Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Pecuarias
Print version ISSN 0120-0690On-line version ISSN 2256-2958
Rev Colom Cienc Pecua vol.23 no.4 Medellín Oct./Dec. 2010
Revisiones de literatura
Role of stearoyl CoA desaturase on conjugated Linoleic acid concentration in bovine milk: review
Papel de la estearoil CoA desaturasa sobre la variabilidad en los niveles de ácido linoléico conjugado en la leche bovina: revisión
Papel do estereaoil CoA desnaturase na variação dos níveis do ácido linoléico conjugado em leite bovino. Revisão
Julián A. Castillo1, Quím; Martha L Pabón1,2*, Quím, MSc, PhD; Martha Olivera3, MV, DrSc Agric; Juan E. Carulla1, MSc, PhD.
1Research Group on Animal Nutrition, Department of Animal Production Sciences, Faculty of Veterinary Medicine and Animal Science, National University of Colombia, Bogotá, Colombia. 2Chemistry Department, Faculty of Sciences. National University of Colombia, Bogotá, Colombia. 3Biogénesis Research Group, School of Veterinary Medicine, University of Antioquia, Medellín, Colombia.
(Received: 2 february, 2010; accepted: 28 september, 2010)
Summary
Interesting health benefits have been attributed to the intake of conjugated linoleic acid, CLA (C18:2 cis9, trans-11), which is the main isomer of linoleic acid, and is present in bovine milk. Among those benefits are: cancer prevention, diminished risk for the onset of type II diabetes and cardiovascular disease, modulation of the immune response, and reduction of preeclampsia risk in primigravid women. Although an adequate nutrition of cows has permitted to increase the amount of CLA in their milk, there is variation in CLA concentrations among cows consuming the same diet. It has been suggested that this variation is due either to changes in the activity of stearoyl CoA desaturase (SCD), changes in the gene expression, or to alterations in the ruminal process of biohydrogenation. Research conducted in semimembranosus muscle and subcutaneous adipose tissue of cattle suggests there are two isoforms of SCD.
Key words: cattle, conjugated linoleic acid, stearoyl CoA desaturase, trans-vaccenic acid, variability.
Resumen
Al isómero mayoritario del ácido linoléico, C18:2 cis-9, trans-11 (Ácido Linoléico Conjugado, ALC) en la leche bovina, se le han atribuido propiedades benéficas para la salud humana, entre las que está su efecto en la prevención del cáncer, disminución de los factores de riesgo para la presentacion de diabetes tipo II y de enfermendades cardiovasculares, modulación de la respuesta inmune como también, en la reducción del riesgo de preeclamsia en mujeres primigrávidas. Aunque se ha logrado elevar la concentración de ALC en leche mediante sistemas de alimentación adecuados, existe variabilidad en su concentración para individuos de una misma especie y sometidos a la misma alimentación. Para explicar dicha variabilidad, se ha sugerido que esta se debe a cambios en la actividad de la Estearoil CoA Desaturasa (ECD), de la expresión genética diferencial o a alteraciones en el proceso de biohidrogenación ruminal. Trabajos realizados en músculo semimembranoso y tejido adiposo subcutáneo en bovinos, sugieren la presencia de dos isoformas de la ECD.
Palabras clave: ácido linoléico conjugado, ácido trans-vaccénico, bovinos, estearoil CoA desaturasa, variabilidad.
Resumo
Ao isómero maioritário do ácido linoléico, C18:2 cis-9, trans-11 (Ácido Linoléico Conjugado, ALC) no leite bovina, tem-se atribuído propriedades benéficas para a saúde humana, entre as quais estão: seu efeito na prevenção do câncer, diminuição dos factores de risco para a apresentação da diabetes tipo II e de doenças cardiovasculares, modulação da resposta imune, como também, a redução do risco da préeclâmsia em mulheres primigrávidas. Embora tem-se logrado elevar a concentração dos ALC no leite mediante sistemas de alimentação adequados, existe a variabilidade na sua concentração para indivíduos da mesma espécie e submetidos a uma alimentação semelhante. Para explicar a variabilidade, tem-se sugerido que é causada por mudanças na actividade do Estearoil CoA Desaturase (ECD), da expressão genética diferencial ou às alterações no processo de biohidrogenação ruminal. Trabalhos realizados no músculo semimembranoso e tecido adiposo subcutâneo em bovinos, sugerem a presencia de duas isoformas do ECD.
