial growth or development, and to regulate their metabolic activity (Prieto, 2010). Among the main microorganisms used as probiotics are lactic acid bacteria and bifidobacteria, but some yeasts such as Sacharomyces boulardii have also been reported (Carnicé, 2006; Gómez, 2019). In this review we will focus on lactic acid bacteria (LAB) as probiotics.
Acid bacteria lactic acid bacteria
LAB have been used in food preservation for many years because they produce different compounds as a result of their metabolism, such as organic acids, hydrogen peroxide and bacteriocins. Likewise, their role as probiotics has also been studied (Agudelo et al., 2015; Gorbeña and Sáenz, 2008).
LAB are a diverse group of Gram-positive microorganisms (cocci or bacilli), with a length (depending on the genus) of 0.5 - 0.8 µm (Carr et al., 2002; Ibrahim and Raman, 2021), that are catalase negative (although in some cases some pseudo catalase can be found), and oxidase and benzidine negative (Vázquez et al., 2009). They are facultative anaerobes, do not form spores, lack cytochromes, are not toxigenic or pathogenic, and are tolerant to acidity, although some can grow at a pH of 9.6. They also grow at different temperatures and can be classified as mesophilic (optimum growth temperature of 20 to 25 °C) and thermophilic (temperature of 40 to 45 °C) (Parra Huertas, 2010). Some have the ability to be aero-tolerant and grow in the presence of oxygen (McKee and McKee, 2016). LAB synthesize ATP through carbohydrate fermentation, and are divided into two large homofermentative and heterofermentative groups based on their biochemical characteristics. Among the metabolites produced by LAB are lactic acid, as well as other acids in smaller proportions such as propionic, acetic, citric, succinic, and formic acids, in addition to hydrogen peroxide, diacetyl, acetaldehyde, reuterin, and bacteriocins (Heredia et al., 2017). Some LAB produces carboxylic acids (Kaiting, 2021).
Criteria for considering a probiotic strain
Among the main criteria for considering a LAB strain to be probiotic are growth at a pH below 4.0, exert control over pathogenic bacteria, survive in the gastrointestinal tract, tolerate bile salts, an ability to adhere to intestinal mucus and epithelial cells, and display co-aggregation and self-aggregation (Ramírez-Chavarín et al., 2013), in addition to not having antibiotic resistance genes, immunostimulation without proinflammatory effect, displaying resistance to phages, and having antimutagenic and anticarcinogenic properties (Rondon et al., 2015).
Beneficial effect of probiotics
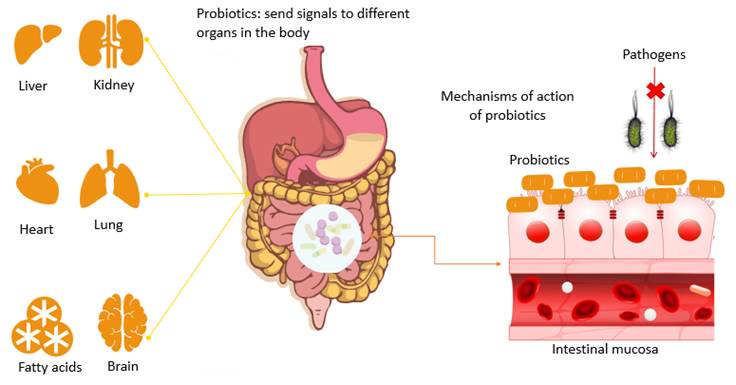
Probiotics are responsible for mediating a wide variety of health problems, as they are involved in regulating intestinal homeostasis, suppressing pro-carcinogenic enzymatic activities, interfering with the ability of pathogenic bacteria to infect the intestinal mucosa, improving nutrient bioavailability, reducing symptoms of lactose intolerance, positively influencing the urogenital flora, reducing infections in the gastrointestinal tract, and improving the regulation of intestinal motility (Cunningham et al., 2021; Veiga et al., 2020). Probiotics establish competition for both nutrients and for adherence sites of digestive tract cells with other (pathogenic) bacteria and stimulate the local and systemic immune system by activating macrophages and elevating immunoglobulin concentrations (Prats Capote, 2007).
