Introduction
A recent trend in the global polymer market is the increasing demand for polymers in numerous industries. However, these materials are highly resistant to extreme environmental conditions and their low degradability can lead to severe environmental contamination (Jem & Tan, 2020). In this context, it is necessary to promote the production of biodegradable polymers that can be decomposed by bacteria or other living microorganisms due to their potentially hydrolyzable ester bonds. The biodegradable polymers are a good alternative for replacing oil-based plastics and research in this field has been of growing interest in the last decades. Biodegradable polymers are composed of plants starch, sugar, and cellulose, or produced by living organisms. Some of the most utilized are polylactic acid, poly(vinyl alcohol), cellulose, and starch (Singh et al., 2021).
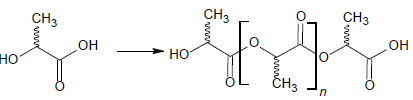
The conversion of the lactic acid monomer (LA) to poly(lactic acid) (PLA) is shown in figure 1. PLA is a biodegradable, biocompatible, recyclable, and compostable polymer made from renewables such as wheat, corn, and rice. The lactic acid obtained from fermentation is optically active, so there are three different PLA isomers types: L (+) or D (-) isomers, or a mix of them (Cunha et aL, 2022).
PLA structure consists of isomers mixture of D and L, just L, or just D, and belongs to the family of aliphatic polyesters. The structural base is 2-hydroxy propionic acid (lactic acid) (Garlotta, 2001; Jem & Tan, 2020; Rasal et al., 2010). The microstructures of the four different PLA stereoisomers that depend on their tacticity are shown in figure S1, https://www.raccefyn.co/index.php/raccefyn/article/view/1770/93. Heterotactic PLA is usually obtained from a racemic-lactide mixture of D-lactide and L-lactide, while syndiotactic PLA is obtained when meso-lactide is the raw material in the polymerization reaction (Montané et al., 2020).
The mechanical properties of PLA may vary depending on the molecular weight of the polymer and the degree of crystallinity (tacticity). High-strength and high-modulus PLA is easily processed as a thermoplastic polymer. PLA is one of the few polymers whose stereochemical structure can easily be modified by polymerizing controlled D-lactide, L-lactide, or meso-lactide to form random or block stereo-copolymers (Figure S1, https://www.raccefyn.co/index.php/raccefyn/article/view/1770/93). The molecular weight is directly controlled by stoichiometry and the addition of hydroxylic compounds (i.e., lactic acid, water, alcohols) (DeStefano et al., 2020; Garlotta, 2001).
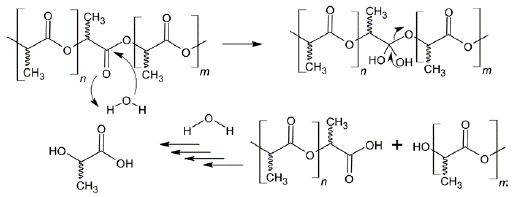
The hydrolytic degradation of the polymer matrix is affected by its degree of crystallinity. It has been shown that highly crystalline PLA will take months, sometimes years, to hydrolyze fully to lactic acid, whereas an amorphous sample is degraded in weeks. PLA is degraded by simple hydrolysis of the ester bond (Figure 2). The degradation products of PLA are also non-toxic to humans and the environment (Garlotta, 2001; Jem & Tan, 2020; Rasal et al, 2010).
There are two degradation mechanisms depending on the synergic effects of reaction kinetics and transport phenomena. If the characteristic time of water penetration is lower than the hydrolysis time scale, homogeneous or bulk degradation occurs. In this situation, the entire matrix is almost uniformly subjected to hydrolysis reactions and the volume of the device remains approximately constant. In contrast, when the degradation rate is faster than water diffusion, heterogeneous or surface degradation takes place; thus, only the surface experiences the hydrolysis reaction, whereas the bulk remains unaffected (Ali et al., 2023).
