Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Caldasia
Print version ISSN 0366-5232
Caldasia vol.35 no.1 Bogotá Jan./June 2013
Comunicación vocal del tití gris (Saguinus leucopus) en estado silvestre
JESUALDO ARTURO FUENTES
ENRIQUE ZERDA - ORDÓÑEZ
JOAO MUÑOZ - DURÁN
Department of Biology, Indiana University, Indiana 47405, USA.jearfuen@indiana.edu
Department of Biology, Universidad Nacional de Colombia, Bogotá D.C., Colombia.ezerdao@unal.edu.co; jvmunozd@unal.edu.co
ABSTRACT
The white-footed tamarin, Saguinus leucopus, is an endemic primate of Colombia whose vocal communication has been little studied. For a period of a month we applied behavior sampling with continuous record, recording tamarins' vocalizations and the behavioral contexts associated with them. The study took place at the "José Celestino Mutis" forest in Mariquita, Tolima department (Colombia). We compiled a vocal catalogue showing some acoustic properties of the vocalizations (i.e. duration and frequency). We identified 19 phonemes associated with different behavioral contexts. Young individuals emitted vocalizations of higher frequency than adults. We discuss the relevance of the tamarins' acoustic displays on their social and ecological interactions.
Key words. Behavior, bioacoustics, Callitrichidae, primates, vocal catalogue.
RESUMEN
El tití gris, Saguinus leucopus, es un primate endémico de Colombia cuya comunicación vocal ha sido poco estudiada. Por un periodo de un mes aplicamos muestreo de comportamiento con registro continuo, grabando vocalizaciones de los titíes y contextos comportamentales asociados a ellas. El estudio se llevó a cabo en el bosque municipal "José Celestino Mutis" de Mariquita, departmento del Tolima (Colombia). Compilamos un catálogo vocal en el que se muestran algunas propiedades acústicas de las vocalizaciones (vale decir, duración y frecuencia). Identificamos 19 fonemas asociados a diferentes contextos comportamentales. Los individuos jóvenes emitieron vocalizaciones de mayor frecuencia que los adultos. Discutimos la relevancia de los despliegues acústicos de los titíes en términos de sus interacciones sociales y ecológicas.
Key words. Bioacústica, Challitrichidae, catálogo vocal, comportamiento, Saguinus.
Recibido: 20/08/2012
Aceptado: 04/04/2013
INTRODUCTION
Sound is one of the most advantageous and fundamental forms of communication and arboreal primates commonly use it since visual cues are hardly perceived in dense forests (Audesirk & Audesirk 1997). Sound allows animals to transmit and share specific information about their environment and elicit specific responses from their kin (Smith 1982). Vocalizations may convey a broad range of information, including the identity and location of the communicator, which in turn may incite subsequent behaviors of other individuals (Smith 1982).
The white-footed tamarin, Saguinus leucopus (Günther 1877), is an endemic primate of Colombia with populations distributed in forests from zero to 1 600 m above sea level in the Magdalena River Basin in Antioquia, Caldas and Tolima departments (Alberico et al. 2000, Muñoz & Cuartas 2003). Studies of captive individuals have provided ethological and acoustic information for this species (García 1996, Defler 2003, Leal 2004). Studies in the wild have provided information about distribution, habitat use, home range, diet, population assessments, conservation and behavior, with vague references to vocal communication (Hershkovitz 1977, Mantilla & Díaz 1992, García 1996, Vargas & Solano 1996, Fajardo 2000, Cuartas 2001, Del Valle 2004, Poveda & Sánchez – Palomino 2004, Valle 2004, Negrete 2005, Alba-Mejía et al. 2010). Only few vocal studies of free range individuals have been conducted on this species, using urban groups as sampling subjects (Rueda 2003, Rueda & Zerda 2009).
Rueda & Zerda (2009) found sounds associated with specific behavioral contexts such as feeding, contact (inside and outside of the visual range), traveling, aggression, play, alarm and threat. Similar uses have been reported for other Neotropical primates, including species within the Saguinus genus (Wilson 1980, Smith 1982, Ghazanfar et al. 2002, Hauser et al. 2002, Weiss & Hauser 2002, Miller et al. 2004, Miller et al. 2005). However, it remains to be seen if these sounds and their respective contexts are the same way in wild groups, and how vocal communication relates to behavioral interactions in free ranging individuals. Differences in terms of rearing history between wild and captive primates may generate the development of abnormal behaviors in the latter (Birkett & Newton-Fisher 2011), so there are not strong reasons to expect that the use and frequency of vocalizations will be equivalent in wild, urban and captive groups of white-footed tamarins. The availability and distribution of resources, as well as the complexity of social interactions may differ between wild and captive individuals, and these differences may have profound implications in their behavioral repertoire (Maier 2001, Birkett & Newton-Fisher 2011). Therefore, even if vocalizations in captive and urban groups are well described, these do not necessarily inform about their occurrence in wild individuals.