Palavras chave: ácido linoléico conjugado, ácido trans-vaccénico, bovinos, estearoil CoA desnaturase, variabilidade.
¤ To cite this paper: Castillo JA, Pabón ML, Olivera M, Carulla JE. Role of stearoyl CoA desaturase on conjugated Linoleic acid concentration inbovine milk. Review. Rev Colomb Cienc Pecu 2010; 23:493-500.
* Corresponding author: Martha L.Pabón. Department of Animal Production Sciences, Faculty of Veterinary Medicine and Animal Science, National University of Colombia, Bogotá, Colombia. E-mail: mlpabonr@unal.edu.co
Introduction
Nutraceuticals and functional foods have become a fast growing research topic in recent years. Bovine milk can fit into the nutraceuticals category if its fat has a particular lipid profile. It is interesting to highlight that there is natural presence of CLA in the lipid fraction of milk. This compound is a geometric and positional isomer of linoleic acid cis-9, cis-12, LiA), which has been regarded (C18:2 as beneficial for the human health. Such benefits include its cancer prevention properties (Belury, 1995, Bauman and Griinari, 2001), action on type II diabetes (Belury and Vanden, 1999, Yu et al., 2002; Belury, 2003), positive effects on the cardiovascular system (Nicolosi et al., 1997; Kritchevsky, 1999, Munday et al., 1999) and modulation of immune cells response (Akahoshi et al., 2002; Iwakiri et al., 2002,Yang and Cook, 2003 ;Akahoshi et al., 2004).
Furthermore, in a study conducted in Colombia, it was reported that CLA also has positive effects on primigravid women with risk of preeclampsia (Herrera et al., 2004).
It is known that there is high variability in the content of CLA in bovine milk. This occurs for animals of the same breed, and even under the same diet. It has been suggested that the causes for these variations are changes in the activity of SCD, the gene coding for the enzyme and the biohydrogenation process (Peterson et al., 2002).
The objective of this review is to provide a possible explanation to the variability observed in CLA concentration in bovine milk in terms of the activityand geneticsof SCD.
Conjugated Linoleic Acid (CLA)
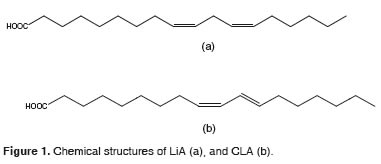
This acid belongs to a family of geometric and positional isomers of LiA. Unlike natural fatty acids, its double bonds are conjugated, which is defined for the presence of alternating doublesingle-double bonds in the carbon structure. In other words, the double bonds are located every two carbons (O ‘Shea et al., 1998, Bauman et al., 1999) (Figure 1). Near 20 different positional and geometric isomers of CLA have been reported. Those isomers have several positions for the double bonds in the18-carbon chain. Some of these geometric configurations are: cis-trans, trans-cis, cis-cis and trans-trans (Sehat et al., 1998).
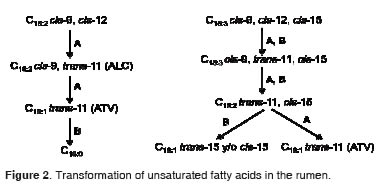
Conjugated linoleic acid is derived from a partial bio-hydrogenation of LiA in the rumen, a process which also generates trans-vaccenic acid (C18:1 trans-11, TVA) from CLA. This TVA can be generated also from α-linolenic acid (C18:3 cis-9, cis-12, cis-15, LnA). This TVA is an intermediate compound which is absorbed and subsequently dehydrogenated by SCD between carbons 9 and 10, to produce CLA (Figure 2). It has been reported that 91% of milk CLA is endogenously synthesized by SCD (Kay et al., 2004). It is also known that SCD has increased activity in the mammary gland of lactating cows and in adipose tissue of growing ruminants (Bickerstaff and Annison, 1972; Kinsella, 1972).
