INTRODUCTION
Nutraceutical foods have received increasing global attention because of their antioxidant properties and presence of compounds that can prevent or alleviate the harmful effect of excessive oxygen free radicals (Hurst et al., 2010; You et al., 2011). Plants in the genus Vaccinium possess a high content of antioxidants and phenolic compounds in the fruits, making this group of plants of great interest for human health (Hurst et al., 2010; Istek and Gurbuz, 2017; Liu et al., 2013; Miranda, 2021). This genus includes around 400 species (Bustillo, 2018), such as blueberry (Vaccinium corymbosum) and Colombian blueberry (Vaccinium meridionale Swartz), both species with fruits of high nutrient value and rich in antioxidant compounds (Luteyn and Pedraza-Peñalosa, 2008; Garzón et al., 2010).
The root system of plants in the genus Vaccinium has a low capacity for absorbing nutrients and water because it lacks root hairs and is superficial and fibrous. So, arbuscular mycorrhizal fungi (AMF) have a fundamental role in nutrition and acclimatization (Vega et al., 2009). AMFs are known to establish a symbiotic association with plant roots, an association widely distributed in terrestrial ecosystems and different plant taxa, including the genus Vaccinium(Vega et al., 2009; Arriagada et al., 2012; Gao et al., 2012). During symbiosis, fungi acquire carbon from host plants to complete their life cycle and, in response, provide multiple benefits to plants, including improved nutrition and tolerance to factors that cause biotic and abiotic stress (Smith and Read, 2008). The mycorrhizal association offers a possible solution for environmental sustainability, as an alternative to overcome the loss of biological fertility in soils (Nadeem et al., 2014; Ganugi et al., 2019).
In its natural habitat, the blueberry (Vaccinium sp.) establishes itself in acid soils with high contents of organic matter. When it grows in soils with a pH greater than 6.5, it can have slow growth and exhibit mineral nutrient deficiencies (Liu et al., 2017). However, when these plants are inoculated with AMF, they have been found to improve responses to stress because of a high pH (Yang et al., 2020). The blueberry plants of the O'Neill variety inoculated with AMF (Glomus mosseae) under conditions of a high pH presented lower transpiration and increased photosynthetic efficiency; this was achieved by increasing the photochemical efficiency of photosystem II, expressed as significant increases in the Fv/Fm ratio when compared to the non-inoculated plants under the same stress condition. Additionally, these plants increased the amino acid contents and biosynthesis of secondary metabolites (Yang et al., 2020). The pH, soil moisture, and nutrient availability influence the colonization of AMF as well as the number of spores in the soil, with the richness of AMF tending to increase as pH and soil moisture levels become higher (Boeraeve et al., 2019).
AMF offer alternatives to improve tolerance to various abiotic stress factors, such as drought in citrus (Wu et al., 2013) and sunflower (Gholamhoseini et al., 2013); salinity stress in tomato plants (Ebrahim and Saleem, 2017), cape gooseberry (Miranda et al., 2011) and wheat (Talaat and Shawky, 2014); high temperatures and acidity (pH) in olive trees (Porras-Soriano et al., 2009), in plants (Selvakumar et al., 2014); and stress from pathogens and pests in tomatoes (Torres-Vera et al., 2014). The positive effect of mycorrhizae on the growth, development, and nutrition of plants in the Ericaceae family is particularly evident in alkaline soils with an altered chemical composition (Zydlik et al., 2019).
Multiple studies have shown that AMF facilitate the plant access to phosphorus through exploration of large volumes of soil with hyphae, transporting this nutrient to the periarbuscular membrane of the root cortical cells, thus, affecting the direct absorption of P through the roots (Ferlian et al., 2018). The genus Glomus increases the absorption of nutrients, such as N, K, and P in olive plants (Porras-Soriano et al., 2009; Chatzistathis et al., 2013); AMF also intervene in the acquisition of micronutrients such as Zn, Cu and Fe in significant amounts in wheat plants (Hussain et al., 2018; Ganugi et al., 2019) and increase the chlorophyll contents in tomato plants under conditions of abiotic stress because of a water deficiency (Ebrahim and Saleem, 2017). AMF improved the quality of the fruits in tomato plants inoculated with Funneliformis mosseae and Rhizophagus irregularis, increasing the contents of N, P and Cu in plants, in addition to antioxidants, carotenoids, and volatile compounds, as compared to the fruits of non-inoculated plants (Hart et al., 2015).
