Introduction
Chemists have historically been interested in understanding phenomena at the nanoscale. However, nanosciences have emerged on the border of basic sciences such as physics, chemistry, and biology to provide a deeper understanding of nanoscale phenomena and nanotechnology to extrapolate knowledge and formulate practical solutions that tackle real problems. It is logical, therefore, that pure sciences converge with nanoscience and nanotechnology, other more established disciplines, such as biotechnology and bioinformatics, and those with a long history, such as polymer synthesis and cell synthesis biology and experimental biology (Bongomin et al., 2020). In this context, nanotechnology searches to manipulate matter desirable on an atomic scale to produce new materials and structures with distinctive characteristics to understand nanoscale phenomena and improved devices to take advantage of the diminished dimensions and potentialize intelligent solutions in different areas (Baig et al., 2021). In healthcare, nanotechnology offers tremendous opportunities for point-of-measurement (Bayda et al., 2019), data optimization and transfer, building information exchange networks, and unprecedented disease diagnosis and intervention approaches (Tsikala Vafea et al., 2020).
Transformative diagnostic goes beyond innovative, intelligent devices at the point-of-care (POC) to massive diagnostic test devices and smart data coupled to informatics approaches that use big data analytics (BDA), internet of things (IoT), machine learning (ML), deep learning (DL), blockchain analysis (BA), artificial intelligence (AI), augmented reality (AR), system integration, cloud and fog computing, and smartphones, among other cutting-edge converging/disruptive technologies. Appropriately selecting such dynamic tools leads to understanding disease's molecular basis for patient stratification, human biology, and disease behavior, and disseminating information at a large scale within a healthcare system. Therefore, it often includes molecular or cellular analysis by genomics and proteomics (Priyadharshini & Teran, 2016), medical imaging, nanoparticle-based theranostics (therapeutics and diagnostics), or toxgnostics (personalized drug toxicity), among others, to know a person's health status thoroughly (Vásquez & Orozco, 2022). Today more than ever, it is plausible to visualize and analyze physiological processes, gather in vivo, ex vivo, and in vitro data by advanced imaging, and discover new patterns and connections by AI and other computational tools. Additionally, new molecular biology provides vast genetic information, such as expanding clustered, regularly interspaced short palindromic repeats (CRISPR) technology and new mRNA approaches. Mimicking complex human anatomy in a chip via tissue engineering and organoid technologies is within disrupting conventional drug discovery models, gene therapy, and regenerative medicine studies. Furthermore, combining clinical data and risk factors with genomics, proteomics, and imaging provides a pathology diagnosis and information about the prognosis, prediction, and recurrence of the disease and the susceptibility and survivability of the patient (Cruz & Wishart, 2006).
This paper discusses the convergence of disruptive technologies in generating innovative solutions in healthcare. It selects illustrative examples of the role of electro chemical nanobiosensors in precisely detecting pathogens and how to elucidate an individual's health status in a personalized manner by monitoring a panel of biomarkers at different molecular levels. Next, it offers a perspective on how electrochemical nanobiosensors can be explored for diagnostics, prognosis, and prediction of the risk of complications and how intelligent sensors can go from simple to massive diagnostics through cutting-edge converging/disruptive technologies. Moreover, through descriptive cases, the work underlines the possibilities of functional nanocarriers for intelligent drug delivery as a branch of precision medicine and comments on translational medicine and the current challenges to implementing this new technology in global health systems. Overall, the work shows the potential of detection and diagnostic devices based on biosensors and treatment based on nanoencapsulation of active ingredients to offer personalized therapeutic alternatives as disruptive technologies that, as they progress, may gradually occupy the niches and markets of the currently established diagnostic and treatment technologies.
Converging technologies in the frame of Industry 4.0
Nowadays, the world is at the cusp of the Industry 4.0 paradigm that has emerged globally to revolutionize production systems and transform the industry. Industry 4.0 integrates physical worlds with 'cyberworlds' by introducing new technologies (Lins & Oliveira, 2020; Sony & Naik, 2020) that seek to digitize industrial value chains and promises to become a new economic and social development model. In this context, Industry 4.0 is a data-driven production system that is exponentially progressing while reshaping how people live and work, offering new opportunities for sustainability, quality of life, and the digital economy (Rainnie & Dean, 2019). In fact, Industry 4.0 is becoming the key to improving productivity, promoting economic growth, and ensuring our society's sustainability (Rosin et al., 2019).
Industry 4.0 is undoubtedly a new scenario in which the virtual, physical, biological, and digital spheres merge converging with various disruptive technologies including AI, AR, IoT, 3D printing, drones, robotics, intelligent sensors, and data system-BDA, BA, and cloud computing. It envisions providing unusual solutions in the industry (Frank et al., 2019) and is characterized by the mass use of intelligent objects in highly reconfigurable environments and fully interconnected industrial systems of products and services (Dragicevic et al., 2019). Therefore, Industry 4.0 promises more efficient and automated processes, higher quality and agility in production, more profitable and personalized products, and shorter delivery times.
Healthcare 4.0
The Health 4.0 revolution seeks healthcare by implementing Industry 4.0 technologies to offer efficient health services including high security and privacy in the electronic medical record of patient data allowing remote access and diagnosis by doctors or health personnel in real-time (Jayaraman et al., 2020). Healthcare 4.0 is digital health or digital technologies and information and telecommunication technologies (ICTs) to support health and other health-related fields. Healthcare 4.0 technologies include IoT, big data, 5G, AI, computing (cloud, fog, and edge), and BA (Haddara & Staaby, 2018). Holistically, the World Health Organization (2019) coined Health 4.0 as a discrete digital technology functionality applied to achieve health goals and implement digital health applications and ICT systems including text messages. Figure 1 summarizes the vertiginous evolution of the healthcare system over the last fifty years.
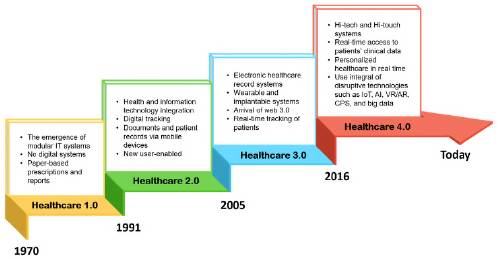
Figure 1 Evolution of the healthcare system in the last fifty years. Modified from reference (Bongomin, et al., 2020).