Mechanisms of action involved in probiotics
The mechanisms of action that give probiotics the ability to provide these benefits to humans are still largely unknown, but may involve modification of intestinal pH (Rondon et al., 2015), antagonism of pathogens through the production of antimicrobial compounds, competition for binding sites and receptors, competition for available nutrients and growth factors by stimulating immunomodulatory cells, increased erythrocyte regeneration, and production of short-chain fatty acids and regulation of gastrointestinal disorder (Cunningham et al., 2021; Figure 1).
Patents on traditional probiotics
There are several patents on the use of traditional probiotics (Table 1).
Table 1 Some patents on the use of traditional probiotics (Boletín Tecnológico: Alimentos funcionales con probióticos, bancos de patentes SIC, 2014).
| Patent number | Year | Title | Applicants |
| EP 2878204 | 2019 | Pectin-containing sour milk drink and method for its production. | Nakano Masatoshi; Nihei Daichi; Kobayashi Yukiko; Rolin Claus; Ushiyama Soko and Mamiya Hiroyuki |
| MAT-MX-2001688 | 2018 | Enterogermina | Sanofi-Aventis de México S.A. de C.V. |
| EP 1884566 | 2018 | Fermented substance with lactic acid bacteria and fermented dairy food product containing it. | Ogasawara Nobuhiroc; Ishii Mayumic; Yoshikawa Masakic; Kudo Tatsuyukic; Akahoshi Ryoishic; Matsui Akihisac; Mizusawa, Susumuc |
| EP 2548948 | 2018 | New lactobacillus classified as Lactobacillus plantarum and its use. | Iino Tohru; Masuoka Norie; Ishikawa Fumiyasu; Yoshimura Koichi and Hayashida Eiji |
| EP 2471544 | 2016 | Lactobacillus casei for reducing the risk of developing cancer. | Toi Masakazu and Ohashi Yasuo |
| EN 2255730 | 2006 | Topical use of probiotic bacillus spores to prevent or control microbial infections. | Ganeden Biotech Inc. |
| ES2243697 | 2005 | Combination of probiotics. | Mayra-Makinen Annika; Suomalainen Tarja; Vaarala Outi |
| MXPA01007144 | 2002 | Use of Lactobacillus salivarius. | Collins John Kevin; Entpr Ireland doing business |
| ES2164299 | 2002 | Pet food containing probiotics. | Cavadini Christof; Ballevre Olivier; Gaier Walter; Nestle |
| ES2176338 | 2002 | Probiotic Compositions. | Brown Ian; Mcnaught Kenneth J; Ganly Robert N; Conway Patricia Lynne; Evans Anthony John; Topping David Lloyd |
New Probiotics
New probiotics are also known as biotherapeutic products that contain live microorganisms, such as bacteria and yeast, and are applicable to the prevention, treatment or cure of human diseases. New probiotics are not considered vaccines, as they are microorganisms isolated mainly from the human intestinal microbiota. To be considered as new probiotics, certain characteristics must be met that are more stringent than those of a traditional probiotic, as they represent a part of the human intestinal microbiome that can be cultivated outside the intestine and offer physiological functions that are not obtained directly by bifidobacteria or lactobacillus, such as the production of butyrate, propionate and other bioactive compounds (Kirmiz et al., 2020).
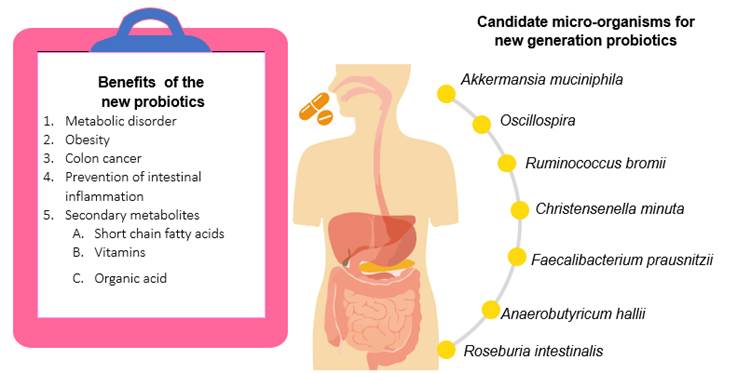
New probiotics can target specific health problems and may have beneficial effects in the treatment of diseases such as hypercholesterolemia, hypertension, non-alcoholic fatty liver disease, diabetes, and obesity. They produce metabolites beneficial to host health such as short-chain fatty acids, acetate, propionate and succinate. The effects of some novel probiotics and some diseases can be treated by such an approach (Figure 2); key species provide protection against pathogenic bacteria (Kumari et al., 2021).