Recent studies have shown how AMF can have a positive effect on blueberry plants, such as an increase in accumulation of N, K and Mg in leaves (Zydlik et al., 2019). In addition, they improve tolerance to stress from low temperatures by increasing the contents of the antioxidant enzymes superoxide dismutase, ascorbate peroxidase and guaiacol peroxidase and proline, an amino acid that generates osmotic adjustment and functions as an osmoprotector (Liu et al., 2017). Brody et al. (2019) found that blueberry plants inoculated with ericoides mycorrhizae fungi produced larger flowers, a greater number of fruits per inflorescence, and fruits with a higher weight than plants without inoculation.
The purpose of this research was to evaluate the effect of fungi of the genera Glomus and Acaulospora on the vegetative growth and nutrition of blueberry (Vaccinium corymbosum) seedlings var. Biloxi in the Andean tropics.
MATERIALS AND METHODS
Plant material and growth conditions
This experiment was carried out in the Colombian Andes, Tabio, municipality of Cundinamarca, at an altitude of 2,569 m a.s.l., located at 4°54′57″ N and 74°05′54″ W, in two consecutive repetitions: January-June 2020 and January-May 2021. Two-month-old blueberry (Vaccinium corymbosum L.) seedlings Biloxi variety were obtained from a meristem cultivation and placed under glass greenhouse conditions, with an average temperature of 14oC, maximum temperature of 28oC and minimum temperature of 6oC, relative air humidity close to 50%, micro-sprinkler irrigation and controlled fertilization.
The seedlings were transplanted into 2 kg plastic pots (20 cm deep and 22 cm diameter) o with a mixture of blond peat (Peat Moos®, pH 4.5), coconut fiber, and washed river sand (1:1:1 v/v/v). One plant per pot was sown with three levels of fertilization: 100, 50 and 0% of the optimal recommendation according to Hirzel (2013); the nutrients were applied weekly with fertigation (Tab. 1).
Table 1. Fertilization levels used in blueberry plants (Vaccinium corymbosum) var. Biloxi for each one evaluated treatments, without inoculation with fungi forming mycorrizal (AMF) of the genera Glomus (AMF1 +) and Acaulospora (AMF2 +).
| Treatments | N | P | K | Ca | Mg | S | Fe | Mn | Cu | Zn | B | Mo |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 100AMF- | 70 | 24 | 45 | 38 | 22 | 38 | 0.20 | 3.40 | 0.07 | 0.15 | 0.10 | 0.04 |
| 50AMF- | 40 | 14 | 27 | 23 | 14 | 23 | 0.10 | 1.70 | 0.04 | 0.08 | 0.05 | 0.02 |
| 0AMF- | 9 | 5 | 8 | 8 | 5 | 8 | 0.05 | 0.85 | 0.02 | 0.04 | 0.03 | 0.01 |
| 50AMF1+ | 31 | 9 | 19 | 15 | 9 | 15 | 0.10 | 1.70 | 0.04 | 0.08 | 0.05 | 0.02 |
| 50AMF2+ | 31 | 9 | 19 | 15 | 9 | 15 | 0.10 | 1.70 | 0.04 | 0.08 | 0.05 | 0.02 |
| 0AMF1+ | 10 | 5 | 8 | 8 | 5 | 8 | 0.05 | 0.85 | 0.02 | 0.04 | 0.03 | 0.01 |
| 0AMF2+ | 10 | 5 | 8 | 8 | 5 | 8 | 0.03 | 0.43 | 0.01 | 0.02 | 0.01 | 0.01 |
Nutrients: N, P, K, Ca, Mg, S, Fe, Mn, Cu, Zn, B, and Mo (mg L-1).
Experiment design
The experiment design used in the two experiments was randomized complete blocks, with seven treatments and eight replicates. The treatments consisted of the combination of three levels of chemical fertilization: 100, 50 and 0, without inoculation with AMF (AMF-) and with inoculation with AMF of the genera Glomus (AMF1 +) and Acaulospora (AMF2 +) as follows: 100AMF-, 50AMF-, 0AMF-, 50AMF1 +, 50AMF2 +, 0AMF1 +, and 0AMF2 +.
The AMF inoculum was previously isolated from mountain soils in the municipalities of San Miguel de Sema, Raquira, and Chinquinquira (Boyaca). The fungi were found in the rhizosphere zone of wild Colombian blueberry plants (Vaccinium meridionale Swartz).