PLA is hydrophobic, so there is no dissolution, but the ester bonds break and cause chain scissions producing small oligomers that diffuse out of the PLA matrix (Figure S2, https://www.raccefyn.co/index.php/raccefyn/article/view/1770/93). The diffusion rate increases as molecular weight decreases. In in vivo environments, additional degradation is facilitated by enzymes. The degradation rate depends on several factors: hydrophilicity and amorphous structure of the polymer increase the degradation rate, while crystalline regions decrease it. Low pH favors the hydrolysis of the ester bond, whereas high pH can neutralize carboxyl end groups and enhance degradation. Additionally, plasticizers can promote water diffusion, enhancing the degradation rate (Casalini, 2017).
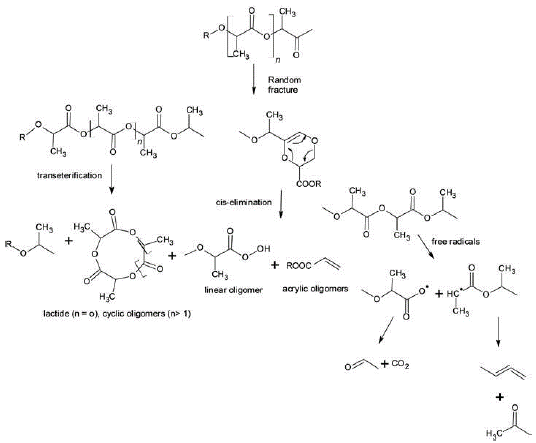
PLA thermal degradation occurs at temperatures above 200°C by hydrolysis, lactide reformation, oxidative main chain scission, and inter or intramolecular transesterification reactions (Figure 3). PLA presents its glass transition and melting temperature at around 55°C and 175°C, respectively (Garlotta, 2001; Li et al, 2023; Li et al., 2019).
PLA synthesis
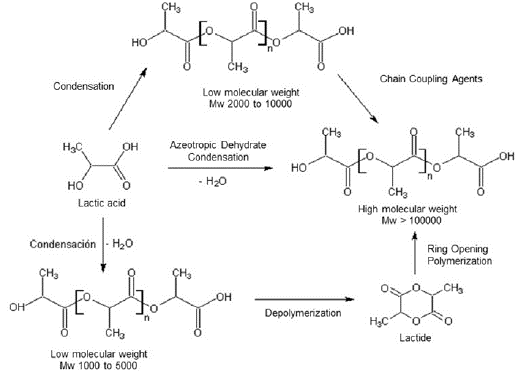
PLA is usually synthesized by direct polycondensation and ring-opening polymerization (Figure 4). Direct polycondensation is the simplest route for lactic acid molecules connecting by carboxyl and hydroxyl groups and producing water as a byproduct. The polymer obtained by polycondensation has a low molecular weight (Mw = 2000 to 10,000) and tends to be brittle and glassy, which limits its usefulness for applications where good mechanical properties are needed; it is also challenging to obtain solid enantiomeric pure PLA. Chain lengthening agents can be used to increase the molecular weight, but this leads to the formation of additional byproducts difficult to remove from the highly viscous reaction mixture (Auras et al., 2011; Balla et al., 2021; Casalini et al., 2019; Hu et al., 2016). A method to overcome these disadvantages is azeotropic dehydrates condensation, where the water is efficiently removed using appropriate azeotropic solvents. The equilibrium between lactic acid, the catalyst, and the polymer is manipulated in an aprotic solvent to produce a polymer with a relatively high molecular weight in one step (Mw > 100,000) (Orozco et al., 2007). However, the polymer thus obtained contains many impurities due to the large concentrations of catalyst used to reach good reaction rates. This method presents many problems during the processes, such as unexpected degradation, hydrolysis rates, and difficulty for achieving reproducibility. Additionally, the PLA obtained is not suitable for medical applications because of the high toxicity or non-biocompatibility of the catalysis (Garlotta, 2001; Hu et al., 2016).
The ring-opening polymerization (ROP) produces a high molecular weight polymer (Mw>100,000). Its first step is the formation of PLA oligomers, followed by the lactide (lactic acid's cyclic ester) synthesis, and finally, the ring-opening of the lactic acid cyclic dimer. Catalysts such as tin octoate (Sn(Oct)2) and p-toluene sulfonic acid are necessary. Sn(Oct)2 is chosen mainly due to its solubility in many lactones, low toxicity, FDA approval, high catalytic activity, and ability to give high-molecular-weight polymers with low racemization (Garlotta, 2001).