This paper describes the vocalizations of the white-footed tamarin in the wild. The species vocal repertoire was characterized, phonemes were associated with behavioral patterns displayed during emission, and acoustic differences between young and adult individuals were identified.
MATERIAL AND METHODS
Study area
The study took place in Mariquita municipality forest, home of about eight groups of tamarins with size ranging from three to fifteen individuals. Mariquita is located in the northeast of Tolima Department (5 º 12' North, 74 º 55' West). The department belongs to the Chocó biogeographical province and is located 328 m above sea level (Zerda 2002). The region has a bimodal climatic regime with two rainy periods from March to May and from October to November. In general the area has a warm humid or equatorial humid climate. The study area is a patch of tropical rainforest that extends for about 123 ha, but it has largely been deforested, so the wooded mountains have given way to pastures and crops on the slopes (Pachón & Bohórquez 1991, Zerda 2002).
Fieldwork
Fieldwork was carried out from March 8th to April 14th of 2006. During the first week we tracked several existing trails in the forest and performed preliminary observations by means of ad libitum sampling using Garmin 60 GPS and Tasco Sonoma binoculars 8 x 40. Tracked trails were used in the remaining weeks for daily walks, which usually began at 6:00 and ended at 18:00, in an attempt to follow these diurnal primates (Defler 2003). These times (6:00-18:00) usually cover the daylight period on the study area, but when the primates were on sight and still active, the ending time was extended until the tamarins settled in sleeping places. Daily walks were performed five days a week (Monday-Friday) and planed using prior observations, usually starting at the place where tamarins were last seen on the previous day. No particular group was followed; tamarins of different groups were located by means of acoustic cues or sampled after random encounters when walking in specific trails. At the end, at least six different groups were sampled. However, these groups split several times during the day. Subgroups of variable size tend to stay relatively close to each other, but not necessarily occupy the same places along several days. Because of this, and since we did not mark any animal in this study, we cannot be sure if some of the subgroups observed belong to the same or different groups for some of the observations. The equipment was ready at all times, and when a group was found, data collection and sound recording were performed using behavior sampling with continuous record, focusing on acoustic displays and related behavioral contexts (Zerda 2004). Both voice annotations of behavioral contexts and vocalizations were recorded using the same equipment to facilitate collection, but in order to avoid sound overlapping, the sampler only made voice annotations when the primates were not vocalizing. A single researcher collected all the data. We recorded animal vocalizations and field observations using a Sennheiser ME 66 shotgun microphone with K6 power module, a Sony MZ-R70 MiniDisc digital recorder and Jwin headband headphones.
Laboratory work
Sounds were processed using Raven 1.2.1 software package for Windows (Cornell Lab of Ornithology 2003-2005) to obtain spectrograms (Window type = Hann; window size = 512 samples; 3 dB bandwidth = 124 Hz; time grid overlap = 50%; hop size = 256 samples; frequency grid spacing = 86.1 Hz). Vocalizations were grouped according to duration, frequency, and graphic isomorphy. The following information was obtained for each vocalization: duration, frequency (maximum and minimum), behavioral context in which the sound was emitted, and details of the sound sequence in which the vocalization was immersed. Since we did not perform this study under captive conditions and wild subjects were not captured at any time during sampling, additional details such as age, sex, individual or group identity could not be registered. Young individuals were identified by their body size and position in the group while travelling, as they were usually left behind or accompanied by an adult member of the group. These adults carried them intermittently. Of course, it would be desirable and highly informative to have such additional details at hand, but since our main focus is centred in the general association between sounds and behaviors (as reflected in the sampling method used, see Fieldwork for further information), the lack of those details should not distort the main findings of this work. Of course, conclusions regarding detailed information content in specific cues are avoided, since we do not have data on the identity of the emitters to support them.
Good quality recordings were isolated and filtered, then named according to the form observed in spectrograms and signal properties. We then compiled a vocalization catalogue complementing that proposed by Rueda & Zerda (2009).
Behavioral contexts
Five behavioral contexts were associated with vocalizations:
- Exploration: Moving and searching small areas usually ending in foraging.
- Alarm: Restless and attentive displays to particular stimuli that calls the attention of other tamarins. Trigger stimulus and resultant responses are variable. We distinguished the following alarm contexts:
2.1. Curiosity: Although restless, tamarins show no indications of conspicuous exaltation; i.e. they stand upright and move very short distances, with occasional sudden glances in addition to bodily contortions that allow them to extend their field of view. They may approach the disturbance source or, rarely, run away. This reaction does not always involve all individuals of the group; some of them may be engaged in different activities. This is a typical warning display during encounters with humans.
2.2. Threat: Tamarins gather around animals that are known to predate on small mammals (e.g. ocelots, Leopardus pardalis (Linnaeus 1758)), moving continuously and exhibiting their lower canines.
2.3. Escape: Quick movement away from a possible predator. This occurs when birds of prey fly over the canopy, causing tamarins to move to lower branches close to the tree trunk.