Industry 4.0 in healthcare is the expression of the convergence of technologies in various application areas including medical education, research, and training (MERT), medical devices and equipment (MDE), pharmaceuticals, and drug discovery and delivery (PDDD), detection, diagnosis, prediction, prognosis, prevention and treatment (DDPPPT), telemedicine and medical record (TMR), health center management and process optimization (HFMPO), surgery, medical imaging, monitoring, and dentistry (Bongomin et al., 2020). Technological convergence in DDPPPT, for example, includes synthetic biology, robotics, IoT, drones, cloud computing, blockchain, AI, and big data while in MERT it includes 3D printing, AI, AR, big data, blockchain, drones, simulation, and robotics.
Nanoscience and nanotechnology in healthcare 4.0
Although studying phenomena at the nanoscale has traditionally been an object of chemistry, nanoscience has emerged as a science that specifically devotes to this field. In the frame of nanoscience, nanotechnology searches to manipulate matter on a near-atomic scale to produce new structures, unprecedented materials, and enhanced devices to understand such phenomena better. In healthcare 4.0, nanotechnology is helping to exploit the initial point-of-measurement, data optimization, and building an information exchange network, to mention only some of its main possibilities.
Nanomaterials have enhanced electrical, mechanical, and optical properties compared to their homologous counterparts at a larger scale enabling some mechanisms to work more efficiently. For example, the high sensitivity and, therefore, more accurate data points are often due to nanomaterials' high electrical conductivity and charge carrier mobility. In intelligent (bio)sensors, the sensing mechanism invokes a change in the nanomaterial's thermal, mechanical, optical, or electrical conductivity depending on the (bio)sensing mechanism, but always with a measurable output signal. The sensitivity of the resulting devices is often high, as very slight changes in temperature, mass, conductivity, or optical properties generate a detectable response.
Nanomaterials are building blocks that can be assembled as nanocarriers to encapsulate drugs. Nanocarriers can be functionalized with ligands to promote the site-specific direction of drugs reducing side effects and enhancing the efficacy and efficiency of therapeutic regimens. In addition, nanoparticles can be specifically uptake by cells for cell labeling and diagnosis; alone or coupled with fluorophores they can serve as contrast imaging to support the diagnosis and be co-encapsulated into nanocarriers for chemo-, thermo-, or photo-therapy (Becerra et al., 2022).
Intelligent nanobiosensors device-based diagnostic
Nanobiosensors are small devices that provide qualitative, semi-quantitative, or quantitative information about a specific target analyte or biomarker in a particular sample (Quinchía et al., 2020). Electrochemical nanobiosensors integrate a transducer platform decorated (or not) with nanomaterials and functionalized with biomolecules to interact with the target rapidly and concentration-dependently. The transducer converts physical, (bio)chemical, or biological information into an electrical signal of simple readout. The biomolecules, also called bioreceptors, are in direct contact with the transducer and are responsible for the specific interaction with the target. The output system is commonly filtered and amplified through proper circuitry for the final signal display, even in pocket devices.
The transducers are often modified with nanomaterials to take advantage of matter's unique electronic, optical, thermal, and catalytic properties at the nanometer scale. Nano-materials can be of zero dimensions (0D), such as quantum dots (QDs), fullerenes, gold nanoparticles (AuNPs), magnetic nanoparticles (MNPs), and other NPs; 1D including nanowires, nanorods, single wall carbon nanotubes (SWCNT), multiwall carbon nano-tubes (MWCNT); 2D such as clays, MoS2, black phosphorous, transition metal oxides, layered double hydroxides, and 3D, involving metallic organic frameworks, amorphous carbon, and nanoconjugates, among many others (Soto & Orozco, 2022b). Also, nano-materials at the transducer platforms have an enhanced area/volume ratio offering a proper 'nanoenvironment' for hosting many bioreceptors while helping keep their conformation, native structure, and functionality. Besides modifying the transducers, nanomaterials can also be part of the amplification system of the bioreceptor-target biorecognition event. For example, nanozymes, enzyme-mimicking synthetic NPs, are emerging as amplification systems of higher efficiency, cost-effectiveness, mass production, and long-term stability compared with their homologous naturally occurring enzymes (Cajigas & Orozco, 2020).
Bioreceptors are functional biomolecules anchored at the transducer platforms to interact specifically with the target. Most common bioreceptors include enzymes, anti bodies, DNA strands, but other biomolecules, including peptides, aptamers, and (glycol) proteins, organelles, and whole cells have served as bioreceptors in nanobiosensing approaches (Echeverri & Orozco, 2022b).
Bioreceptors ensure that the biorecognition event is specific and minimize unspecific interactions. Biomolecules are contacted to the transducer platforms by entrapment, ionic interaction, decoration layer-by-layer, and supramolecular and covalent interactions, one of the more comprehensive approaches for linking bioreceptors producing more stable junctions (Fernández & Orozco, 2021).
Electrochemical nanobiosensors are particularly interesting in Healthcare 4.0 because they can be designed and assembled on demand with ultra-high sensitivity, selectivity, and specificity for plenty of molecular targets. They are amenable to miniaturization, require small volumes of reagents and samples, and can be automatized for continuous, in-place monitoring, even in remote settings.
Nanobiosensors can be mass-produced for easy operation and interpretation of output information, even for not-specialized personnel or users, and ubiquitous testing boosted by smartphones. They can be used not only for detecting a target analyte or biomarker but also for diagnosis, prognosis, and knowing the course of diseases. In this context, intelligent diagnostics, which is based on smart data from massive diagnostic test devices analyzed by BA, can play a critical role in Healthcare 4.0, as it benefits from diagnostics linked to new digital Industry 4.0 technologies such as IoT, BDA, ML, DL, BA, AI, AR, neural network (NN) cybersecurity, system integration, cloud and fog computing, and, especially, from intelligent sensors and smartphones (Ting et al., 2020). These innovative tools hold the potential to rapidly generate biomedical data and opportunities not only for diagnosis but prevention, control, treatment, surveillance, and disease management. For instance, point-of-care (POC) devices (Dincer et al., 2019) can be connected with epidemiologic modeling, real-time online databases, virtual clinics, AI-assisted diagnostics, and prognostics to classify medical conditions automatically, as well as distribution of patients' medications (Ting et al., 2020).