In the laboratory of the Santana farm located in Tabio, Cundinamarca, the inoculum was multiplied using trap oat (Avena sativa) and marigold (Calendula officinalis) plants under semi-controlled conditions. A mixture of blond peat (Peat Moss®), coconut fiber and matured vegetable compost (1: 1: 1 v/v/v) was sieved and used as substrate in a glass greenhouse with controlled irrigation for the inoculum multiplication. This substrate had the following characteristics: pH: 5.58; 14.7 meq/100 g electrical conductivity, 44% humidity saturation, 19% organic matter, and 0.68 g cm-3 apparent density. After six months, the substrate was sieved and two fungi of the Glomus and Acaulospora genera were isolated from the inoculum obtained for a second multiplication cycle. The morphological identification was based on spore characteristics such as: spore type formation (glomoide, acaulosporoide, entrophosporoide, gigasporoide, and scutellosporoide), sporocarp formation, size and color, structure and wall staining, and germination characteristics. The classification keys of INVAM (International Culture Collection of Arbuscular & Vesicular-Arbuscular Mycorrhizal Fungi http://invam.caf.wvu.edu/cultures/cultsearch.htm) were used, as well as the Schüßler website (http://www.amf-phylogeny.com/), which offers detailed descriptions of most internationally accepted HFMA species. Finally, the definitive inoculum was obtained, which was used in the present experiment. The inoculum had concentrations between 70 and 90 spores/g-plant for each genus. The morphological characterization of the spores and sporocarps of HFMA from rhizospheric soil samples of wild sour crops began with isolation and quantification by decanting and suspending spores in a sucrose gradient following the methodology proposed by Gerdemann and Nicholson (1963). For the inoculation of mycorrhizal treatments, 10 g of inoculum were used per plant.
Root colonization and dependence on AMF
To calculate the colonization percentage (CP) and mycorrhiza dependency (MD), the root fraction was carefully washed with distilled water and cut into 1 cm long segments. The segments were bleached in a 10% KOH solution for 15 min. Subsequently, staining was carried out with 0.05% Trypan blue in lactic acid (v/v) (Phillips and Hayman, 1970). The CP was estimated with the method described by Giovannetti and Mosse (1980). The presence of arbuscular mycorrhizal structures (AMF) was recorded with an optical microscope, and 100 fields per root and three repetitions per plant were analyzed, for a total of 12 readings per treatment. The CP was calculated according to the following equation: CP = (length of colonized roots / length of observed root) × 100. The MD = (dry weight of inoculated plants / weight of uninoculated plants) × 100 (Van der Heijden and Kuyper, 2001).
Growth parameters
During the experiment, measurements were taken for plant height (PH), number of basal branches (BB), and number of apical shoots (AS) at 20, 56, 91, 121, and 153 days after inoculation (dai). At 153 dai, the experiment ended, and the blueberry plants were divided into roots, stems, and leaves. The leaf area (LA) was determined using a portable leaf area meter (CI-202). To determine the dry mass, the plant material was dried at 70°C for 40 h in an oven. Each plant organ was weighed, and the ratio root:shoot (R/S) was calculated.
Relative chlorophyll contents in leaves
The relative chlorophyll content (RCC) was determined with a CCM-200 chlorophyllometer (Opti-Sciences,Hudson, NH). Measurements were taken between the 4th and 5th leaf of the main stem, with 12 measurements per leaf, and the values were expressed as Chlorophyll Content Index: CCI (Castañeda et al., 2018).
Florescence of chlorophyll a
To have information on the status of photosystem II (PSII), the fluorescence of chlorophyll a was measured with a Junior PAM fluorometer (Portable Junior PAM; Gademann Instruments GmbH, Effeltrich, Germany). The plants were adapted to the dark for 2 h before the measurement, which was carried out between the 4th and 5th leaves of the selected stem. The maximum quantum yield variable of the PSII (Fv/Fm) was recorded (Ebrahim and Salem, 2017).
Foliar analyses
The foliar analyses were carried out on all plants in each treatment at 153 dai at the end of the experiment. The foliar nitrogen analysis was done with Micro-Kjeldahl using the volumetric technique, in accordance with the Colombian technical standard (NTC 370, Colombia INCONTEC, 2011). For the quantification of phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), sulfur (S), the detection method with Microwave Assisted Extraction (MAE) was used, with nitric acid dissolved in water, HNO3: H2O2 and the ICP-OES Avio 200 inductively coupled plasma Optical Emission Spectroscopy technique (Perkin Elmer, Santiago). The K contents was evaluated with an Atomic Absorption 3100 Spectrophotometer (Perkin Elmer, Santiago) with an air-acetylene flame with direct aspiration, and the S was measured with barium sulfate turbidimetry (Aguilera et al., 2010).
Statistical analysis
The results were analyzed using the statistical program IBM® SPSS® Statistics 25. To determine the statistically significant differences between each treatment according to the randomized complete block experiment design, the comparison tests of Duncan were applied, with a significance level of P≤0.05.