2.4. Latent: Similar to the "curiosity" alarm context, but more agitated and with the display of erratic movements. Some individuals appear restless and often focus their sight on specific areas. This behavioral context occurs either after or before stressful events such as agonistic encounters or threat displays.
- Contact: An individual signals its presence to others. This may occur in two ways:
3.1. Intra-group: Group members tend to gather or to keep social cohesion during activities such as travelling or exploration.
3.2. Inter-group: Causes repulsion or disputes between the individuals involved. Usually rounding up and harassment occur involving two or more individuals.
- Social: Physical contact that occurs between two or more individuals, either when playing with members of the same group or during agonistic disputes with individuals from different groups.
- Affiliation: Care attention solicited primarily by young individuals. These requests usually end with an adult feeding or carrying the young.
Data analysis
We used descriptive statistics to determine basic characteristics of vocalizations in terms of frequency and duration. To test for the relationships between phonemes and behaviors, we used a two-tailed Fisher's exact test using R (R Development Core Team 2009) with the 'stats' package (R Development Core Team 2009). For r by c tables, p – values were computed with Monte Carlo simulations (100 000 replicates) when tables were too large. Some associations were explored further by means of Correspondence Analysis using R with the 'ca' package (Greenacre & Nenadic 2010). To compare similar vocalizations in terms of quantitative values and graphic isomorphy, but emitted by different class ages (i.e. young vs. adults), we carried out Kolmogorov - Smirnov tests using PAST version 1.79 (Hammer et al. 2001). We determined statistical significance at the 5% level.
RESULTS
Description of vocalizations
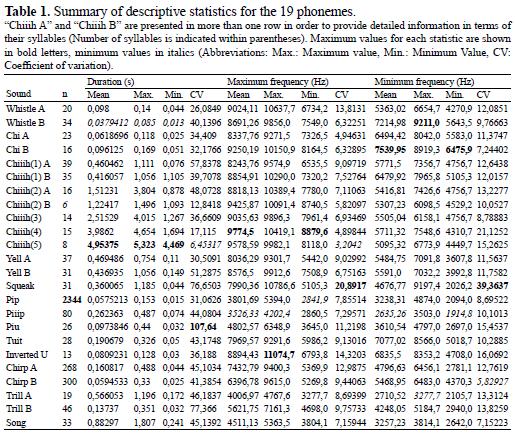
We registered 3 483 vocalization occurrences ranging from 1 914.8 to 11 074.7 Hz (Table 1, Figure 1). Rueda & Zerda (2009) found fourteen phonemes, five of them are presented here in a very similar way ("Whistle A", "Chiiih A", "Yell A", "Squeak", "Pip"), but four phonemes do not receive the exact same treatment in this study ("Whistle B", "Chiiih B", "Yell B", "Inverted U") due to divergent criteria regarding their classification (i.e., number of syllables, individual class age; Table 1, Figure 1). We present seven new phonemes ("Chi A", "Chi B", "Tuit", "Chirp B", "Trill A", "Trill B", "Song") and the remaining three ("Piiip", "Piu", "Chirp A") are reported elsewhere (Rueda, 2003).
Relationships between phonemes and behavior
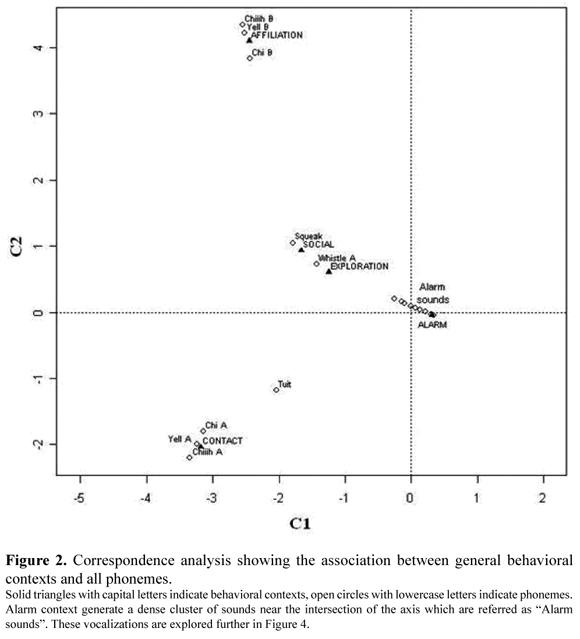
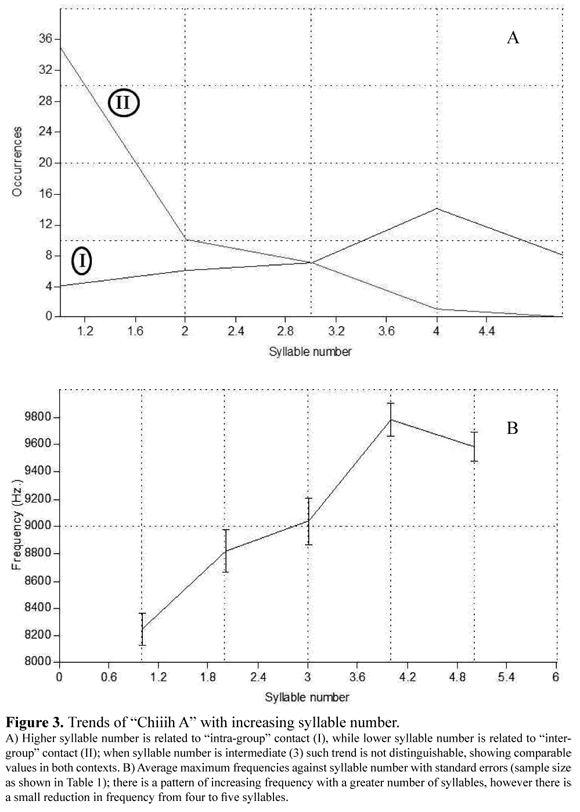