Characteristics of new probiotics
For a probiotic to be considered new, it must meet certain characteristics, which are listed in Table 2.
Table 2 Comparative table between traditional and new probiotics (Modified from Castañeda-Guillot, 2019; Castañeda-Guillot, 2021; Martin and Langella, 2019; Tzu-Lung et al., 2019).
| Traditional probiotics | New probiotics |
|---|---|
| Isolated from variable sources, from human intestine and fermented foods, to plants and other ecosystems. | Isolated mainly from the indigenous human intestinal microbiome. |
| Used for different processes, in medical treatments, and in the production of fermented and enriched foods. | Focused on the treatment of specific and systemic diseases, regulation of inflammation, metabolic diseases, obesity, help in the production of vitamins of the host, increase the immune system, a single strain can help in different diseases. |
| Used as additives, binders, flavorings, food preservatives, starter cultures for fermented products, and as treatments for some specific diseases. | Development of specific drugs, production of secondary metabolites such as vitamins. They are divided into immunobiotics, psychobiotics, oncobiotics and pharmabiotics. Functional components such as paraprobiotics, postbiotics, probioceuticals or probiotaceuticals are in development. |
Classification of new probiotics
Among the new probiotics we can mention immunobiotics, psychobiotics, oncobiotics, pharmabiotics, paraprobiotics, postbiotics, probioceuticals and pseudoprobiotics (Martin and Langella, 2019). The characteristics of each of these are described below.
Immunobiotics
Defined as probiotic microorganisms with favorable immunological action for the organism. Immunobiotics increase the humoral immune response and promote better functioning of the intestinal barrier, stimulate resistance to pathogenic organisms and decrease the hypersensitivity reaction (Aviña et al., 2006). The activity on non-specific immune modulation allows immunobiotics to increase the host immune response and facilitate the elimination of pathogenic germs in the intestine by releasing proinflammatory cytokines such as Tumor Necrosis Factor-alpha (TNF-α). It has been reported that immunobiotics can stimulate macrophages and increase phagocytosis by early activation of the inflammatory response prior to antibody production (Isolauri et al., 2001; Molina et al., 2016).
Psychobiotics
Defined as a special class of probiotics that when ingested exert mental health benefits through an interaction with the gut microbiota, stimulating the production of short-chain fatty acids, enteroendocrine hormones, anti-inflammatory cytokines and neurotransmitters such as gamma amino butyric acid, serotonin, acetylcholine, catecholamines and norepinephrine, which helps regulate mood, cognitive, memory and learning functions (Sharma and Shukla, 2016; Zendeboodi et al., 2020). An ability to modulate the expression of neurochemical receptors such as endocannabinoids are also attributed to psychobiotics. Table 3 lists the most commonly used psychobiotics in neurological conditions.
Table 3 Most commonly used psychobiotics in neurological conditions (Adapted Misra and Mohanty, 2019; Nataraj et al., 2020).
| Neurological condition | Psychobiotic strains |
| Anxiety | Lactobacillus fermentum NS9, L. casei Shirota, L. rhamnosus JB-1, L. helveticus ROO52, B. breve 1205, B. infantis, B. longum 1714, B. longum NCC3001, B. longum R0175 |
| Depression | L. acidophilus, L. acidophilus W37, L. brevis W63, L. casei, L. casei Shirota, L. casei W56, L. gasseri OLL2809, L. helveticus NS8, L. lactis S19, L. lactis W58, B. infantis, B. bifidum, B. bifidum S23, B. lactis W52, B. longum R0175 |
| Stress | L. casei Shirota, L. helveticus, L. helveticus R0052, L. plantarum PS128, L. rhamnosus, B. infantis, B. longum R0175 |
Oncobiotics
Oncobiotics are probiotics that enhance the response to cancer immunotherapy, helping the immune system to eliminate tumor cells through cytotoxicity stimulated by biological agents aimed at preventing apoptosis or lymphocyte inhibition (Zitvogel et al., 2016). There are studies that suggest that oncobiotic probiotics play an important role in the immune response after chemotherapy and radiotherapy treatment, thus helping the host to avoid future relapses (Gopalakrishnan et al., 2018).