POC systems are small, portable, simple devices that can be used in decentralized outside settings, close to the individual, and even at home. Wise diagnostic tests and devices in Healthcare 4.0, including POC devices, should adjust to the REASSURED criteria proposed by WHO in 2018: real-time, easy sample collection, affordable, sensitive, specific, user-friendly, rapid, equipment-free, and delivered to end users (Pérez et al., 2022; Pérez & Orozco, 2022).
Furthermore, devices fulfilling the REASURED criteria are well-intricated with new digital Industry 4.0 technologies. For instance, real-time connectivity features are crucial to reading and communicating the test results and receiving feedback for proper decision-making, remote monitoring of patient's clinical progress, treatment process, and therefore, faster and better disease control. Briefly put, in REASSURED-based diagnostics, intelligent data analysis from millions of decentralized diagnostic devices worldwide can promote intelligent diagnostics through digital technologies such as BDA, ML, DL, NN, BA, and AI. Additionally, real-time diagnostic connectivity can be supported by quick response (QR) international standard codes (Scherr et al., 2017) using fog servers with lower latency than cloud ones. Remarkably, smartphones are ubiquitous, efficient, easy-to-use tools that can promote the migration from batch to innovative (bio) sensing toward intelligent diagnostics. Apart from miniature computers with fast operating systems, 6.8 billion people (94% of the world's population) use smartphones regularly, even in low-income settings without suitable healthcare systems (Xu et al., 2015). This reveals the prominent role of smartphones in telemedicine and the remote monitoring of patients through plenty of IoT devices, including intelligent thermometers, patches, and nanobiosensors (Chamola et al., 2020; Morales-Narváez & Dincer, 2020).
Nanobiosensors based on hybrid materials
Nanobiosensors based on nanohybrids and nanocomposites have attracted increased attention because they provide enhanced sensitivity, selectivity, robustness, and simplicity to the functional interfaces (Cajigas & Orozco, 2020; Soto & Orozco, 2022b). Nano-composites combine two or more materials of different properties resulting in novel physicochemical properties with one of the dimension constituents at the nanoscale or, instead, the nanocomposite structure exhibiting a nanometric phase separation of the individual components. Therefore, nanocomposites present mixed properties based on the original properties of each nanomaterial constituent (Ashby et al., 2013). Similarly, organic and inorganic building blocks are combined in hybrid nanomaterials (Alemán et al., 2007) with a serial interface between the structural components (Ashby et al., 2013) and emerging improved physicochemical properties distinct from the components alone. Metallic nanostructures (Arduini et al., 2016), silicon nanomaterials (Ahmed et al., 2021; Hasanzadeh et al., 2012; You et al., 2020; Zhang et al., 2013), carbon nanostructures, and semiconductor polymers (Barsan et al., 2015; Duan et al., 2016; Y.Liu et al., 2015; Noreña-Caro & Álvarez-Láinez, 2016; Promphet et al., 2015) are the most common hybrid nanomaterials applicable to electrochemical biosensing to date.
Recently, we reviewed the role of hybrid materials on nanobiosensors (Cajigas & Orozco, 2020; Soto & Orozco, 2022b). Besides, a multifunctional hybrid material was developed based on iron NPs coated with graphene and supported on carbon nanotubes. The new hybrid was exhaustively characterized from the material (Gallego et al., 2017) and electrochemical (Soto et al., 2018) points of view. For this purpose, the material was deposited on the surface of a screen-printed carbon electrode (SPCE) (Figure 2A) (Soto et al., 2018), and its electrochemical properties were evaluated in each assembly stage. The results showed that the hybrid material had improved electrochemical properties compared to each component acting alone. In addition, the outer layer of graphene, covering the iron NPs, served as protection to prevent oxidation and maintain its catalytic properties for longer. Finally, it was clear that the electrochemical response of the hybrid material was proportional to changes in the concentration of hydrogen peroxide (H2O2) in the order of mM and had a higher sensitivity compared to the response of the individual components.
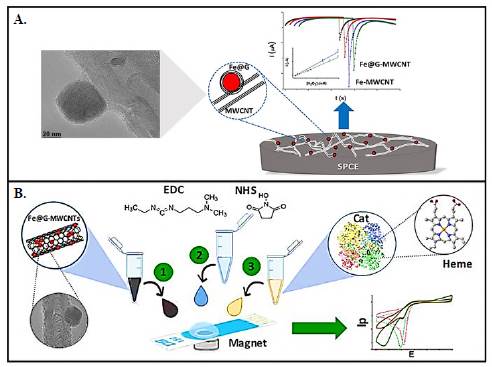
Figure 2 Nanobiosensors based on hybrid materials. A) Multifunctional hybrid material based on iron NPs coated with graphene and supported on carbon nanotubes for H2O2 sensing (Reproduced with permission, Copyright © 1999-2023 John Wiley & Sons, Inc. B) Catalase (Cat)-functionalized hybrid nanomaterial to detect H2O2 with enhanced sensitivity (Reproduced with permission, Copyright © 2023, Elsevier B.V.)
The conjugation of biomolecules with hybrid nanomaterials has offered opportunities to assemble nanobiosensors of much more improved electrochemical performance. In this context, to improve the analytical performance of the first nanohybrid structure, a novel catalase (Cat)-functionalized hybrid nanomaterial was developed to detect H2O2 with enhanced sensitivity (Figure 2B) (Soto et al., 2021). The rationally assembled nanobiosensor used Fe@G-MWCNTs deposited at an SPCE functionalized with Cat by covalent coupling. TEM, X-ray diffraction-, X-ray photoelectron- and thermogravimetric analysis were used to characterize the physicochemical properties of the hybrid nano-biomaterial and better understand its electrochemical behavior. The results evidenced enhanced detection of H2O2 (linear range from 0.1 to 7 mM, limit of detection (LOD) of 28.2 pM and sensitivity of 0.059 pA/(pM.cm2) concerning the hybrid nanomaterial laking Cat (linear range of 0.5 to 9.8 mM, LOD 0.65 mM and sensitivity of 7.97 pA/(mM.cm2).