RESULTS AND DISCUSSION
Colonization and dependence on HFMA
At 153 dai, the levels of colonization and the degree of mycorrhizal dependence of the treatments with plants inoculated with AMF were evaluated (Fig. 1A). A higher colonization with AMF was observed in the treatments with a mean level of fertilization of 50AMF1+ (45%), followed by 50AMF2+ (40%), while the treatments with low levels presented values significantly lower than 0AMF1+ (25%) and 0AMF2 + (19%). The dependence of blueberry plants to form symbiotic associations was high for the low fertilization treatments 0AMF1+ (255%) and 0AMF1+ (220%), when compared to the half fertilization treatments 50AMF1+ (145%) and 50AMF2+ (140%) (Fig. 1B).
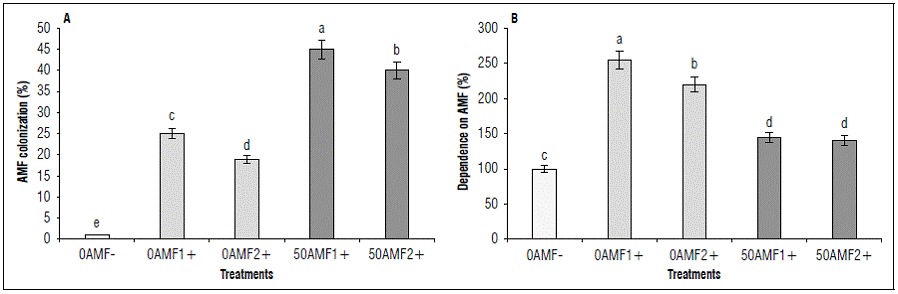
Figure 1. Percentage of colonization (a) and dependence of blueberry plants (Vaccinium corymbosum) var. Biloxi to AMF (b) at 150 days after inoculation (dai), with two genera of fungi Glomus (AMF1 +) and Acaulospora (AMF2 +), with two levels of fertilization (50 and 0). Different letters show significant differences at P<0.05 according to Duncan's test.
At 153 dai, the levels of colonization and the degree of mycorrhizal dependence of the treatments with plants inoculated with AMF were evaluated (Fig. 1A). A higher colonization with AMF was observed in the treatments with a mean level of fertilization of 50AMF1+ (45%), followed by 50AMF2+ (40%), while the treatments with low levels presented values significantly lower than 0AMF1+ (25%) and 0AMF2 + (19%); while the treatment without mycorrhization was 0%. The dependence of blueberry plants to form symbiotic associations was high for the low fertilization treatments 0AMF1+ (255%) and 0AMF1+ (220%), when compared to the medium fertilization treatments 50AMF1+ (145%) and 50AMF2+ (140%) (Fig. 1B). Degree of mycorrhizal dependence of the treatments with plants inoculated with AMF were evaluated (Fig. 1A). A higher colonization with AMF was observed in the treatments with a mean level of fertilization of 50AMF1+ (45%), followed by 50AMF2+ (40%), while the treatments with low levels presented values significantly lower than 0AMF1+ (25%) and 0AMF2 + (19%). The dependence of blueberry plants to form symbiotic associations was high for the low fertilization treatments 0AMF1+ (255%) and 0AMF1+ (220%), when compared to the medium fertilization treatments 50AMF1+ (145%) and 50AMF2+ (140%) (Fig. 1B). The results showed the establishment of symbiosis with both genera of AMF, Glomus and Acaulospora, expressed both in percentage of colonization and in mycorrhizal dependence, being higher for the genus Glomus than for Acaulospora for the blueberry plant (Vaccinium corymbosum) variety Biloxi. This agrees with findings for other plants, where preferences for the fungus type can occur depending on the variety and the edaphic condition or type of stress to which the plant is subjected.
The existence of specific interactions between the fungus and the host plant implies a previous selection of different associations to maximize the benefits of the symbiosis (Ortas et al., 2011, 2012). Colonization percentages and dependence on G. mosseae in blueberry plants varied with cultivar, thus, the Britewell cultivar had higher parameters than the Misty cultivar for optimal growth at low temperatures (Liu et al., 2017).