Role of SCD genotype in the variability of CLA
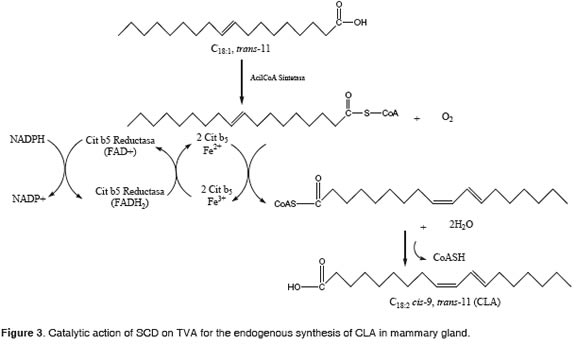
The creation of a cis double bond between carbons 9 and 10 of a saturated fatty acid having between 10 to 18 carbons is an important step in the synthesis of unsaturated fatty acids (Ntambi, 1995, Bauman et al., 1999). The ferric ion, coordinated with SCD, NADPH, cytochrome b5 reductase, cytochrome b5 and oxygen, catalyzes the dehydrogenation o f TVA (Figure 3) (Ntambi, 1995; Yahyaoui et al., 2002).
The bovine gene that codifies for SCD has 5,331 base pairs and is located on chromosome 26 (Taniguchi et al., 2004).
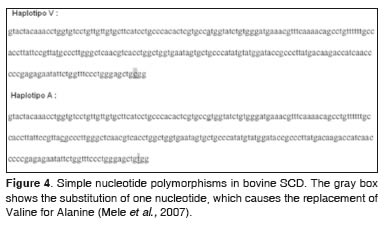
The entire length of the gene has been reported in a study in which 20 Japanese black breed steers were used. The same researchers reported they found eight simple nucleotide polymorphisms (SNPs) (Figure 4) (Campbell etal., 2001).
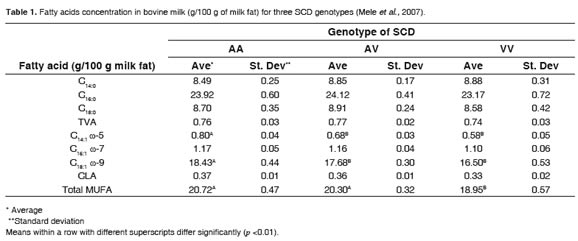
Three SNPs have been reported that resulted in two haplotypes detected in the fifth exon. The third SNP caused the substitution of valine (V allele) for alanine (A allele), on the 293 residue of the SCD enzyme (Taniguchi et al., 2004). Besides, they found the relative frequencies of the genotypes of the enzyme were: 27% for AA, 60% for AV and 13% for VV (Mele et al., 2007). They also found that AA genotype was associated with 9.3, 37.9 and 11.7% more total monounsaturated fatty acids (MUFA; C18:1ω9 and C14:1ω5, respectively, in regard toVVgenotype (Table 1).
In addition, it was established that the contribution of the genotypes of SCD to the total variation of the other fatty acids was not significant. The same authors, found that the ratio C14:1 ω5 / (which is an estimate of SCD activity) in AA C14:0 genotype cows was the highest compared with AV and VV genotypes. No significant differences were reported for C16:1 ω7/C16:0 and C18:1 ω9/C18:0 ratios. The ratio C14:1 ω5/C14:0, clearly consistent with the results found for the genotypes studied, suggests there is an effect of genetic variability in the overall compositionof fatty acidsin milk.
Although, as mentioned, there were significant differences in the profiles and concentration of fatty acids in milk fat within the same breed, it was also found that while AA genotype produced more than 12% CLA compared with VV, this difference was not significant (Mele et al., 2007). In other words, the genetic factor did not satisfactorily explain the dispersion values registered for CLA under those conditions. However, this effect cannot be ruled out entirely, because there was an experimental constraint, which was the sample size of the population. Although there have been other studies evaluating the effect of SNPs on the SCD and the fatty acids profile in bovine milk fat (Moioli et al., 2007), there are scarce reports determining the effect ofgeneticvariabilityofSCDonCLAconcentration.
Stearoyl CoA desaturase activity
The dehydrogenation index of a fatty acid is considered as a rough measure of the SCD activity. Several indexes have been proposed, such as the ratio: product / substrate (Lock and Garnsworthy, 2003, Thompson et al., 2003), substrate / product (Chouinard et al., 1999) or product / (substrate + product) (Kelsey et al., 2003; Royal and Garnsworthy,2005).