Since lactide is a cyclic ester, its ring can be opened by nucleophilic attack on the ester bond to start polymerization. Suitable initiators (nucleophiles) are water and alcohol, including the hydroxyl group of lactic acid. One ester linkage of a lactide ring is cleaved by the reaction of the OH group of the R-OH initiator, creating a new R-O-C(O)-ester group and an OH end group (Figure S3, https://www.raccefyn.co/index.php/raccefyn/article/view/1770/93) (Auras et al., 2011).
Recent research in ring open polymerization is focused on the use of powerful metalfree organocatalysts such as guanidine and amidine, which improve reaction efficiency under atmospheric pressure at room temperature while preventing residual metal contamination. The absence of metal contamination is advantageous in biomaterials for drug delivery (Lohmeijer et al., 2006); this alternative pathway uses bifunctional organocatalysis as thiourea-tertiary amines. The carbonyl group of lactide monomers is activated toward electrophilic attack by the thiourea via hydrogen bonding, and the initiating propagating alcohols are activated as nucleophiles by the tertiary amine (Figure S4, https://www.raccefyn.co/index.php/raccefyn/article/view/1770/93). The lactic acid (LA) is polymerized quickly in a controlled manner. There is a good correlation between theoretical and observed molecular weight with linear relationships between conversion, molecular weight, and low dispersity (Fukushima & Nozaki, 2020).
Drug delivery systems
PLA has been widely studied for medical applications because of its biodegradability and biocompatibility properties. In 1970, PLA products were approved by the US Food and Drug Administration (FDA) for direct contact with biological fluids. It has been employed to manufacture tissue engineering, scaffolds, covering membranes, various bio-absorbable medical implants, sutures in dermatology and cosmetics, and well-delivery system materials (DeStefano et al., 2020). Since PLA biodegradation products are easily cleared from the body, its use does not induce severe immune responses and eliminates the need for additional surgeries to remove the device, improving patient recovery and optimizing health system costs (Casalini et al., 2019; Gagliardi et al., 2021).
PLA is specifically used to prepare nanoparticles for drug delivery whose importance resides in reducing harmful side effects, minimizing premature degradation of the active principle, and increasing the amount of active principle in the required site. Due to their nanometric size, they improve the permeation efficiency of the active ingredient at the site of action, resolve solubility problems, ease administration, increase bioavailability, and allow the administration to sites in an appropriate minimum dose by different routes (oral, nasal, transdermal, parenteral, pulmonary) (Palacio et al., 2016a, 2016b; Vllasaliu et al., 2014).
The characteristics of nanoparticles can be modulated by synthesis. For example, the preformed polymer molecular weight is very important because it influences the particle size, the encapsulation degree, the adsorption, the physicochemical interaction of the drug or therapeutic agent, the release rate, and the z potential. A different behavior has been reported for nanoparticles prepared from PLA, where a low molecular weight polymer produces larger nanoparticles compared to nanoparticles prepared from a higher molecular weight PLA (Palacio et al., 2011).
Nanoparticles obtained from preformed polymers
Various methods have been used to prepare polymeric nanoparticles. The best one to prepare PLA nanoparticles is chosen depending on the physicochemical characteristics of a drug and the needs of its application. Drugs' physicochemical characteristics are relevant to achieve high loading capacity and reducing the number of nanocarriers required for administration dependent on drug-polymer interaction.
An adequate preparation method depends on the properties needed for the application. Drug molecules are either bound to the surface or encapsulated inside the nanoparticles. They can be incorporated into the nanocarriers with the polymer through covalent bonds (chemical encapsulation) or through hydrophobic, hydrophilic, or electrostatic interactions (physical encapsulation). The type of encapsulation largely depends on the desired release profiles and the polymer-active ingredient interaction. PLA nanoparticles have been prepared by solvent evaporation, solvent displacement, salting out, and solvent diffusion (Casalini et al., 2019; Khalid & El-Sawy, 2017; Kumari et al., 2010; Pinto Reis et al., 2006).