Pharmabiotics
Pharmabiotics are probiotics that can function as pharmacological active ingredients with preventive and curative potential. Pharmabiotics are used for the adjunctive treatment of various diseases such as acute diarrhea and irritable bowel syndrome. Studies have determined that specific pharmabiotics can improve many lower gastrointestinal tract symptoms in adults (Belkaid and Hand, 2014; Navarro et al., 2021).
Paraprobiotics or phantom probiotics
Taverniti and Guglielmetti (2011) described paraprobiotics as inactive, dead, and non-viable cells, or cell fractions, from intact or broken probiotics that provide health benefits to the recipient. Other authors have shown that cells from probiotics, even when dead or inactivated, continue to have a beneficial effect on the host, such as decreasing the inflammatory response, as well as preventing the adhesion of pathogenic bacteria (Zendeboodi et al., 2020). They are also attributed with attenuation of colitis, stimulation of the intestinal immune system, anti-adhesion capacity against several pathogens in CaCo-2 experimental models (Zhang et al., 2005; Nataraj et al., 2020). Some paraprobiotics produce compounds such as teichoic acid, peptidoglycan-derived muropeptides, exopolysaccharides and biosurfactants bound to their cell wall (Nataraj et al., 2020).
Postbiotics
Defined as a complex mixture of molecules that are part of the products or by-products derived from probiotic metabolism and must be cell-free (Aguilar-Toalá et al., 2018; Salminen et al., 2021). Postbiotic molecules have a known chemical formula, prolonged storage stability, as well as an ability to trigger various treatment mechanisms for diseases such as inflammation, adhesion of pathogens to the gastrointestinal tract, obesity, coronary artery disease, cancer, and oxidative stress (Aguilar-Toalá et al., 2018). Some examples of postbiotic molecules are enzymes, peptides, proteins, exopolysaccharides, organic acids, serine protease inhibitors (serpin), short-chain fatty acids, and cell wall components such as teichoic acid, peptidoglycan, hydrogen peroxide, reuterin, diacetyl, bacteriocins, phenol, amino acids, benzoic acid, phenylactic acid, and vitamins (Jastrząb et al., 2021). There is scientific evidence attributing different antimicrobial, antioxidant and immunomodulatory functions to postbiotics, properties that could positively affect the homeostasis of the gut microbiota, as well as signaling pathways and metabolic pathways of the host, thus having effect on specific physiological, immunological, neuronal, hormonal, biological, regulatory and metabolic reactions (Sharma and Shukla, 2016; Salminen et al., 2021).
Probioceuticals
Probioceuticals are biologically active substances derived from the metabolism of probiotics that are attributed with health benefits, as well as the treatment and prevention of diseases. An example of probioceutical compounds are exopolysaccharides which are long-chain polymers of sugars with antioxidant and immunomodulatory properties (Kumar et al., 2020; Adebayo et al., 2018).
Pseudoprobiotics
Pseudoprobiotics are viable, but not active, probiotic cells that were inactivated by environmental factors such as high or low temperatures, extreme pH, lack of nutrients, low water activity, so they have low or no growth rates. Based on these characteristics they cannot be classified as traditional or true probiotics since they need to be viable and active cells. The advantages that these pseudoprobiotics have is that they can function in an inactive manner as paraprobiotics or be activated and used as traditional probiotics, with the advantages that both classifications give them (Zendeboodi et al., 2020; Blinkova et al., 2014).
Candidate microorganisms for new probiotics
Among the species that have been reported as new probiotics are Akkermansia miciniphila (Castañeda-Guillot, 2019), Ruminococcus bromii (Kumari et al., 2021), Roseburia intestinalis (Kasahara et al., 2018), Anaerobutyricum hallii (Kumari et al., 2021), Faecalibacterium prausnitzii (Castañeda-Guillot, 2019; Kumari et al., 2021), Oscillospira (Yang et al., 2021) and Christensenella minuta (Castañeda-Guillot, 2019). The main characteristic of these microorganisms is that they are isolated from the human gastrointestinal tract. Therefore, they must be resistant to a low pH in the intestinal tract, have no resistance to antibiotics and exercise pathogen control, although they may be sensitive to oxygen.