Nanobiosensors for the detection of pathogens
Along with multiple-target analytes and biomarker monitoring, pathogen detection is paramount in precision medicine. It is part of the arsenal of tools required to know the status of a patient affected by a disease. Furthermore, pathogen detection searches to identify specific microorganism biomolecules or molecular changes in the host (Zhang & Guo, 2020). In the following paragraphs, some cases illustrating the role of pathogen detection in precision medicine it is presented. Electrochemical nanobiosensors have been used to detect viruses (Alzate et al., 2020; Alzate, et al., 2022; Alzate, et al., 2022; Cajigas et al., 2020, 2022), parasites (Echeverri et al., 2020) and bacteria (Vásquez et al., 2017) with high sensitivity, low LOD, and straightforwardness, demonstrating their potential for disease diagnosis and monitoring the host-pathogen ecosystem.
For example, we developed a dual electrochemical magneto-nanogenosensor for differential diagnosis of the Zika virus and its discrimination from homologous arboviruses such as dengue and chikungunya (Alzate et al., 2020; Alzate et al., 2022; Cajigas et al., 2020). The diagnostic tool integrated magnetic nanoparticles as a support platform with highly selective DNA strands as bioreceptors, which responded to changes in the concentration of Zika genetic material. The nanogenosensor was assembled in a sandwich format, as shown in figure 3A. A DNA strand anchored on the surface of the magnetic nanoparticles A(1) hybridizes the genetic material of the target virus with a DNA strand (2) that is labeled with an enzyme attached to an antibody (3). The resulting nanobioconjugate was transferred to a screen-printed gold electrode (SPAuE) (B) for the corresponding electrochemical reading (C). The enzyme produced an electrical current proportional to the concentration of the virus's genetic material present in samples from the patients. It was possible to sensitize the magnetic nanoparticles with specific DNA probes for Zika, Dengue, or Chikungunya, which, once transferred to the SPE compartments, allow the discrimination of the Zika virus from its homologs in a single test.
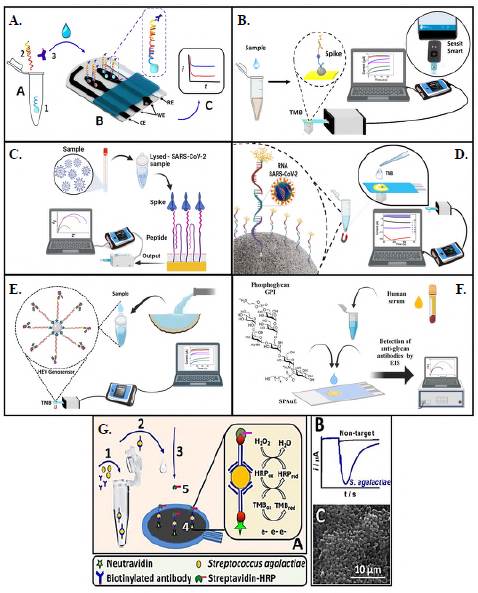
Figure 3 Nanobiosensors for the detection of pathogens. A) Dual chronoamperometric magneto-nanogenosensor for differential diagnosis of the Zika virus and its discrimination from homologous arboviruses such as dengue and chikungunya. B) Electrochemical immunosensor for amperometric detection of SARSCoV-2. C) Electrochemical biosensor based on peptides immobilized on SPAuEs for the rapid and specific detection of unlabelled spike protein from SARS-CoV-2 by EIS. D) Electrochemical genosensor for detecting viral RNA from SARS-CoV-2 by chronoamperometry. E) Highly sensitive and specific electrochemical genosensor to detect genotype 3 of hepatitis E virus (HEV) in wastewater samples by chronoamperometry. F) Synthetic glycosylphosphatidylinositol-based biosensor for the detection of toxoplasmosis by EIS. G) First amperometric immunosensor for the rapid detection of Streptococcus agalactiae (A: Sketch of the concept with all the assembling steps from 1 to 5. B: Typical chronoamperometric layout, and C: SEM image of bacteria confined at an SPCE surface. (All images were reproduced with permission, Copyright © 2023, Elsevier B.V.)
An electrochemical immunosensor was recently developed for the amperometric detec tion of SARS-CoV-2 (V.Vásquez et al., 2022). The sandwich-type immunosensor used magnetic particles that take advantage of the high-affinity spike-ACE2 proteins interaction and a poly-horseradish peroxidase (HRP) enzyme complex to amplify the resultant signal. The particles were confined at SPAuE and the reaction was followed by chronoampero-metry reaching 22.5 ng/mL LOD in 5 uL samples. The device's high performance was also tested in a pocket potentiostat (Figure 3B) with identical results compared to a laboratory potentiostat in line with the concept of POC. For this same purpose, the first electrochemical biosensor based on peptides immobilized on SPAuEs was developed for the rapid and specific detection of unlabelled spike protein from SARS-CoV-2 by electrochemical impedance spectroscopy (EIS) (Soto & Orozco, 2022a). The biosensor obtained an 18.2 ng/mL LOD in spike protein standard solutions and 0.01 copies/mL of lysed particles in 15 min (Figure 3C), which is considered clinically relevant. It is noteworthy that both devices detected the SARS-CoV-2 in positive samples (by RT-PCR) with no signal in negative samples (from healthy individuals) highlighting the potential of the devices to detect the spike protein from SARS-CoV-2 and viral particles from clinical samples.
Using magnetic particles as a supporting platform, an electrochemical genosensor was developed by covering it with thiolated capture probes (Figure 3D) for detecting viral RNA from SARS-CoV-2. The RNA was sandwiched between the capture and biotinylated signal probes modified with enzyme complexes achieving an 807 fM LOD and high specificity to discriminate from SARS-CoV, MERS, and HKU1 homologous viruses (Cajigas et al., 2022). These examples demonstrate the significant advantages of applying electrochemical biosensors to different molecular levels in SARS-CoV-2 infection as a diagnostic component in COVID-19 precision medicine.
The following example describes a highly sensitive and specific electrochemical genosensor to detect genotype 3 of the hepatitis E virus (HEV) in wastewater samples (Figure 3E) (Alzate et al., 2022). Highly specific DNA target probes were designed to hybridize a target sequence of HEV between a biotinylated capture probe and a labeled signal probe in a sandwich-type format. An enzyme-labeled antibody allowed straightforward electrochemical detection. The specificity of the probes was determined in silico and by PCR and qPCR assays demonstrating efficient amplification of two targets. The genosensor electrochemical response was target concentration-dependent from 300 pM to 2.4 nM with a sensitivity of 16.93 LiA/nM, a 1.2 pM LOD, high reproducibility, and differential against HEV genotype 3 viral genomes.