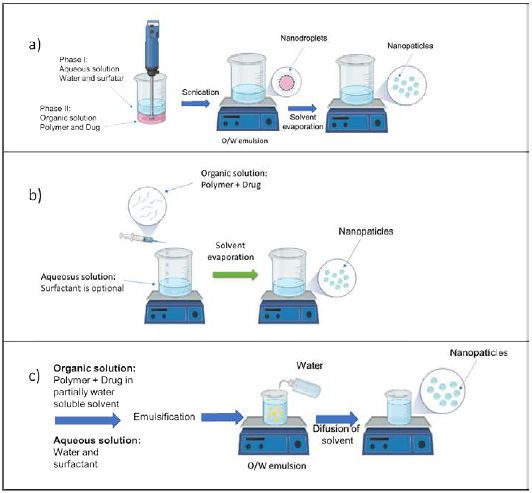
Emulsification-solvent evaporation
The emulsification-solvent evaporation method involves two steps: first, the emulsification of the PLA solution into an aqueous phase and, second, the evaporation of the polymer solvent to induce polymer precipitation as NPs. In this method, a polymer organic solution containing the dissolved drug is dispersed into nanodroplets using a dispersing agent and high-energy homogenization in a nonsolvent. The polymer precipitates in the form of nanospheres in which the drug is finely dispersed in the polymer matrix network. The solvent is subsequently evaporated by increasing the temperature under pressure or by continuous stirring (Pinto Reis et al., 2006) (Figure 5a).
Solvent displacement or nanoprecipitation
Solvent displacement and interfacial deposition are similar methods based on spontaneous emulsification of the internal organic phase containing the dissolved polymer into the aqueous external phase. Solvent displacement involves the precipitation of a preformed polymer from an organic solution and the diffusion of the organic solvent in the aqueous medium in the presence or absence of a surfactant. PLA is dissolved in a water-miscible solvent of intermediate polarity leading to the precipitation of nanospheres. This phase is injected or poured in a controlled manner (drop-by-drop addition) into an aqueous solution into or a stirred aqueous solution. Nanoparticles are formed immediately by rapid solvent diffusion. Lastly, the solvent is removed under reduced pressure or magnetic stirring (Pinto Reis et al., 2006; Tabatabaei Mirakabad et al., 2014) (Figure 5b).
Salting-out
Salting-out is based on the separation of a water-miscible solvent from an aqueous solution via a salting-out effect. This procedure can be considered a modification of the emulsification/solvent diffusion process. Initially, the polymer and the drug are dissolved in a solvent, such as acetone. The resulting solution is then emulsified into an aqueous solution containing an electrolyte salt and a colloidal stabilizer. Dilution with water or aqueous solution of the oil/water emulsion promotes diffusion of acetone into the aqueous phase, which leads to the formation of Nps. (Pinto Reis et al., 2006) (Figure 5c).
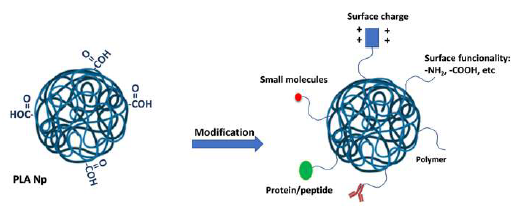
PLA modification
For drug delivery applications, the nanocarrier surface properties are of major importance to modulating cell affinity, aggregation features, drug release, interaction with the active principle, and biodistribution. Besides, they can facilitate and improve the pharmacokinetics of nanocarriers, enhancing the physicochemical properties of the active principle and/or its biocompatibility and mucosal delivery (Gagliardi et al., 2021; Kumari et al., 2010; Vllasaliu et al., 2014). Also, PLA can be chemically modified through its carboxylic and hydroxyl groups in lactic acid monomer to incorporate new surface functional groups (amino, hydroxy, carboxyl, sulfonic groups) (Figure 6), or it can be mixed or linked with other polymers to improve certain characteristics, or blend with other chemicals or copolymerized with other monomers (Rasal et al., 2010). In most cases, the modifications are made before NPs preparation. For example, for oral administration, Nps must adhere to the mucus; however, strong interaction with mucus could increase retention at the mucosal surface and Nps could be trapped and rapidly removed from the gastrointestinal (GI) system. These interactions are driven by hydrogen bonding, Van der Waals interactions, polymer chain interpenetration, hydrophobic forces, and electrostatic/ionic interactions. It seems, therefore, that the Nps surface plays an important role in particle uptake (Palacio et al., 2016a). The modification of the surface of the PLA Nps can be done before or after preparing the particles (Orozco et al., 2013).