Patents for new probiotics
Table 4 shows some of the patents for new probiotics.
Table 4 Patents of new probiotics (Adapted from Eligo Bioscience Announces Successful Outcome in US Patent Interference against SNIPR Biome on CRISPR-Cas antimicrobials, 2021; SNIPRBIOME A CRISPR COMPANY, 2022).
| Patent number | Year | Title | Applicants |
| EP/V027743/1 | 2021 | Next-generation probiotics: the development of oral microbe-based formulations for microbiome-altering applications. | Institute of Clinical Sciences, University of Birmingham |
| WO2014124226 | 2021 | Eligobiotics | Eligo Bioscience; Rockefeller University |
| US 10,953,090 Β2 | 2021 | Selectively altering microbiota for immune modulation. | Jasper Clube, London; Christian Grondahl, Copenhagen; Morten Sommer, Copenhagen; SNIPR Technologies Limited, London (GB) |
| US 10,920,222 Cl | 2021 | Treating and preventing microbial infections. | Morten Sommer; Virginia Martinez; Eric Van Der Helm; Jakob Krause Haaber; Ana Dc Santiago Torio; Christian Grøndahl; Jasper Clube, SNIPR BIOME APS |
| EP 3 291 679 B1 | 2021 | Altering microbial populations and modifying microbiota. | SNIPR Technologies Limited |
| US 9, 701, 964, B2 | 2017 | Altering microbial populations and modifying microbiota. | Jasper Clube; Morten Sommer; Christian Grondahl; Erick Van Der Helm; Rubén Vázquez Uribe |
Studies on probiotics
Studies using traditional probiotics
Several authors have studied the role of probiotic LAB. Ramírez-Chavarín et al. (2013) evaluated the probiotic potential of ten thermotolerant LAB strains, which were studied for tolerance to low pH and bile salts, co-aggregation and self-aggregation, adhesion to epithelial cells, concluding that the thermotolerant LAB studied are promising for their probiotic potential. Sanchez and Tromps (2014) characterized 72 LAB strains by growth at different pH, temperature and tolerance to high sodium chloride concentrations, selecting 14 strains as probiotic candidates which were tested for hydrophobicity, self-aggregation and microbial antagonism. The results showed that strains such as Lactobacillus spp., Leuconostoc spp., Lactococcus spp., Streptococcus spp. and Pediococcus spp. have microbial antagonism to Bacillus cereus and Staphylococcus aureus at an inhibition rate of 63.6%, to Salmonella typhimurium at 75.7% and to Pseudomonas aeruginosa at 72.7%. Jurado and Fajardo (2017) evaluated the susceptibility of two strains of lactic acid bacteria to different antibiotics, the inhibition effect of Lactobacillus gasseri and its supernatant on Staphylococcus epidermidis, the growth of the lactic acid strain at different pH and different temperatures, resistance to bile salts and bovine bile, established fermentation kinetics and determined the count of viable microorganisms for each condition. They concluded that both strains have resistance to the antibiotics gentamicin and dicloxacillin, with L. gasseri showing growth inhibition of S. epidermidis, reaching the maximum growth peak at 12 h, at pH 4.2, and a lactic acid percentage of 1.26. Zachary et al. (2020) evaluated the mechanisms of adhesion to intestinal mucus of different probiotic strains, for which they immobilized the mucus on the surface of the plate and tested the use of different cell concentrations, finding that surface proteins and cellular components influence mucoadhesion and were heterologously expressed or altered in Lactococcus lactis and Escherichia coli, concluding that adhesion to mucus depends on the type of strain, as well as on the concentration of cells.
Studies with new probiotics
In recent years, studies on new probiotics have been developed. Molina et al. (2016) evaluated the use of the immunobiotic Lactobacillus rhamnosus and its influence on the immunological alterations that occur naturally in aging mice, demonstrating that this strain is capable of improving the phagocytic activity of macrophages. Therefore, they concluded that L. rhamnosus may have application as an immunomodulator given its positive influence on aging and help in reinforcing intestinal and systemic immunity.
O'Toole et al. (2017) sought a sustainable route to the delivery of novel probiotics from a pharmaceutical perspective, where this relatively new concept overlaps with live biotherapeutic products. Through the development of improved cultivation methodologies, accessibility to genome and metagenome sequencing will allow these probiotics to be targeted.