For the detection of toxoplasmosis, a transducer platform was functionalized with a synthetic glycosylphosphatidylinositol (GPI) glycan (Figure 3F) (Echeverri et al., 2020) distinguishing disease states in human sera (Götze et al., 2014). This means that a label-free electrochemical glycobiosensor for detecting anti-GPI IgG and IgM antibodies in serum from toxoplasmosis seropositive patients was established. This biosensor used the synthetic GPI phosphoglycan bioreceptor anchored at the SPAuE through a linear alkane thiol phosphodiester. The antigen-antibody interaction was detected and quantified by EIS. The resulting device showed a linear dynamic range of anti-GPI antibodies in serum ranging from 1.0 to 10.0 IU mL-1, with a 0.31 IU mL-1 LOD. The method also has great potential for detecting IgG antibodies related to other multiple medical conditions characterized by antibody overexpression. Moreover, the electrochemical biosensor has the additional advantage of a simple, portable, and cheaper tool for a closer-to-the-patient diagnosis.
The first amperometric immunosensor for rapidly detecting Streptococcus agalactiae (Vásquez et al., 2017) is a helpful example of detecting a pathogen in its ecosystem. The biosensor consists of a biotinylated antibody (AT) (Figure 3G) with two functions: to selectively interact with the antigens expressed in the bacterial cell wall (A2) and to immobilize the resulting bioconjugate (A3) at the SPEs surface coated with neutravidin (A4). When the bioconjugate reacts with the streptavidin-HRP complex (A5), an electrochemical signal was produced proportional to the concentration of bacteria present in a given sample (Figure 3G, B, signal in blue) and to the minimum signal generated with a control sample (signal in black). Figure 3G, C shows an electron microscopy (SEM) image with the bacteria confined at the SPE surface. The immunosensor allowed the selective detection of S. agalactiae cells compared with the response in samples containing other bacteria that can coexist in the same environment in 90 min. Highly sensitive quantification of bacteria was also achieved in a concentration range of 101 to 107 CFUml-1 with a 10 CFUml-1 LOD in buffered solutions doped with bacteria and the detection of bacteria in water samples from a lake in Huila, Colombia (2°41'6"N, 75°26'24"W), demonstrating its practical utility.
These examples show the detection of different pathogens for disease diagnosis and in environmental matrixes potentially useful for surveillance systems and disease control in a specific population.
Nanobiosensors for the detection of cancer-related biomarkers
Smart biosensors may play a crucial role in developing decentralized analysis systems that can bring pathogen detection and microorganism diagnostic capabilities from laboratories to remote settings and even at home. They also have promising testing solutions to monitor other disease-related biomarkers. For example, nanobiosensors for detecting a panel of biomarkers of colorectal cancer (CRC) offer opportunities for early diagnosis, prognosis, predictions of the course of the disease, metastasis, or response to treatment (Quinchía et al., 2020). For this purpose, selecting the panel of biomarkers is crucial, i.e., ideally, biomarkers of different molecular levels in line with the concept of precision medicine.
In the context of nanobiosensors for CRC-related biomarker detection, we reported the first electrochemical immunosensor for detecting p-1,4-GalT-V (Figure 4A) (Echeverri & Orozco, 2022a). A label-free electrochemical immunosensor coupled an anti-p-1,4-GalT-V antibody covalently at a mixed self-assembled monolayer (SAM)-coated SPAuE surface. This sensitized platform captured the p-1,4-GalT-V biomarker from human serum samples with high specificity. The clinically relevant response, monitored by EIS, was protein concentration-dependent showing a linear dynamic range from 5 to 150 pM, with a sensitivity of 14 Q pM-1 and a 7 pM LOD. This outstanding performance highlights its great potential for including it in a biomarker signature for the early diagnosis/prognosis of CRC.
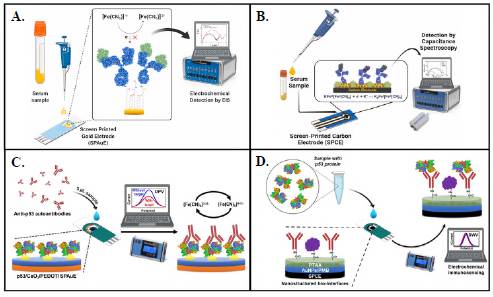
Figure 4 Nanobiosensors for the detection of cancer-related biomarkers. Electrochemical immunosensor for detecting P-1,4-GalT-V by A) EIS and B) ECS. (Reproduced with permission, Copyright © 2023, Elsevier B. C) Nanocomposite-decorated SPAuE for the label-free electrochemical detection of anti-p53 autoantibodies by DPV. D) Poly(thiophene acetic acid)/Au/poly(methylene blue) nanostructured interface at SPCE for label-free detection of p53 protein by SWCV. (Reproduced with permission, Copyright © 2023 Springer Nature Switzerland AG.)
However, to increase the sensitivity and reduce the LOD of this biosensor, we reported the first capacitive nanobiosensor for detecting p-1,4-GalT-V (Figure 4B) (Echeverri et al., 2023). To this end, we nanostructured the surface of the SPCEs with gold nanorods (AuNRs) and Prussian blue (PrB). AuNRs increased the SPCE surface area for physical adsorption of the capture antibody and promoted the deposition of PrB as a redox-active compound at the SPCE surface. The molecular biorecognition event perturbed the PrB redox density-of-states changing surface capacitance (Cu) that correlated well with increasing biomarker concentrations from 50 to 400 fM, 1.5 uF fM-1 cm-2 sensitivity, and 20 fM LOD. The label-free reagentless electrochemical device detected the glycoprotein by electrochemical capacitance spectroscopy (ECS) in unpretreated human serum samples with high specificity and ultrasensitivity. The device can be used as a CRC diagnosis/ prognosis tool in a decentralized setting with minimal patient sample manipulation and rapid response.