Phonemes were related to general behavioral contexts (Fisher, n = 3 483, p = 1*10-5, MC replicates = 1*105). Associations with sounds tend to be strong (Figure 2). "Whistle A" was associated with "exploration"; "Chiiih B", "Chi B" and "Yell B" with "affiliation"; "Squeak" with "social"; "Chiiih A", "Chi A" and "Yell A" with "contact". Furthermore, the number of syllables of the "Chiiih A" phoneme varied depending on two contact situations (Fisher, n = 92, p = 1.395*10-5). "Chiiih A" is composed by one to five syllables and tends to increase sound frequency with a higher number of syllables (Figure 3). "Chiiih A" of one, two or three syllables tended to be emitted in a combined fashion. "Contact" contexts were associated with "Chiiih A" of one syllable (Fisher, n = 39, p = 0.004012) and two syllables (Fisher, n = 16, p = 0.01136) depending on phoneme combinations, but this association was not significant for "Chiiih A" of three syllables (Fisher, n = 14, p = 0.06993). During "intra-group" contact contexts, "Chiiih A" of one and two syllables was always emitted with no other vocalization. In contrast, during "inter-group" contact contexts this vocalization was more frequently emitted in combination with other vocalizations (80% for one syllable "Chiiih A" and 70% for two syllables "Chiiih A").
Specific vocalizations were linked to the particular alarm strategies described above (Fisher, n = 3 169, p = 1*10-5, MC replicates = 1*105). Some alarm vocalizations showed conspicuous relationships (Figure 4): "Inverted U" with "escape"; " Chirp A" with "threat"; and "Trill A", "Trill B" and "Song" with "latent". "Pip", "Piiip" and "Piu" were associated to "curiosity"; however, they were also emitted during the "threat" and "latent" contexts, but these last associations were not as strong. Similarly, "Chirp B" had a strong association with "curiosity", although it was also slightly associated with "threat".
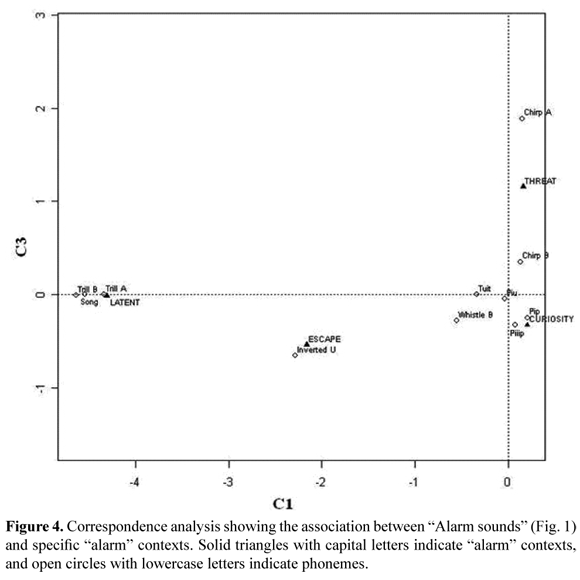
The behavioral association of other sounds was weak. That is the case of "Whistle B" that occurred in "alarm" contexts and, with a lower frequency, "exploration" (Figure 2). Furthermore, this vocalization was recorded in two different "alarm" categories: "latent" and, less frequently, "curiosity" (Figure 4). This contextual plasticity was associated with phoneme combinations (Fisher, n = 34, p = 0.04448): During "exploration" this vocalization always occurs with no other vocalizations, but during "alarm" it may occur by itself (41.4%) or combined with other vocalizations (58.6%). "Tuit" occurred in "contact" and "alarm" contexts (Figure 2). For the latter, it was frequent during "curiosity" and was recorded in fewer occasions during "threat" (Figure 4). "Tuit" was combined with other sounds depending on the context in which it occurred (Fisher, n = 28, p = 7.62*10-5): It was usually emitted by itself or combined with sounds such as "Pip", "Piu" and "Chirp B" during "alarm" contexts, but it was emitted repeatedly or combined with sounds such as "Chi A", "Chiiih A" and "Yell A" during "contact" contexts.