The main products of SCD activity present in the mammary gland of ruminants are C14:1 ω-5, C16:1 ω-7, C18:1 ω-9 and CLA, which are derived from C14:0, C16:0, C18:0 and ATV, respectively. Taking into account that C14:0 is mostly obtained from de novo synthesis in the mammary gland, the best indicator of SCD activity is the ratio C14:1 ω-5/C14:0 (Lock and Garnsworthy, 2003).
It is known that diet is the main factor influencing SCD activity in the bovine mammary gland (Kelsey et al., 2003). According to Lock and Garnsworthy (2003), high levels of SCD activity were observed for cows fed forage-based diets. On the other hand, cows fed grain-forage diets resulted in lower SCD activity. Similarly, Daniel et al. (2004) reported high SCD activity in the mammary glandof sheep fed high forage diets.
It has been reported that cobalt inclusion in feed supplements, and abomasal infusion of CLA have resulted in a reduction of SCD activity (Chouinard et al., 1999;Taugbøl et al., 2008).
Besides the effect of the diet, it is known that also breed has a significant effect on SCD activity. It has been reported that Holstein cows present higher SCD activity than Brown Swiss (Kelsey et al., 2003). In a similar work, Vasta et al. (2009) found that Brown Swiss and Jersey breeds exhibited lower SCDactivity in comparison with Holsteincows.
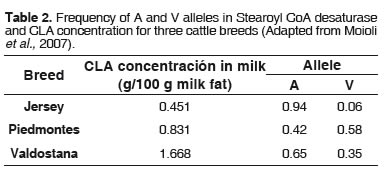
A relationship was established between the proportion of A and V alleles and the activity indexes of SCD in Jersey, Piedmont and Varlostana cattle (Table 2). The researchers found that V allele increased C14:1 ω-5/C14:0 and C10:1 ω-1/C10:0 indexes. They reported no increase in CLA production for Jersey cattle, and no effect of V allele was detected, which is entirely consistent with its frequency in SCD ofthisbreed (Moioli et al., 2007).
It has been established that milk yield, milk fat, parity, and lactation stage do not have a significant effect on SCD activity, which explains why these physiological effects have received little attention (Kelsey et al., 2003; Soyeurt et al., 2008).
Besides the specific dehydrogenation rates of fatty acids, a direct relationship between SCD activity and its expression has been proposed (Vasta et al., 2009). There is little research concerning SCD expression in mammary gland. Literature is more abundant regarding protein expression in muscleandadipose tissue of cattle.In a recent paper Dance et al. (2009) reported the effect of breed on fatty acid composition and SCD expression in bovine muscle and subcutaneous adipose tissue. They concluded that changes in SCD expression could contribute to the MUFA and CLA variations observed into the same breed.
The additional reports found on SCD in ruminants are limited to the enzyme activity in relation to its mRNA (Jamberenghi et al., 2007, Pavan and Duckett, 2007). There is a report about the effect of feeding systems and tannin supplementation on the relation between intramuscular fat content, fatty acid composition and SCD expression in lamb longissimus dorsi (Vasta et al., 2009). Changes in the activity of SCD may be related to variations in the expression of mRNA and / or protein. The authors found that SCD expression was not affected by tannin supplementation, and also reported no association between SCD expression and the levels of MUFA and CLA (p> 0.05). Additionally, they found that feeding concentrate diets affected the MUFA / SFA ratio (saturated fatty acids) but did not affect CLA / TVA ratio, which agrees with a previous report conducted on milk fatty acid composition (Mele et al., 2007.) As a possible explanation for this inconsistency, it is argued that CLA and MUFA biosynthesis could be catalyzed by two SCD isoforms. The presence of more than one isoform has been previously reported in other species (Thida et al., 1986, Miyazaki and Ntambi, 2003). Today, we know that there are two isoforms of SCD in ruminants, and both have been found in cattle (Ward et al., 1998, Chung et al., 2000; Lengies and Corl, 2007). Regarding research in other species, four isoforms have been found in mice (Kaestner et al., 1989, Zheng et al., 2001, Miyazaki et al., 2003), two in humans (Zhang et al., 1999, Wang et al., 2005 ), and many counterparts in rats, sheep, goats and pigs (Lengi and Corl, 2007). It is not clear why such isoformsexist.