It is clear that detecting autoantibodies (Aabs) can be a potential approach with predictive value for early cancer diagnosis and preventive and curative treatments before the tumor progresses to the late stages (Tan et al., 2009). Thereon, we reported a nanocomposite-decorated SPAuE for the label-free electrochemical detection of anti-p53 autoantibodies (Figure 4C) (Cruz-Pacheco et al., 2022). The nanoimmunosensor was prepared by in situ electropolymerization of 3,4-ethylenedioxythiophene (EDOT) on SPAuE in the presence of CeO2 NPs. The p53 antigen was covalently linked to the resul tant Ce/PEDOT/SPAuE platform for determining anti-p53 autoantibodies by differential pulse voltammetry (DPV) in a linear range from 10 to 1000 pg mL-1 and with a 3.2 pg mL-1 LOD. The nanoimmunosensor offered high specificity, selectivity, and long-term storage stability with great potential to detect anti-p53 autoantibodies in serum samples. Incorporating organo-functional nanoparticles into polymeric matrices can provide a simple-to-assemble, rapid, and ultrasensitive approach for on-site screening of anti-p53 autoantibodies and other disease-related biomarkers with low sample volumes.
Increased serum levels of the tumor suppressor protein p53 have become a promising biomarker for diagnosing CRC (Aydin et al., 2018). We electrochemically assembled step-by-step a poly(thiophene acetic acid)/Au/poly(methylene blue) nanostructured interface at SPCE for label-free detection of p53 protein (Figure 4D) (Cruz-Pacheco et al., 2023). The conductive properties of the polymeric interface increased with an additional layer of poly(methylene blue) electropolymerized in the presence of gold nanoparticles. Anti-p53 antibodies were covalently immobilized through the poly(thiophene acetic acid) moieties at the resultant nanoarchitecture as bioreceptors. Under optimal conditions, p53 was specifically and selectively detected by square-wave cyclic voltammetry (SWCV) in a linear range between 1 and 100 ng mL-1 with a LOD of 0.65 ng mL-1. In addition, the electrochemical nanoimmunosensor detected p53 in spiked human serum samples and CRC cell lysates; the results did not exhibit significant differences compared to standard spectrophotometric methods. The resultant p53 nanoimmunosensor is simple-to-assemble, robust, and has the potential for point-of-care biomarker detection applications.
Nanobiosensors for diagnostics, prognostics, and assessing the risk of complications in patients
Some biomolecules can indicate the presence, severity, or type of disease. These biomolecules are commonly named biomarkers and play a fundamental role in diag nosing and predicting disease severity and future complications (Zhang & Guo, 2020). However, unlike single biomarkers, a panel of biomarkers should be analyzed in the con text of precision medicine as commented. For example, pathogen detection requires theidentification of specific microorganism biomolecules or host molecular changes, including detecting RNA, antigens, host-generated antibodies, or whole cells. This information can be combined with monitoring inflammatory, hematological, biochemical, and endothelial biomarkers, among others, searching to elucidate different pathways of clinical manifes tation of a disease or disease complications such as a high inflammatory response, low white blood cell and lymphocyte counts, and abnormal coagulation parameters. Such complications are associated with proteins and genetic factors expressed differently in each person and with disease progression and severity (V.Vásquez et al., 2022).
In this sense, it is worth mentioning the cytokine storm inflammatory response produced in response to, for example, viral infections and their progression (Alosaimi et al., 2020). Excessive release of inflammatory biomarkers in response to infections may trigger sepsis and complications, including lung injury and, eventually, fatal outcomes (Kumar et al., 2020). Furthermore, changes in inflammatory cytokines levels such as interleukin 1 (IL-1), interleukin 6 (IL-6), interleukin 8 (IL-8), interleukin 10 (IL-10), and tumor necrosis factor-alpha (TNF-a) and chemokines such as C-X-C motif chemokine ligand 10 (CXCL10), chemokine ligand 3 (CCL3), and monocyte chemoattractant protein 1 (MCP1) have been related with severe infections (Liu et al., 2020; Mariappan et al., 2021). High levels of IL-6, IL-10, and TNF-a have also been reported in patients with post-acute sequelae of infection after diagnosis (Peluso et al., 2021). Finally, while vitamin D is a biomarker most frequently related to susceptibility to infections, lymphocyte count (lymphopenia), procalcitonin (PCT), IL6, and C-reactive protein (CrP) have been related to disease severity, and the D-dimer, cTn, and LDH biomarkers to the risk of death, (Malik et al., 2021).
Although they are not positioned at the clinical level yet, nanobiosensors have shown outstanding potential not only for diagnostics but for prognosis and determining the course of the disease at the research level. For example, a low-cost, portable, and wireless multiplexed biosensor platform was reported for the rapid and ultrasensitive detection of nucleocapsid proteins as indicative of viral infection and IgG and IgM antibodies of the immune response and CrP of disease severity (Torrente-Rodríguez et al., 2020). In relevant physiological ranges, the device also showed a highly selective and rapid response (1 to 10 min) in blood and saliva samples. This illustrates the implementation of electrochemical biosensors at different molecular levels for diagnosis, progression, and disease severity in line with precision medicine.
Nanocarrier-based therapeutics
Proper detection and timely diagnosis are at the forefront of measures to face diseases. However, success depends on other essential processes that include the selection of proper drug regimens involving the type of drug, dose, and frequency (Sánchez et al., 2020). Encapsulating drugs into biocompatible and biodegradable polymeric nanocarriers, among nanocarriers from other materials, helps to overcome the limitations related to high lipophilicity, low water solubility, and poor biodistribution of some drugs. Furthermore, the loading capacity and encapsulation efficiency can be modulated depending on the formulation's polymer characteristics, synthesis method, and other components. Besides, this strategy protects the cargo from degradation and reaction with other drugs or molecules in the human body in its journey to the therapeutic target. Therefore, it is expected to improve the efficiency and efficacy, decrease the doses and frequencies required to reach the therapeutic doses, and increase its effectiveness, thereby preventing the appearance of side effects and the development of, for example, the resistance of microorganisms to therapeutic agents.
Most often, nanosystems utilized in drug encapsulation include lipid nanoparticles, nanoemulsions, nanosuspensions, nanogels, liposomes, niosomes, and polymeric nano-particles (Colorado et al., 2020). Among these, niosomes are vesicular systems that have drawn attention due to their chemical stability, cost-effectiveness, and preparation simplicity without harsh solvents (Aditya et al., 2017; Fidan-Yardimci et al., 2019). In this sense, it is worth mentioning our evaluation of the metabolic activity of anthocyanins (ACNs) encapsulated into niosomes in a diet-induced murine obesity model to increase their oral bioavailability. The ACN-loaded NPs with low dispersity, a negative surface charge, and 57 % encapsulation efficiency ameliorated insulin resistance and glucose intolerance and reduced animal weight and plasma insulin, glucose, leptin, and total cholesterol levels in obese mice.