Growth variables
By increasing fertilization, an increase in the total DM was observed at 153 dai (Fig. 2), where the complete fertilization treatment without inoculation (100AMF-) presented significantly higher values (10.67 g), with respect to the other treatments with lower doses of fertilization, without inoculation, 50AMF- (8.21 g) and 0AMF- (7.23 g) and inoculated 50AMF1+ (9.20 g), 50AMF2+ (8.02 g) , 0AMF1+ (6.44 g) and 0AMF2+ (7.27 g) at 153 dai (Fig. 2). These values correspond to the DM of the roots (3.68, 3.36 and 3.37 g), stems (4.13, 2.77 and 2.33 g) and leaves (2.86, 2.08 and 1.52 g), for the uninoculated treatments, 100AMF-, 50AMF- and 0AMF-, respectively. The mycorrhizal treatment with Glomus, 50HFM1 + presented a significantly higher DM of the plant (9.20 g) in relation to the other treatments without inoculation, 50AMF- (8.21 g), 0AMF- (7.23 g), and inoculated 50AMF2 + (8.02 g), 0AMF1 + (6.44g) and 0AMF2 + (7.27g).
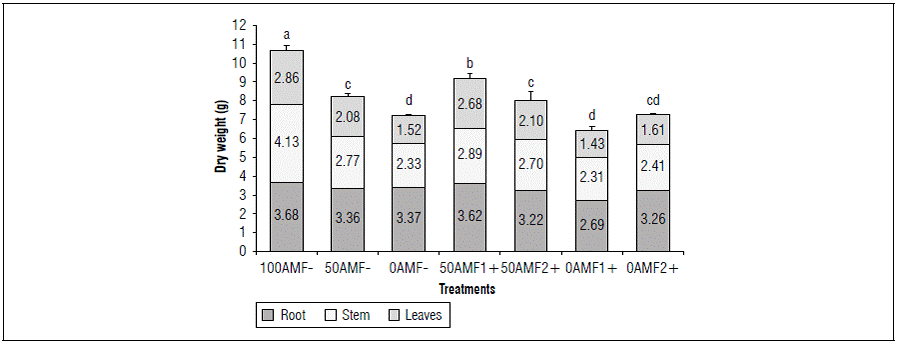
Figure 2. Distribution of dry biomass in blueberry plants (Vaccinium corymbosum) var. Biloxi 153 days after inoculation, with two genera of fungi, Glomus (AMF1+) and Acaulospora (AMF2+) and three levels of edaphic fertilization (100, 50, 0). Different letters show significant differences at P<0.05 according to Duncan's test. The standard deviation bars represent full dry weight.
These results indicate that the plants inoculated with Glomus (50AMF1+) significantly exceeded the DM with the same level of fertilization without inoculation (50AMF-) and had significantly lower DM values than the 100AMF- treatment at 153 dai. On the other hand, the plants inoculated with each of the fungal genera and with low fertilization (severe deficiency) 0AMF1+ 0AMF2+ did not present significant differences in relation to the 0AMF- and 50AMF- treatments. This indicated that inoculation with HFMA with a medium availability of nutrients (moderate deficiency) contributed to the accumulation of biomass, but this effect might not be observed when the nutrients have low concentrations (severe deficiency) (Tab. 1).
The response of specific growth variables, such as PH, BB, AS and LA, were significantly higher in the 100AMF- treatment, except for the R/S ratio, where they presented the highest values, which were to the 50AMF1 + and 50AMF2 + treatments and to the 50AMF2 + treatment for BB (Tab. 2).
Table 2. Effect of the inoculation of mycorrhizal fungi (AMF) with strains of the genus Glomus (AMF1+) and Acaulospora (AMF2+) on blueberries (Vaccinium corymbosum) var. Biloxi with three levels of fertilization (100, 50 and 0), on some variables of plant growth: plant height (PH), number of basal branches (BB), number of apical shoots (AS), leaf area (LA) and the relationship, root/shoot, at 153 days after inoculation (dai).
| Treatments | Plant height (cm) | Basal branches (No.) | Apical shoot (No.) | Leaf area (cm2) | Ratio: Root/Shoot |
|---|---|---|---|---|---|
| 100AMF- | 68.2±7.7 a | 5.7±0.7 a | 20.7±4.7 a | 744.2±66 a | 0.79±0.13 a |
| 50AMF- | 43.9±4.7 c | 3.4±0.5 d | 10.9±2.5 c | 565.4±70 c | 0.62±0.07 b |
| 0AMF- | 35.3±5.1 d | 4.1±0.4 c | 14.4±2.1 b | 235.1±17 e | 0.46±0.06 d |
| 50AMF1+ | 51.5±10.0 b | 4.9±0.4 b | 15.6±3.5 b | 654.2±55 b | 0.74±0.07 a |
| 50AMF2+ | 43.3±4.6 c | 6.1±1.2 a | 15.0±1.3 b | 644.1±46 b | 0.66±0.17 ab |
| 0AMF1+ | 35.3±4.1 d | 4.0±0.3 c | 15.4±1.5 b | 355.1±17 d | 0.54±0.11 c |
| 0AMF2+ | 40.1±4.4 cd | 4.8±0.7 bc | 16.1±2.8 b | 335.1±18 d | 0.50±0.06 c |
| ANOVA | *** | *** | *** | *** | *** |
Different letters indicate significant differences for n = 8 according to Duncan's test (P≤0.05). Significance level: *** (P<0.001).