Additionally, nanocarriers can improve passive and active targeting. Passive targeting is a strategy that enables the Nps to be directed to a specific target through modulation of their size, shape, charge, hydrophobicity, and stiffness. The active targeting approach is based on the surface conjugation with ligands or their adsorption to direct the nanocarrier to targets on the surface of cells (e.g., ligand-receptor, antibody-antigen, or lectin-carbohydrate interactions) (Gagliardi et al., 2021; Palacio et al., 2016a) (Figure 7).
PLA copolymers
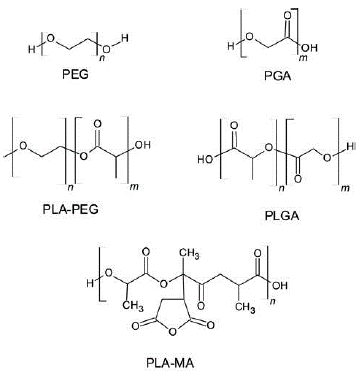
Copolymerization is one of the important methods of PLA chemical modification. Chemical binding is more effective because blending requires additional purification steps to incorporate chemicals or additives and coverage density is limited by steric hindrance, increased reaction time, and production cost (Puthumana et al., 2020). PLA-based copolymers can be of "block" or "graft" architecture. The PLA needs to have at least one reactive function to obtain these blocks or graft copolymers. However, PLA has only reactive functions at its chain ends, typically alcohol and carboxylic acid functions (Coudane et al., 2022). The copolymer properties depend on the copolymerization method, the structure of the block copolymer, and the different comonomers added. PLA-polyglycolide and PLA-polyethylene glycol are the most widely used synthetic biodegradable polymer for biomedical applications (DeStefano et al., 2020; Palacio et al., 2011).
PLA modification with PEG
Polyethylene glycol (PEG) or polyethylene oxide (PEO) is a non-ionic hydrophilic polyether (Figure 8). PEG is biocompatible, presents low immunogenicity, and is soluble in water and organic solvents. After its administration, it is eliminated from the body through the kidneys (Harris, 1992).
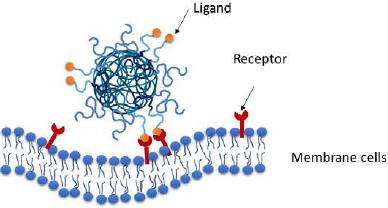
Figure 7 Active targeting: Nps interacting with specific targets on the surface of cells (ligand-receptor)
PLA can be copolymerized with PEG by bulk chemical modification using its carboxyl and hydroxyl groups. In PLA-PEG copolymer, the hydrophilic/hydrophobic molar ratio can affect colloidal stability, degradation, release profiles, particle size, and encapsulation efficiency (Xiao et al., 2010; Yang et al, 2010). The physical properties of different commercially available forms, such as the degree of crystallinity and the molecular weight (MW), influence the chemical composition and structure of PLA (Xiao et al, 2010; Yang et al., 2010).
Nanocarriers have different morphologies. For instance, those prepared from copolymers such as PLA-PEG can exhibit nanostructures such as micelles, nanoparticles, or vesicles, due to their amphiphilic nature. Specifically, it has been reported that the different arrangements mentioned above can be obtained depending on the fraction of PEG in the copolymer (Ahmed & Discher, 2004; Tang & Pikal, 2004). Additionally, the hydrophilic/ hydrophobic ratio can influence the particle size of the nanocarriers, the colloidal stability, biodistribution, and drug release, and even the PEG crown conformation (Palacio et al., 2016a; Riley et al., 1999; Vllasaliu et al., 2014; Xiao et al., 2010). Diblock copolymers with small hydrophilic block PEG (PEG volume fraction, fEO <20%) and large PLA MW blocks exhibit a strong propensity for sequestering their immobile hydrophobic blocks into solid-like particles. An increased fEO of 20-42% generally shifts the assembly towards more fluid-like vesicles or other ''loose'' micellar architectures. However, for fEO > 42% micelles are obtained generally (Ahmed & Discher, 2004).