Modulations
We compared adult and young tamarin vocalizations showing similar graphic isomorphy (Figure 1). We found differences between the two age classes in "Chi A" and "Chi B" length (DN = 0.663043, p = 0.000210423), and in maximum (DN = 0.763587, p = 1.05974*10-5) and minimum frequency (DN = 0.61413, p = 0.000773311). "Chiiih A" and "Chiiih B" differ in maximum (DN = 0.463736, p = 0.000414855) and minimum frequency (DN = 0.383883, p = 0.00598587), but not in length (DN = 0.178755, p = 0.554332). No differences were found for duration (DN = 0.273758, p = 0.132745) or minimum frequency (DN = 0.170009, p = 0.672374) between "Yell A" and "Yell B", but there were differences between adults and young for maximum frequency (DN = 0.37925, p = 0.0109759). In general terms, young individuals tend to produce vocalizations of higher frequency than adults (Table 1).
DISCUSSION
Saguinus leucopus vocalizations appear to be associated with specific behavioral contexts. "Exploration" was linked to foraging activities. The tendency of white-footed tamarins to exchange information about food sources is controversial (García 1996, Leal 2004, Negrete 2005). We found signals ("Whistle A", "Whistle B") associated with "exploration" that may be related with such information exchange. Similar vocalizations have already been reported for other primate species recognized for showing prosocial preferences, including some in the genus Saguinus (Evans 1968, Roush & Snowdon 2001, Snowdon & Boe 2003, Leal 2004, Prieto 2004, Rueda & Zerda 2009, Cronin et al. 2010). Both "Whistle A" and "Whistle B" have relatively high frequency and short duration which are features consistent with anti-predatory qualities (Table 1); i.e., they will not be easily detected by individuals of other species and, if they are (e.g. species capable of perceiving high frequency sounds), their duration will not last enough for a potential predator to locate the source of the sound.
Several phonemes were associated with the "alarm" context. Acoustic communication for discriminated response to alarm situations has been reported for several primate species (Evans 1968, Bradbury & Vehrencamp 1998, Prieto 2004, Fichtel et al. 2005, Sproul et al. 2006, Sugiura 2007). Alarm sounds are usually related to escape and threat behaviors (Evans 1968, Bradbury & Vehrencamp 1998). "Inverted U" shows features of escape vocalizations: it has a high frequency (Table 1), is not emitted in combination with other vocalizations, is seldomly repeated, and it generates a quick escape reaction (Bradbury & Vehrencamp 1998). Meanwhile, "Chirp A" displays features of threat vocalizations (Bradbury & Vehrencamp 1998): it is repeatedly emitted (91% of total emissions were repeated), and it shows modulations and harmonics (64% of total emissions were highly modulated, and 71% showed conspicuous harmonics). It also generates clustering around a danger source.
Other vocalizations show further specificity to alarm contexts. For example, while "Pip" and "Piiip" are more associated to "curiosity", "Chirp A" is more related to "threat". On the other hand, "Chirp B" occurs less specifically in both alarm contexts ("curiosity" and "threat"). Variability in vocalizations may be associated with specific behavioral contexts and strategies, and provides non-linear elements that make them less likely to be overlooked (Fitch et al. 2002, Rukstalis et al. 2003, Yin and McCowan 2004). "Chirp B" is similar to "Pip" in its short duration, but it is also similar to "Chirp A" in its high frequency. These similarities may be indicative of graduated emissions between extreme alarm contexts such as "curiosity" and "threat", where the latter corresponds to a more conspicuous response. Such graduated emissions, and variations in behavioral responses due to modifications in alarm generating stimuli, may indicate motivational changes (Evans 1968, Wilson 1980). In fact, it has been suggested that high or variable-pitched vocalizations with increasing frequency (i.e. "Chirp B") express aggression combined with fear, while low-pitched vocalizations without marked frequency variation (such as "Pip" and "Piiip", Table 1) express aggression and low fear levels (Bradbury & Vehrencamp 1998, Fichtel et al. 2005). Further studies with captive animals would be relevant to address these features in detail.
The "latent" alarm context, associated with vocalizations such as "Trill A", "Trill B" and "Song", may be another example of transitional behavior responses where vocalizations could serve in keeping the group alert to potential or latent threats, such as agonistic disputes or predation risks.
"Chiiih A" is perhaps the most characteristic vocalization for this species and its cohesive function has already been proposed (Hershkovitz 1977, Rueda 2003, Del Valle 2004, Negrete 2005, Rueda & Zerda 2009). Our results also suggest a territorial function for this vocalization that was suspected before (Hershkovitz 1977). Long calls are common for primates and other mammal species (Evans 1968, Bradbury & Vehrencamp 1998, Ghazanfar et al. 2002); "Chiiih A" fulfils both contact and territorial signal attributes for an animal of small size: it is long and has high frequency (Table 1), making it a strong sound that can propagate for relatively long distances, providing information on the location of the vocalizing individual (Evans 1968, Bradbury & Vehrencamp 1998, Ghazanfar et al. 2002, Sugiura 2007). Rueda (2003) suggested that syllable number in this long call could be related to the distance between tamarins, motivational state and specific contexts, such as a male calling to his offspring. Our data partially support this suggestion regarding its specific context functionality. Our results suggest that "Chiiih A" vocalizations that include a larger number of syllables are less likely to be combined with other vocalizations and tend to be associated to "intra-group" contact; in contrast, "Chiiih A" vocalizations composed of a lower number of syllables have a greater tendency to be combined and used for "inter-group" contact association. In Saguinus oedipus (Linnaeus 1758) it has been shown that variations in long call emissions (including syllables) may have an effect on signal recognition and mate attraction (Ghazanfar et al. 2002, Miller et al. 2004, Miller et al. 2005). Rueda (2003) suggested that a reduction in the number of syllables increases sound frequency. This could not be confirmed in this study; on the contrary, our results show the opposite tendency (Figure 3).