The mycorrhizal treatment with Glomus 50AMF1+ showed significantly higher PH values (51.5 cm) with respect to the treatments 50AMF- (43.9 cm), 0AMF- (35.3 cm), 50AMF2+ (43.3 cm), 0AMF1+ (35.3 cm) and 0AMF2+ (40.1 cm) but not with 100 AMF- (68.2 cm). On the other hand, the plants inoculated with Acaulospora 50AMF2+ and Glomus 50AMF1+ obtained higher values in BB and LA in relation to the treatments 50AMF-, 0AMF-, and 0AMF1+ but not with 100AMF-. These results indicated the beneficial effects of inoculation with Glomus (50AMF1+) on PH, while inoculation with Acaulospora (50AMF2 +) favored BB.
For AS, most of the treatments (0AMF-, 50AMF1+, 50AMF2+, 0AMF1+ and 0AMF2+) behaved similarly, while 100AMF- was significantly higher and 50AMF- was significantly lower. This result showed that inoculation with AMF of both genders in the medium and low doses had no effect on the variable AS. Finally, for the R/S ratio, the control treatment 100AMF- and the mycorrhizal treatments (50AMF1+ and 50AMF2+) were significantly higher than the treatments without inoculation 50AMF- and 0AMF- and the inoculated treatments 0AMF1+ and 0AMF2+. The R/S ratio in treatments 0AMF1+ and 0AMF2+ (severe stress) were higher than 0AMF-. These results highlighted the role of inoculation with AMF in stimulating the root development to compensate for the nutrient deficiency when there are medium levels of nutrients in the substrate.
Similar results have been reported in many species, such as sunflower (Gholamhoseini et al., 2013), apple, cherry (Grzyb et al., 2015), pear (Świerczyński et al., 2015), and olive (Porras-Soriano et al., 2009), where AMF offer alternatives to improve tolerance to various drought or salinity stresses with increases in biomass and improvement of plant growth, when comparing inoculated with non-inoculated plants.
Relative chlorophyll contents in leaves
It was observed that the relative content of chlorophylls was significantly higher in 100AMF- at 20 dai (28.12 CCI), 91 dai (38.60 CCI) and 153 dai, (34.91 CCI) than in the other treatments, except at 153 dai where no differences were observed between this treatment 100AMF- (34.91 dai) and the treatments with nutrient deficiency and inoculation with Glomus 50AMF1+ (32.37 CCI) and with Acaulospora 50AMF2+ (33.72 CCI) (Fig. 3).
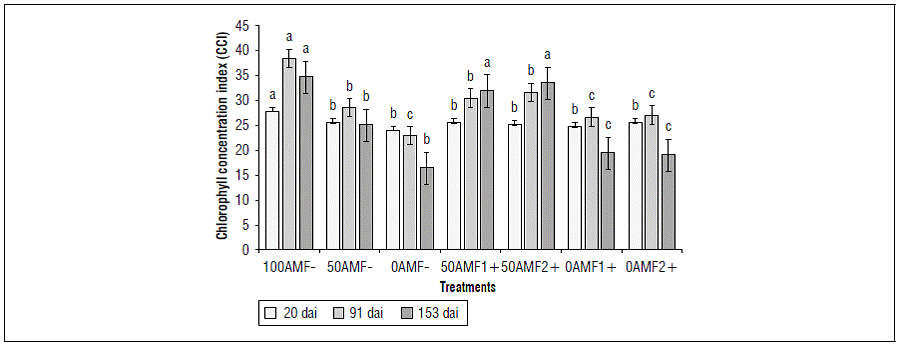
Figure 3. Effect on the chlorophyll concentration of blueberry plants (Vaccinium corymbosum) var. Biloxi at 153 days after inoculation (dai), with two fungal genera, Glomus (AMF1 +) and Acaulospora (AMF2 +) and three levels of edaphic fertilization (100, 50, 0). Different letters show significant differences at P<0.05 according to Duncan's test.
All treatments with a low nutrient content had the lowest CCI, especially at 91 and 153 dai, 0AMF- (23.25 and 16.66 CCI), 0AMF1+ (26.95 and 19.73 CCI) and 0AMF2+ (26.91 and 19.45 CCI). The inoculated treatments with low fertilization (0AMF1+ and 0AMF2+) did not have a positive response to CCI symbiosis since the plants were subjected to extreme nutrient deficiency conditions.