Often, polymeric nanocarriers are also a choice when encapsulating drugs. Properties of nanocarriers from synthetic polymers can be readily tunned on demand by modulating molecular weight, polymer concentration, and polymer composition using surfactants and/ or energy inputs (or not), depending on the fabrication method. In this field, aiming to contribute to a new arsenal of alternatives to fight intracellular infections, we developed a therapeutic strategy based on encapsulating itraconazole (ITZ) into functionalized NPs for their targeted and controlled release into macrophages (Figure 5A) (Mejía et al., 2021). NPs were based on poly (lactic acid-co-glycolic acid) (PLGA) polymers of different compositions, molecular weights, and lactic acid-to-glycolic acid ratios self-assembled by the high-energy nanoemulsion method. Results showed improved drug-loading capacity and encapsulation efficiency by lowering the pH and using a mixture of surfactants, initial immediate ITZ release, followed by a prolonged release phase that fitted better with a Fickian diffusion kinetic model and high thermal stability. In addition, NPs were also stable, efficient, and reproducibly functionalized with F4/80 and mannose by the carbodiimide approach (Mejía et al., 2022). Overall, in-vitro assays showed the nanosystem's efficacy in eliminating the Histoplasma capsulatum fungus and paved the way to design highly efficient nanocarriers for drug delivery against intracellular infections.
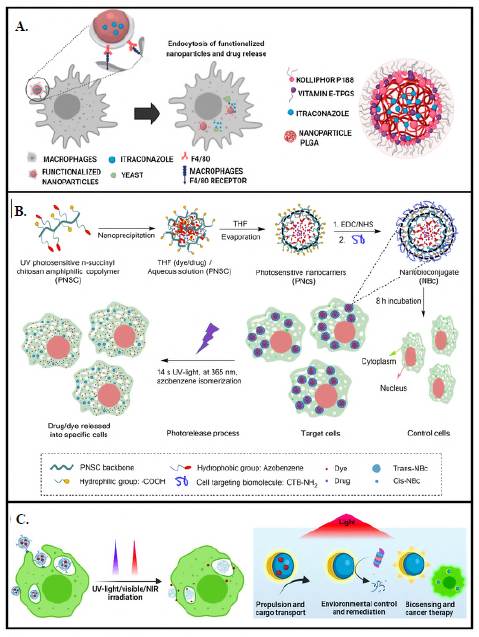
Figure 5 Nanocarrier-based therapeutic. A) Therapeutic strategy based on encapsulating itraconazole (ITZ) into functionalized NPs for their targeted and controlled release into macrophages. (Reproduced with permission, open-access articles are distributed under the terms of the Creative Commons Attribution License (CC BY). Copyright © 2023 Frontiers Media S.A.). B) Photosensitive functional chitosan backbone to assemble a photosensitive polymeric nanocarrier for intracellular administration of therapeutic principles. (Reproduced with permission. Copyright © 2023 Springer Nature Limited.) C) Micromotor-immobilized enzymes to improve their long-term stability and reusability by protecting them from UV light irradiation and accelerating substrate degradation. (Reproduced with permission. Copyright © 1996-2023 MDPI.)
Apart from synthetic polymers, natural polymers are common raw materials when encapsulating drugs. Although modulation of their properties is a little more challenging than their synthetic counterparts, they are amenable to cost-effective derivatization. Derivatization aims to introduce functionalities for coupling with other polymers, ligands, and particles or becoming susceptible to external stimuli, including temperature, pH, media, and light (Becerra et al., 2022).
Functionalization can be achieved on the polymer pre- or post-assembling the nano-carriers using the proper linking chemistry (Fernández & Orozco, 2021). For example, we introduced a photosensitive functionality into a chitosan backbone to assemble a photosensitive polymeric nanocarrier for intracellular administration of therapeutic principles (Figure 5B) (Mena-Giraldo et al., 2020). Ultraviolet (UV) photosensitive nanocarriers were then functionalized with transmembrane peptides (TPs) for intra-cellular and site-specific drug delivery. The resulting nanobioconjugate encapsulated Nile red and dofetilide, the gold standard against auricular fibrosis, with high efficiency and biocompatibility. Furthermore, the cargo was released by exposing it to UV for a few seconds without phototoxicity. Additionally, fluorescence microscopy experiments showed that the nanobioconjugate had a higher affinity for the target cells evidenced by a higher extent of cell uptake concerning bare nanobioconjugates after 8 h of incubation. Overall, the results demonstrated that administering active principles in a spatiotemporally controlled manner through functional nanocarriers is a strategy to improve the effective ness of therapeutic regimens, with the potential to reduce their side effects.
Taking advantage of the chitosan backbone derivatized with a photostimulable molecule, we proved the concept of immobilizing enzymes into micromotors to improve their long-term stability and reusability by protecting them from UV light irradiation and accelerating substrate degradation (Figure 5C) (Mena-Giraldo & Orozco, 2022). Micromotors convert different kinds of energy into movement, enhancing the kinetics and thermodynamics of the involved reactions. The movement of micromotors made of photosensitive polymers speeds up the tasks they were designed for, adding value to the inherent advantages of these naturally existing macromolecules, apart from photo stimulation. In this context, we reported on a photosensitive polymeric Janus micromotor (JM) for UV-light protection of enzymatic activity and accelerated motion-degradation of substrates. The UV-photosensitive modified chitosan JMs co-encapsulated fluorescent labeled proteins, enzymes, magnetite, and platinum nanoparticles for magnetic and catalytic motion. The JMs protected the enzymatic activity and accelerated the enzyme-substrate degradation by magnetic/catalytic propulsion. Protein-immobilized photosensitive JMs offer the potential to improve the enzyme's stability and substrate degradation efficiency.
Precision medicine
For more efficient and effective disease diagnosis and intervention, cutting-edge tech nologies, devices, therapeutic approaches, and practices are evolving depending on the patient's biology and molecular disease basis. Precision medicine is expected to revolutionize healthcare because it offers tremendous opportunities to assess disease risk and predict response to treatment by understanding a person's health status and healthcare decision-making.