Sounds of young animals seeking attention from their parents or other adult members are well known in several species of primates, other mammals and birds (Evans 1968, Prieto 2004). In this study, such vocalizations were associated with the "affiliation" context. Young vocalizations resembled adult vocalizations associated with "inter-group" contact contexts. Poorly defined vocalizations of immature individuals are variable both in structure and meaning, and often behave as a "parody" of those vocalizations emitted by older individuals, thus allowing them to learn and develop their own repertoire (Bradbury & Vehrencamp 1998, Roush & Snowdon 2001, Rueda 2003). Youngsters also add non-linear components to their calls making them more conspicuous (Fitch et al. 2002). Frequencies were significantly higher for "affiliation" sounds, indicating juveniles' tendency to produce higher frequency sounds. Anatomical structures of young individuals are not fully developed and are smaller, so the oscillation and the displaced air volume are lower, generating high-frequency and low-amplitude sounds (Bradbury & Vehrencamp 1998). This works as an anti-predatory strategy, because such sounds are difficult to detect by other species but not by adult individuals from the same species (Evans 1968, Paulo & Sánchez 2006, Rueda & Zerda 2009). This is advantageous for youngsters during travel because adult members are informed about their presence and location (Bradbury & Vehrencamp 1998, Rueda & Zerda 2009).
The "social" context was mainly characterized by evident proxemic violations either between same group individuals (games or short quarrels) or between different group individuals (fighting). "Squeaks" were emitted by the assaulted individual in this context, but its meaning or receptors (e.g. the attacker or individuals of the same group, in the case of inter-group fights) are unknown.
Vocalization emission does not occur in isolation. Combination of vocalizations can vary according to the context (Wilson 1980, Fernández - Juricic et al. 1998). For instance, "Chiiih A", "Whistle B" and "Tuit" may be emitted in several ways and, in turn, may be associated with different contexts. Combinations become a key factor in terms of information transfer (Evans 1968, Ghazanfar et al. 2001, Rueda 2003). The fact that some combined sounds are units of information contributes to the acoustic communication complexity for the species from a single sound repertoire (Ghazanfar et al. 2001).
Acoustic communication is a key element in primates' lives as it provides relevant information for social interactions and various ecological contexts, which may enhance survivorship probabilities. The study on free ranging individuals allowed to gain additional insights in the role of vocalizations during activities associated with foraging and aggression, to expand our knowledge of the long calls in this species, and to recognize the relevance of concomitant cues (e.g. modulations and sound combinations) to increase the functionality of the vocal repertoire. These new pieces of information may be consequence of the methodological approach to some extent, but they could also result from inherent features of the vocal repertoire in the study subjects, since captive animals can show regular behaviors seen in their wild counterparts, but abnormal behavior may still be endemic in captivity (Birkett & Newton-Fisher 2011). Further work on wild populations will allow us to better understand life history, behavior and ecology of white-footed tamarins. Studies on captive individuals, including playback experiments, will help to clarify the role of particular variables (e.g. intensity, harmonic structure, temporal cues, spontaneous number discrimination of auditory stimuli) that are difficult to control in the field. The study of such variables may be critical to understand how sound modulation can provide additional pieces of information embedded in acoustic signals, such as identity recognition and motivational state.
ACKNOWLEDGEMENTS
We thank Thomas Richard Defler and Mark Duffy for valuable suggestions on the manuscript. We also thank the Universidad Nacional de Colombia for equipment supplied and the Universidad de Antioquia for its support. To the Mariquita municipality, and especially Nory Campiño and Rodrigo Patiño, for their help during the field phase. To Paulo Pulgarín, Abel Díaz and Luz Helena Rueda Campiño for their technical help and support. We also thank several anonymous reviewers whose thoughtful comments helped to greatly improve this manuscript.