The beneficial effect of inoculation with Glomus and Acaulospora appeared under nutrient deficiency once the symbiosis was established at 153 dai, when the plants began a process of recovery from the condition of nutrient stress with the induction of chlorophyll synthesis in blueberry plants. Recent studies have shown how inoculation with AMF has a significant increase in chlorophyll content in different plant species, such as strawberry plants (Mikiciuk et al., 2019) and tomatoes (Ebrahim and Saleem., 2017), as compared to plants without inoculation. This is due to an increase in the absorption of nutrients by mycorrhizae, mainly magnesium and nitrogen (Zhu et al., 2017). Similar results were reported for blueberry cultivar O'Neal with an increase in chlorophyll content when inoculated with Glomus mosseae (Yang et al., 2020).
Fluorescence of chlorophyll a
For the variables derived from the fluorescence of chlorophyll a in blueberry plants (Vaccinium corymbosum) at 153 dai, Glomus (AMF1+) and Acaulospora (AMF2+) had a reduction in Fv/Fm in all nutritional treatments at 20 dai (0.61 - 0.64), versus 100AMF- (0.75). These differences were highly significant according to Duncan's test (Fig. 4). These results indicated the stress in the nutrient deficient blueberry plants with and without inoculation. During the first 20 dai, the inoculated treatments presented stress symptoms with Fv/Fm values lower than 0.65 since the symbiotic association had not yet been established. It is noteworthy that the plants without inoculation and with a low level of nutrients (0AMF-) recorded the lowest chlorophyll fluorescence value (0.52).
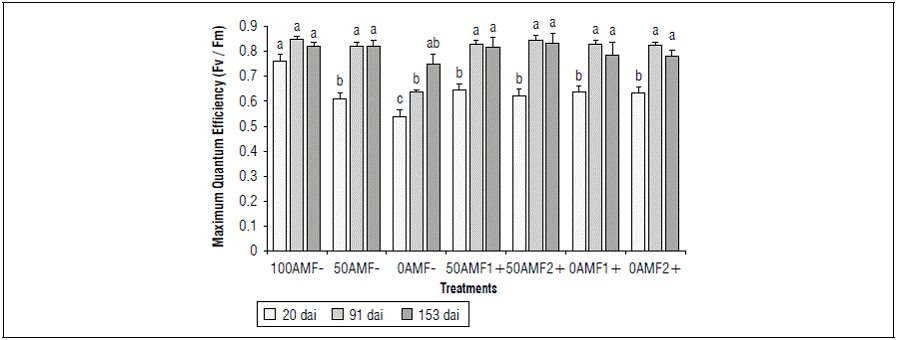
Figure 4. Effect of photosynthetic quantum efficiency in blueberry plants (Vaccinium corymbosum) var. Biloxi at 20, 91 and 153 days after inoculation (dai), with two genera of fungi, Glomus (AMF1 +) and Acaulospora(AMF2 +) and three edaphic fertilization levels (100, 50 and 0). Different letters show significant differences at P<0.05 according to Duncan's test.
At 91 and 153 dai, the 0AMF treatment recorded lower Fv/Fm values (0.63 and 0.74) than the other treatments, which presented values higher than 0.77 at 153 dai. For the 50AMF1+, 50AMF2+ and 100AMF-Fv/Fm treatments, it was higher than 0.80 at 153dai, which indicated the integrity of the photosynthetic apparatus and therefore the absence of stress. However, these differences were not statistically different.
According to studies, the blueberry O'Neill variety inoculated with Glomus mosseae under high pH stress conditions showed less respiration and an increase in photosynthetic efficiency by increasing the photochemical efficiency of photosystem II, expressed in the significant increase in the Fv/Fm ratio compared to non-inoculated plants under the same stress condition. Additionally, the amino acid contents and the biosynthesis capacity of secondary metabolites increased (Yang et al., 2020). Yang et al. (2020) found that seven-month-old blueberry plants associated with Glomus mosseae had no symptoms of stress, with values of 0.753 for photosynthetic performance (Fv/Fm) at pH 4.2. In the present study, values lower than 0.750 were obtained only at 20 dai, while at 91 and 153 dai, the Fv/Fm values exceeded 0.75, which is consistent with the study by Yang et al. (2020).
