Services on Demand
Journal
Article
Indicators
 Cited by SciELO
Cited by SciELO  Access statistics
Access statistics
Related links
 Cited by Google
Cited by Google  Similars in
SciELO
Similars in
SciELO  Similars in Google
Similars in Google
Share
DYNA
Print version ISSN 0012-7353
Abstract
PAEZ-MEZA, Manuel; PEREZ-SIERRA, Omar and CUELLO-DELGADO, Yeris. Apparent molar volume and modeling of volumetric properties of ionic liquid aqueous solutions 1-butyl-3-metilmidazolium chloride [Bmim+][Cl-] at various temperatures. Dyna rev.fac.nac.minas [online]. 2014, vol.81, n.186, pp.120-125. ISSN 0012-7353. https://doi.org/10.15446/dyna.v81n186.39554.
Densities of the aqueous solutions of ionic liquid 1-butyl-3 metilmidazolium chloride [Bmim+][Cl-] were determined using a vibrating tube densitometer Anton Paar DMA 5000 at a temperature range between (283.15 - 218.15) K. The apparent molar volumes 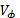 of aqueous chloride1-butyl-3-methylimidazolium were calculated and adjusted to the Pitzer ion interaction model, obtaining the limiting apparent molar volumes
of aqueous chloride1-butyl-3-methylimidazolium were calculated and adjusted to the Pitzer ion interaction model, obtaining the limiting apparent molar volumes 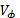 and Pitzer volumetric parameters
and Pitzer volumetric parameters 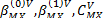 at temperatures of 283.15, 288.15, 293.15, 298.15, 303.15, 313.15 and 318.15K, proving that this model adequately represents the experimental volumetric data below an ionic strength of 0.6018 mol / kg. Finally the limiting apparent molar expansibilities
at temperatures of 283.15, 288.15, 293.15, 298.15, 303.15, 313.15 and 318.15K, proving that this model adequately represents the experimental volumetric data below an ionic strength of 0.6018 mol / kg. Finally the limiting apparent molar expansibilities 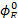 were calculated from the limiting apparent molar volumes at various temperatures and the results are discussed in terms of the interactions occurring in solution.
were calculated from the limiting apparent molar volumes at various temperatures and the results are discussed in terms of the interactions occurring in solution.
Keywords : apparent molar volume; Pitzer parameters; density; limiting expansibilities; ionic liquid.