LITERATURE CITED
1. Alba-Mejía, L., O. Montenegro-Díaz, & P. Sánchez-Palomino, 2010. Área de acción de tres grupos de tití gris (Saguinus leucopus) en el bosque de Bellavista (Victoria, Caldas). Final inform, Corporación Autónoma Regional de Caldas (CORPOCALDAS), Bogotá, D.C. [ Links ]
2. Alberico, A., A. Cadena, J. Hernández, & Y. Muñoz. 2000. Mamíferos (Synapsida: Theria) de Colombia. Biota Colombiana 1(1): 43-75. [ Links ]
3. Audesirk, T. & G. Audesirk. 1997. Biología, la vida en la tierra. 4th ed. Prentice–Hall, México D.F. [ Links ]
4. Birkett, L. P. & N. E. Newton-Fisher. 2011. How abnormal is the behavior of captive, zoo-living chimpanzees? PLoS ONE 6(6): e20101. doi:10.1371/journal.pone.0020101. [ Links ]
5. Bradbury, J. & S. Vehrencamp. 1998. Principles of Animal Communication. Sinauer, Sunderland, Massachussets. [ Links ]
6. Cronin, K. A., K. K. E. Schroeder, & C. T. Snowdon. 2010. Prosocial behaviour emerges independent of reciprocity in cottontop tamarins. Proceedings of the Royal Society B: Biological Sciences 277(1701): 3845–3851. [ Links ]
7. Cuartas, C. 2001. Distribución parcial del tití gris (Saguinus leucopus, Callitrichidae) en el departamento de Antioquia, Colombia. Neotropical Primates 9(3): 109–113. [ Links ]
8. Defler, T. 2003. Primates de Colombia. Conservación Internacional, Bogotá D.C. [ Links ]
9. Del Valle, C. 2004. Estudio del comportamiento social de dos grupos de Saguinus leucopus en el bosque y la zona urbana de Mariquita, Tolima. BSc thesis, Universidad Nacional de Colombia, Bogotá [ Links ].
10. Evans, W. 1968. Communication in the Animal World. Thomas Y. Crowell Company, New York. [ Links ]
11. Fajardo, A. 2000. Caracterización sistemática de las especies colombianas transinterandinas del género Saguinus (Hoffmannsegg, 1807) Primates: Callitrichidae. MSc thesis. Universidad Nacional de Colombia, Bogotá [ Links ].
12. Fernández – Juricic, E., M. Martella & E. Alvarez. 1998. Vocalizations of the blue – fronted amazon (Amazonia aestiva) in the Chancaní Reserve, Córdoba, Argentina. The Wilson Bulletin 110(3): 352–361. [ Links ]
13. Fichtel, C., S. Perry & J. Gros – Louis. 2005. Alarm calls of white – faced capuchin monkeys: an acoustic analysis. Animal Behaviour 70: 165-176. [ Links ]
14. Fitch, W. T., J. Neubauert & H. Herzel. 2002. Calls out of chaos: the adaptive significance of nonlinear phenomena in mammalian vocal production. Animal Behaviour 63: 407–418. [ Links ]
15. García, R. 1996. Rehabilitación, reproducción y reintroducción de un grupo de tití gris cautivo (Saguinus leucopus – Orden: Primates, Familia: Callitrichidae) a un ambiente natural. BSc thesis. Universidad de Antioquia, Medellín. [ Links ]
16. Ghazanfar, A. A., J. I. Flombaum, C. T. Miller & M. D. Hauser. 2001. The units of perception in the antiphonal calling behavior of cotton – top tamarins (Saguinus oedipus): playback experiments with long calls. Journal of Comparative Physiology A 187: 27–35. [ Links ]
17. Ghazanfar, A. A., D. Smith–Rohrberberg, A. Pollen, & M. D. Hauser. 2002. Temporal cues in the antiphonal long – calling behaviour of cottontop tamarins. Animal Behaviour 64: 427–438. [ Links ]
18. Greenacre, M. & O. Nenadic. 2010. ca: Simple, Multiple and Joint Correspondence Analysis. R package version 0.33. Available at: http://CRAN.R-project.org/package=ca [ Links ]
19. Hammer, Ø., Harper, D. and Ryan, P. 2001. PAST: Paleontological Statistics software for education and data analysis. Paleontologia Electronica 4(1): 1-9. [ Links ]
20. Hauser, M., S. Dehaene, G. Dehaene – Lambertz & A. Patalano. 2002. Spontaneus number discrimination of multi – format auditory stimuli in cotton – top tamarins (Saguinus oedipus). Cognition 86: 23–32. [ Links ]
21. Hershkovitz, P. 1977. Living New World Monkeys (Platyrrhini). Vol. 1. University of Chicago Press, Chicago. [ Links ]
22. Leal, A. 2004. Identificación de los patrones de comportamiento y conformación de un grupo social de tití gris (Saguinus leucopus) en proceso de rehabilitación. BSc thesis. Universidad Francisco José de Caldas, Bogotá D.C. [ Links ]
23. Maier, R. 2001. Comportamiento animal: Un enfoque evolutivo y ecológico. McGraw Hill Interamericana, Madrid. [ Links ]
24. Mantilla, L. C. & S. Díaz. 1992. Fray Diego García, su vida y obra científica en la expedición botánica. Academia Colombiana de Ciencias Exactas, Físicas y Naturales, Bogotá [ Links ].