Nishida et al. (2017) analyzed the relationship that the parapsychobiotic Lactobacillus gasseri has with stress and sleep quality. A group of 21 men and eleven women were orally administered the parapsychobiotic for 5 weeks, finding that sleep quality can improve with the administration of L. gasseri, particularly in men, shortening sleep latency and increasing the sleep duration. A decrease in the growth of strains such as Bacteroides vulgatus was observed, responsible for intestinal inflammation, suggesting that the L. gasseri strain is a good ally against stress and its side effects, as well as improving sleep.
Salva and Alvarez (2017) studied the role of microbiota and immunobiotics in granulopoiesis (the process of renewal of granulocytic cells circulating in the blood that are part of the body's defense system), which occurs in the bone marrow of immunocompromised hosts. The authors determined that dietary supplementation with immunobiotics is an interesting alternative to improve granulopoiesis in stationary and emergency states, improving the respiratory innate immune response and resistance against respiratory pathogens in immunocompromised hosts.
Chang et al. (2019) conducted a review of existing information on novel probiotics and how they influence most diseases such as chronic intestinal inflammation, colitis, obesity, metabolic syndromes, diabetes mellitus, liver disease, cardiovascular diseases, cancer, neurodegenerative diseases, using new strains such as Prevotella copri, Christensenella minuta, Parabacteroides goldsteinii, Akkermansia muciniphila, Bacteroides thetaiotaomicron, Faecalibacterium prausnitzii and Bacteroides fragilis.
Kumar et al. (2020) studied the probioceutical produced by Pediococcus acidilactis, in its exopolysaccharide form and its influence on human health. They analyzed the Pediococcus acidilactis genome and identified ten genes responsible for the production of exopolysaccharides, which are linear homopolysaccharides (α-glucans) with few α-(1→3) branches, which have antioxidant activity, reducing power, and anticancer activity, suggesting this probioceutical can be used as an antioxidant and anticancer agent.
Akter et al. (2020) suggested the use of paraprobiotics in the treatment for people with weak immune systems as live probiotics could do more harm than good regarding host health, highlighting their long shelf life, modulation of immune responses, biological response modification, anti-inflammatory and antiproliferative properties.
Different methods of extraction of paraprobiotics and postbiotics produced by bacilli have been studied, such as heat treatment, enzymatic treatments, solvent extraction, radiation, sonication, supercritical CO2, chromatography, centrifugation, dialysis and lyophilization (Teame et al., 2020).
Moradi et al. (2020) analyzed the applications of postbiotics in food preservation, packaging and biofilm control, as well as their use as a biodegradant against chemical compounds in food (e.g., biogenic amines), concluding that further studies on food biosafety are needed to develop international standards and regulations on the use and applications of postbiotics.
Amirí et al. (2021) proposed the use of postbiotics to produce linoleic acid, exopolysaccharides, and bacteriocins using Bifidobacterium lactis, showing how the effect of yeast extract had a beneficial effect on the production yield of postbiotic metabolites.
Recent studies have determined that complementary probiotic therapy can reduce the duration and severity of intestinal diseases, since using the pharmabiotic Lactobacillus acidophilus LB can reduce intestinal pH and inhibit the growth of pathogenic organisms (Navarro et al., 2021).
Michels et al. (2022) investigated the molecular patterns of the immune response using Bifidobacterium lactis, Lactobacillus casei, L. gasseri, L. paracasei and Streptococcus thermophilus. Different doses of these strains on macrophage cell lines stimulated with polysaccharides was examined; the immune response was analyzed after application of these strains as paraprobiotics, concluding that the paraprobiotics had an effect on the production of interleukin in addition to inhibiting free radicals, increasing cell viability, making it likely that these paraprobiotics contribute to improve intestinal homeostasis, immunomodulation and host metabolism. The identification of microbial strains of intestinal origin for the development of new probiotics requires extensive knowledge about the cultivation of these microorganisms, because unlike traditional probiotics these strains are very sensitive to oxygen (De Filippis et al., 2022).
CONCLUSION
The beneficial role of traditional probiotics in human health is undeniable. With the discovery of new probiotics with functions different from traditional probiotics, a door is opened for the development of treatments for different diseases in a specific and systematic way. However, more applied research in humans is needed to determine their biosafety.