The nanobiosensors mentioned before can be incorporated into microfluidic and/or multiparametric systems for monitoring multiparametric biomarkers at different molecular levels. This information can be interpreted not only along with molecular biomarker features, mechanisms, and clinical history of the patient but in the frame of the other cutting-edge converging/emerging technologies mentioned to potentially elucidate an individual's health status in a personalized manner, including diagnostics, prognostics, and potential assessment of the risk of complications in patients in the context of a new paradigm of precision medicine.
Proper and early diagnostics is one of the most critical aspects guiding therapy. The trend today considers the genetic component of the individual and involves multiple approaches, for example, nuclear imaging agents, nanoparticle-based biomarkers, in-vivo contrast imaging (Kelkar & Reineke, 2011), and laboratory tests (Xie et al., 2010) that often combine molecular assays (Kamps et al., 2017) with deep learning algorithms and AI for monitoring disease-related biomarkers (Yahata et al., 2017). The approaches in synergy ensure accurate diagnostics while holding great promise for preventive care.
After diagnosing a disease, unerring treatment at the proper dose and frequency must be pursued. However, not all treatments are adequate for all patients. Therefore, "therapy with the right drug at the right dose in the right patient" (Mancinelli et al., 2000) is promoted by precision medicine. The potential treatment can be defined based on efficacy, accuracy, and cost-effectiveness, considering the patient response based on its genome. Besides, tailored drug production, tuning composition, dose, or administration route, among other features, can be supported by computational and mathematical models to predict drug interactions, and known pharmacodynamics and pharmacokinetics, in the frame of precision medicine. Similarly, functional nanocarriers (Fernández & Orozco, 2021; Mena-Giraldo & Orozco, 2022) for site-specific and controlled drug delivery, as an object of study of precision medicine, promise to revolutionize healthcare. Additionally, theranostics is emerging as a precision approach to treating diseases such as cancer with the same (or similar) devices for diagnosis and therapy.
Translational medicine: from laboratory to clinic and back
Translational medicine (TM) research involves applying basic scientific principles and concepts to address complex clinical research questions in the clinic. Commonly referred to as a bench-to-bedside process, basic sciences such as biology, (bio)chemistry, and physics provide a conceptual understanding of complex phenomena in anatomy, physiology, and pathology, among other medical areas, to better understand disease at the cellular, tissue, organ, and systemic level. Therefore, applying concepts and principles from basic science to the biomedical field boosts the generation of innovative detection and diagnosis tools, new treatments, or novel approaches to fight diseases and investigate their mechanisms (Zerhouni, 2007). Translational research goes from in-vitro approaches that search for an interpretation of basic concepts from laboratories to applying its findings outside settings to benefit patients in the clinic. Socializing the information with patients through clinicians and public health officials is another purpose of translational medicine.
TM searches to convert molecular knowledge into practical, targeted diagnostics and therapies and apply interdisciplinary biomedical research to improve the health of patients and society in general. Therefore, it brings physicians, bench scientists, bioengineers, biostatisticians, epidemiologists, patent and regulatory experts, and patients to communicate across disciplines to achieve advances in healthcare actively and back to the lab for feedback (Krueger et al., 2019). TM implies out-of-the-box critical thinking, breaking intellectual and cultural barriers, and being outside the comfort zone with literature and basic science knowledge to apply innovative on-demand disease solutions creatively. It requires a deep understanding of the phenomena, the problem, the idea to face it, and how to build interdisciplinary teams to guide it along the long translational journey. In other words, while basic science designs experiments that validate or reject hypotheses to get knowledge (hypothesis-driven science), the starting point of translational science is health needs (need-driven science) and searches for scientific insights or tools to address them and improve health.
Concluding remarks and current challenges
Nanoscience aims to explain phenomena at the nanoscale level in a task traditionally faced by chemistry and basic sciences. Nanotechnology builds new materials, devices, and tools to exploit such understanding while nanobioengineering combines nanomaterials with biomolecules to design unusual solutions with practical applications, especially in nanomedicine. Among them, nanobioengineered diagnostic and therapeutic approaches and disruptive technologies offer tremendous opportunities that promise to revolutionize Healthcare 4.0. New digital technologies (IoT, Fog, cloud computing, DL, ML, NN, BA, AI, etc.) (Hosseinifard et al., 2021) should be integrated within intelligent system architectures as unified strategies for data collection, interpretation, and dissemination toward Healthcare 4.0. For example, nanobiosensors can be integrated into autonomous microfluidic systems for minimum consumption of reagents, samples, and energy and adapted for multiplexed on-demand monitoring of pathology-related biomarkers in portable devices compatible with the POC concept and massive data dissemination (Echeverri & Orozco, 2022b). In addition, intelligent nanobiosensors may shift to intelligent diagnostics efficiently integrated with smartphones as IoT gateways and other new digital technologies to use the data as the base for decision-making in healthcare systems. Besides, the encapsulation of drugs into intelligent drug delivery systems is positioned at the forefront of precision therapeutics that expect to impact the efficiency and effectiveness of therapeutic regimens in many pathologies.
Therefore, nanotechnology and converging technologies are pivotal in the path to precision medicine. Precision medicine is expected to have a tremendous positive impact on disease management in patients (Vásquez & Orozco, 2022). However, many challenges to discovering, developing, manufacturing, and administering novel diagnostics and therapies compatible with this new paradigm remain unresolved. For example, precision medicine should offer a new foundation to help reduce patients' risks, timelines, and costs toward future clinical trials. The effectiveness of such technologies and their broad usability have been demonstrated step-by-step, but their implementation still depends on the cost-benefit. Therefore, further directions have to shift the technology to more efficient and accessible diagnostic and therapeutic solutions.
There is no doubt about the need to bring such a radically new technology to health systems, but there are many other challenges to face. For example, it is mandatory to provide ubiquitous access to the internet, regardless of resource limitations and the country's income. A sufficient number of fog and cloud servers is required to facilitate information exchange networks while minimizing network/data traffic (Hosseinifard et al., 2021). Intelligent diagnostics' big data should be transformed into smart services. Global protocol standardization, harmonization of international rules, ethical guidelines, and security are highly demanded. Continuous smart data directly from patients or self-users at home, along with self-sampling, self-testing, data sharing, and data analysis from healthcare authorities, would be invaluable. Notwithstanding the remaining significant challenges, multi-disciplinary researchers worldwide are trying to tackle healthcare needs with innovative and more intelligent solutions. Their unified efforts for translational medicine are expected to bring all these new technologies to all in need soon.