25. Miller, C., J. Scarl & M. Hauser. 2004. Sensory biases underlie sex differences in tamarin long call structure. Animal Behaviour 68: 513–520. [ Links ]
26. Miller, C., C. Iguina & M. Hauser. 2005. Processing vocal signals for recognition during antiphonal calling in tamarins. Animal Behaviour 69:1387-1398. [ Links ]
27. Muñoz, J. & C. Cuartas. 2003. Lista de Mamíferos (Mammalia: Theria) del departamento de Antioquia, Colombia. Biota Colombiana 4(1): 65–78. [ Links ]
28. Negrete, J. 2005. Comportamiento y uso de hábitat del tití gris o manos blancas (Saguinus leucopus) en el bosque municipal de Mariquita Tolima. BSc thesis. Universidad de los Andes, Bogotá, D.C. [ Links ]
29. Paulo, C. & P. Sánchez. 2006. Estructura y función del repertorio vocal en grupos silvestres de tití cabeciblanco (Saguinus oedipus, Primate) en un bosque seco tropical de Colombia. MSc thesis. Universidad Nacional de Colombia, Bogotá, D.C. [ Links ]
30. Pachón, G. & A. Bohórquez. 1991. Ecología básica del Bosque Municipal de Mariquita, Tolima. Fundación Segunda Expedición Botánica. Universidad INCCA de Colombia. Editora Guadalupe Bogotá, D.C. [ Links ]
31. Poveda, K. & P. Sánchez – Palomino. 2004. Habitat use by the White – Footed Tamarin, Saguinus leucopus: A comparison between a forest – dwelling group and an urban group in Mariquita, Colombia. Neotropical Primates 12 (1): 6–9. [ Links ]
32. Prieto, R. 2004. Comunicación vocal en un grupo de Ateles fusciceps robustus en cautiverio. BSc thesis. Pontificia Universidad Javeriana, Bogotá, D.C. [ Links ]
33. R Development Core Team. 2009. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, Available at: http://www.R-project.org. [ Links ]
34. Roush, R. S. & C. T. Snowdon. 2001. Food transfer and development of feeding behavior and food – associated vocalizations in cotton – top tamarins. Ethology 107: 415-429. [ Links ]
35. Rueda, L. 2003. Comunicación vocal de dos grupos de Tití Gris (Saguinus leucopus) en Mariquita, Colombia. BSc thesis. Universidad Nacional de Colombia, Bogotá, D.C. [ Links ]
36. Rueda, L. & E. Zerda. 2009. Comunicación Vocal de un Grupo de Tití Gris (Saguinus leucopus) en Mariquita, Colombia. Neotropical Primates 16(1): 37-43. [ Links ]
37. Rukstalis, M., J. E. Fite & J. A. French. 2003. Social change affects vocal structure in a Callitrichid Primate (Callithrix kuhlii). Ethology 109: 327–340. [ Links ]
38. Smith, W. J. 1982. Etología de la comunicación. Fondo de la cultura económica, México D.F. [ Links ]
39. Snowdon, C. T. & C. Y. Boe. 2003. Social communication about unpalatable foods in tamarins (Saguinus oedipus). Journal of Comparative Psychology 117(2): 142-148. [ Links ]
40. Sproul, C., A. Palleroni & M. Hauser. 2006. Cottontop tamarin, Saguinus oedipus, alarm calls contain sufficient information for recognition of individual identity. Animal Behaviour 72: 1379-1385. [ Links ]
41. Sugiura, H. 2007. Effects of proximity and behavioral context on acoustic variation in the coo calls of Japanese macaques. American Journal of Primatology 69(12): 1412-1424. [ Links ]
42. Valle, H. (2004). Estimación del tamaño poblacional del tití gris Saguinus leucopus Gunther 1877 en tres zonas del municipio de Mariquita, Departamento del Tolima. BSc thesis. Universidad del Tolima, Ibagué [ Links ].
43. Vargas, N. & C. Solano. 1996. Evaluación del estado de dos poblaciones de Saguinus leucopus para determinar áreas potenciales de conservación en un sector del valle del Magdalena Medio, Colombia. Neotropical Primates 4(1): 13–15. [ Links ]
44. Weiss, D. & M. Hauser. 2002. Perception of harmonics in the combination long call of cottontop tamarins, Saguinus oedipus. Animal Behaviour 64: 415–426. [ Links ]
45. Wilson, E. O. 1980. Sociobiología, la nueva síntesis. Ediciones Omega S.A. [ Links ]
46. Yin, S. & B. McCowan. 2004. Barking in domestic dogs: context specifity and individual identification. Animal Behaviour 68: 343–355. [ Links ]
47. Zerda, E. 2002. Evaluación ecológica de la avifauna del bosque municipal de Mariquita. Final inform, Universidad Nacional de Colombia, Bogotá, D.C. [ Links ]
48. Zerda, E. 2004. Notas de clase. Comportamiento Animal: Introducción, métodos y prácticas. Universidad Nacional de Colombia, Bogotá D.C. [ Links ]