Concentration of mineral nutrients in plant organs
The 100AMF- treatment had significantly higher concentrations for most of the macronutrients, except for Ca. The treatments inoculated with Glomus 50AMF1+ and Acaulospora (50AMF2+) did not present significant differences from 100AMF- for N and P. Additionally, the 50AMF1+ mycorrhizal treatment presented significantly higher values in the concentrations of N, K, Ca, Mg and S, with respect to the treatments without inoculating 50AMF- and 0AMF-; while 50AMF2+ was higher than 0AMF- (Tab. 3).
Table 3. Concentration of macronutrients (% DM) in blueberry plants (Vaccinium corymbosum) var. Biloxy 153 days after inoculation (dai), with two fungal genera, Glomus (AMF1 +) and Acaulospora (AMF2 +) and three levels of edaphic fertilization (100, 50 and 0). Different letters show significant differences at P<0.05 according to the Duncan's test.
| Treatments | N | P | K | Ca | Mg | S |
|---|---|---|---|---|---|---|
| 100AMF- | 1.11±0.03 a | 0.105±0.009 a | 0.959±0.044 a | 0.961±0.021 b | 0.292±0.024 a | 0.382±0.009 a |
| 50AMF- | 0.87±0.03 b | 0.083±0.007 b | 0.751±0.023 c | 0.961±0.039 b | 0.252±0.008 c | 0.281±0.013 c |
| 0AMF- | 0.74±0.04 c | 0.074±0.003 c | 0.738±0.015 c | 0.985±0.014 b | 0.231±0.008 d | 0.246±0.024 d |
| 50AMF1+ | 0.95±0.01 a | 0.092±0.002 a | 0.824±0.017 b | 1.021±0.052 a | 0.274±0.010 b | 0.332±0.013 b |
| 50AMF2+ | 0.93±0.03 a | 0.089±0.005 ab | 0.879±0.027 b | 1.037±0.025 a | 0.273±0.020 b | 0.315±0.015 b |
| 0AMF1+ | 0.75±0.03 c | 0.072±0.006 c | 0.732±0.035 bc | 1.103±0.012 a | 0.300±0.019 a | 0.205±0.032 e |
| 0AMF2+ | 0.74±0.02 c | 0.072±0.007 c | 0.683±0.007 d | 1.027±0.021 a | 0.286±0.021 a | 0.202±0.020 e |
| ANOVA | *** | *** | *** | *** | ** | *** |
These data corroborate the positive effects of symbiosis on the efficiency of nutrient absorption in blueberry plants with medium levels of fertilization (nutrient deficiency) in response to inoculation with Glomus and Acaulospora. These results showed that blueberry plants can reach an optimal level of N and P, Ca and significantly increase the absorption of K, Mg, and S. Likewise, the inoculation increased the levels of Ca and Mg in the blueberry plants with a severe nutrient deficiency.
Finally, the treatments without inoculation, 50AMF- and 0AMF-, had a gradual decrease in the concentration of all macronutrients in the case of N, 50AMF- (0.87%), 0AMF- (0.74%); P 50AMF- (0.083%), 0AMF- (0.074%); Mg 50AMF- (0.252%), 0AMF- (0.231%); and S 50AMF- (0.281%), 0AMF- (0.246%).
The absorption of nutrients P and N and the transport of water to plants has been reported in pepper plants (Ortas, 2012), soybeans (Abdel-Fattah et al., 2014) and olive trees when inoculated with Glomus in N, P, and K (Porras-Soriano et al., 2009) and in pepper with an increase in the number of shoots, the dry weight of the roots, the content of P and Zn in plants inoculated with G. mosseae, G. intraradices, G. etunicatum , G. clarum, G. caledonium and the mixture of these fungi when compared with uninoculated plants (Ortas et al., 2011). The blueberry fruits of the varieties Britewell and Misty had a higher concentration of soluble sugars, proline, P and K in plants inoculated with G. mosseae than in non-inoculated plants under stress conditions because of low temperatures; these differences were more prominent in the first cultivar (Liu et al., 2017). A positive effect of AMF on blueberry plants was observed when growing in nutrient-poor soils with an increase in the content of N, K and Mg in the leaves (Zydlik et al., 2019).
CONCLUSIONS
The results indicated that inoculation with Glomus might contribute to the accumulation of biomass (total, root, stems, and leaves), improvement of nutrition, reaching adequate levels of N and P, Ca, close to 100%, and the absorption of K, Mg and S in blueberry plants (Vaccinium corymbosum) var. Biloxi under conditions with a nutrient deficiency, with fertilization at 50%. Under conditions with low fertilization 0% (severe stress), inoculation with AMF did not play an important role. These results corroborated the role of inoculation with AMF in stimulating root development and promoting the synthesis of chlorophylls to compensate for the deficiency of available nutrients when there are average levels of nutrients available in the soil.