Nanoparticles can be rapidly removed from the circulation in the body by the reticuloendothelial system (RES). This removal is dictated by the absorption of plasma proteins (opsonins) on the surface of the NPs, which leads to the recognition and sequestration by the Kupffer cells in the liver and macrophages in the spleen. The short circulation time of these systems limits their ability to reach the targeted tissue and their effectiveness. PEG incorporation on the surface of PLA NPs confers them with stealth properties by creating a hydrophilic steric barrier that delays opsonization and rapid recognition by the RES. The effects of PEGylation are highly dependent on the PEG molecular weight, polymer chain architecture, and surface density of the PEG coating, which leads to transitions in PEG conformations ("mushroom" or "brush") at the surface (Figure 9). There is a consensus that stealth properties can be achieved by coating with a high-density PEG with MW ranging from 2K to 10K using a brush regime (Betancourt et al, 2009; Perry et al., 2012; Tobío et al., 2000; Vllasaliu et al, 2014).
PLA modification with PGA
The modification by copolymerization of lactic acid with glycolic acid to obtain poly(D, L-lactide-co-glycolide) (PLGA) produces a linear copolymer (Figure 8). This modification improves biodegradability and lowers the PLA melting point. There are several ways to obtain PLGA (Lu et al., 2023). PLGA of low molecular weight (LMW) is obtained by polycondensation of lactide and glycolide acid at a temperature above 120°C. PLGA of high molecular weight can be obtained by using ring-opening polymerization with a metal catalyst (stannous octoate) (Tabatabaei Mirakabad et al., 2014).
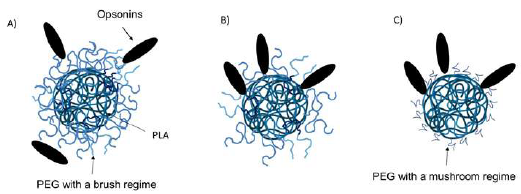
Figure 9 Effect of PEG surface density and its conformation on the opsonization process: A) opsonization is not possible at higher surface density using a brush regime for PEG conformations; B) opsonization occurs when density is low, and C) mushroom regime for PEG conformations
PLGA is an FDA-approved biomaterial characterized by its stability, biocompatibility, and biodegradability. Its non-immunogenic and non-toxic degradation residues make it an ideal choice for drug delivery systems. PLGA is one of the most widely used biodegradable polymers in the development of drug delivery systems. In the body, it undergoes hydrolysis to produce the lactic and glycolic acid biodegradable metabolite monomers, which are easily metabolized in the body via the Krebs cycle producing carbon dioxide and water (Hoyos-Ceballos et al., 2018; Kumari et al., 2010; Pourtalebi-Jahromi et al., 2020; Tabatabaei Mirakabad et al., 2014).
Hoyos-Ceballos et al. (2020) prepared PLGA nanoparticles modified with the blood-brain barrier (BBB) penetrating peptide angiopep-2 (Ang-2) used as a promising active brain-targeting drug delivery system. The accumulation of brain NPs was confirmed through in vivo analysis evidencing the localization of this kind of Nps within the brain cells and their accumulation in different brain areas such as the cortex and hippocampus. This kind of nanoparticle is a potential alternative for the treatment of brain diseases (Hoyos-Ceballos et al., 2020).
PLGA sequence has a significant impact on its degradation rate. Randomly sequenced PLGA degrades quicker than sequenced ones. Moreover, the degradation rate can be modulated by changing the PLA and PGA ratio and the molecular weight.
Lactide is more hydrophobic than glycolide. Employing higher proportions of glycolic acid results in increased hydrophilicity and, hence, the increased degradation rate of the nanoparticle formulation. One exception is the co-polymer with a 50:50 glycolic/lactic acid ratio, which has the fastest degradation rate (half-life [t1/2] about 2 weeks) among PLGA due to the amorphous nature of PLGA 50:50 (Gagliardi et al., 2021).
Grafting of maleic anhydride (MA) on PLA
The use of maleic anhydride grafting (Figure 8) to modify PLA is an excellent alternative due to the high activity of the anhydride group, its low toxicity, and cost-effectiveness (Pan et al., 2005; Orozco et al., 2013). The chemical conjugation of MA with PLA (PLA-g-MA) to improve the covalent conjugation with ovalbumin (OVA) was done via amide linkages between the carboxylic groups of the nanoparticles activated with 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide) and the amino groups of the protein. The PLA-g-MA nanoparticles increased the amount of conjugated OVA by 36 wt% compared to PLA NPs (Orozco et al., 2013).
PLA blending
Blending PLA with other polymers offers convenient options to improve associated properties or to generate novel PLA polymers/blends for target applications. A variety of PLA blends have been explored for various biomedical applications, such as drug delivery. By varying the ratios of PLA polymers/blends, a range of properties, such as drug loading, particle size, and release profiles, can be controlled. Blending eliminates the requirement to develop new polymers or copolymers and can tailor a material with the desired properties via a thermodynamically driven mixing of two or more polymers. Polymer blending provides a faster and more economical means to alter or enhance the properties of a polymer (Nyamweya, 2021; Singhvi et al., 2019). Unfortunately, PLA-based blends exhibit an insufficient performance because the blended polymers are often thermodynamically immiscible, resulting in poor compatibility between the blended components (Coudane et al., 2022; Park et al., 1992).
Palacio et al. (2016) prepared PLA/PEG nanoparticles of 100 nm using the adsorption method and an amphiphilic, non-toxic surfactant of the Pluronic® family (Figure S5, https://www.raccefyn.co/index.php/raccefyn/article/view/1770/93). The Pluronic® surfactants are triblock copolymers with a central lipophilic PPO block bonded to two hydrophilic PEO blocks. This surfactant characteristic confers colloidal stability to the NPs, even in simulated gastric fluids, due to the good hydrophobic interaction between PLA and the PPO middle block. Therefore, these nanoparticles are suitable for encapsulating hydrophobic active principles with good load capacity and delivering them via oral administration. The hydrophilic/hydrophobic balance of the PLA/PEG NPs can be easily modulated by varying the hydrophilic-lipophilic balance (HLB) of the Pluronic® (Palacio et al., 2016b).
Hoyos-Ceballos et al. (2018) prepared nanoparticles of poly(lactic-co-glycolic acid) and Pluronic® F127 (PLGA/PF127) to encapsulate epigallocatechin gallate (EGCG). This polyphenol has shown great therapeutic potential in different diseases such as cancer, diabetes, and neurodegenerative diseases. However, the limitations imposed by its low stability and rapid systemic elimination lessen their bioavailability and therapeutic efficacy. Remarkably, EGCG encapsulated in PLGA/PF127 NPs was found to prevent rotenone-induced ROS generation, loss of mitochondrial membrane potential, and DNA fragmentation in nerve-like cells (Hoyos-Ceballos et al., 2018).
Conclusions
Polylactic acid (PLA) is one of the most popular biopolymers. It can be synthesized from various renewable resources and methods. It is biodegradable and has shown potential as a biomaterial in many medical applications, such as controlled drug delivery, tissue engineering, and various medical implements. Advances in polymer chemistry encourage the study of its application in vaccines or combined chemotherapy and immunotherapy by delivering drugs and specific antibodies together for specific diseases. Today, nanomedicine has made great advances in the development of vaccines and in the treatment of various diseases, especially cancer. PLA nanoparticles offer great opportunities for succeeding in these developments.





![Design, synthesis, and electrochemical studies of a new [60] fullerene pyrrolidine as a precursor for the construction of supramolecular systems](/img/en/next.gif)