Nevertheless, there is evidence of their divergent and specific expression in tissues (Ntambi et al., 1988), and also substrate specificity. An example is Muridae family, where SCD1, SCD2 and SCD4 have shown preference to desaturate palmitoyl-CoA and estaroil-CoA, whereas SCD3 desaturates only palmitoyl-CoA (Miyazaki et al., 2006). This could indicatethat the existence of different SCDisoforms has a physiological role (LengiesandCorl, 2007).
Conclusions
The experimental evidence so far suggests that there is an effect of genetic variability in the overall composition of fatty acids in milk, for animals of the same breed under the same diet. Although the genetic factor did not explain the dispersion registered for CLA values, this factor cannot be entirely ruled out, since there was an experimental constraint, which was the sample size of the population, making it necessary to conduct further work to determine the effect of SCD genetic variability onCLAconcentration.
A marked influence was found for the effect of diet and breed on SCD activity. Experimental evidence shows that genetic variability among breeds is the factorthatexerts such a change in SCD activity.
Based on SCD studies using cattle semimembranosus muscle and adipose tissue, it has been suggested the existence of two isoforms of the enzyme, which may preliminary explain why feeding concentrates significantly affected the MUFA / SFA rate, but not the CLA / TVA rate. Consideration also should be given to the existence of several SCD isoforms in some species which is related to their specific substrates and different tissue expression, features which play perhaps some physiological role.
Acknowledgements
The authors want to thank the Colombian Administrative Department for Science, Technology and Innovation (COLCIENCIAS) for funding this publication, which is a product of the project entitled: "Effect of supplementing lactating cows with rich sources of unsaturated fatty acids on milk yield and lipid profile, lipogenic enzymes transcription, SRBP1 activity, and its relationship with different components of milk fatty acid profile". 1115-452-21319 Code.
References
1. Akahoshi A, Goto Y, Murao K, Miyazaki T, Yamasaki M, Nonaka M, Koji Y. Conjugated linoleic acid you reduce body fats and cytokine Levels of mice. Biosci Biotechnol Biochem 2002; 66:916-920. [ Links ]
2. Akahoshi A, Koba K, Ichinose F, Kaneko M, Shimoda A, Nonaka K, Yamasaki M, Iwata T, Yamauchi Y, Tsutsumi K, Sugano M. Dietary protein modulate the effect of CLAon lipid metabolism in Rats.Lipids 2004;39:25-30. [ Links ]
3. Bauman DE, Baumgard LH, Corl BA, Griinari JM. Biosynthesis of conjugated linoleic acid in ruminants. Proc Am SocAnim Sci1999:1-11. [ Links ]
4. Bauman DE, Griinari JM. Regulation and nutritional manipulation of milk fat: Low-Fat syndrome. Livest Prod Sci 2001;70:15-29. [ Links ]
5. Belury MA. Conjugated dienoic linoleate: A polyunsaturated fatty acid with unique chemoprotective properties. Rev Nutr 1995;53:83-89. [ Links ]
6. Belury MA, Vanden JP. In: Yucawecs MP, Mossoba MM, Kramer JK, Pariza MW, Nelson GJ, editors. Advanced conjugated linoleic acids in reseach, Vol 1 Champaign (IL): AOCS Press, 1999.p.401-411. [ Links ]
7. Belury MA. In: Sebedio JL, Christine WW, Adlof R, editors. Advances in conjugated linoleic acid research, vol. 2. Champaign(IL):AOCSPress, 2003.p.302-315. [ Links ]
8. Bickerstaffe R,AnnisonAR.The effect of intravenous infusions of sterculic acid on milk fat synthesis. Br J Nutr 1972; 27:561-570. [ Links ]
9. Campbell EM, Gallagher DS, Davis SK, Taylor JF, Smith SB. Rapid Communication: Mapping of the bovine stearoylcoenzyme A desaturase (SCD) gene to BTA26. J Anim Sci 2001,79:1954-1955. [ Links ]
10. Chouinard PY, Corneau L, Barbano DM, Metzger LE, Bauman DE. Conjugated linoleic acids alter milk fatty acid composition and inhibit milk fat secretion in dairy cows. J Nutr 1999; 129:1579-1584. [ Links ]
11. Chung M, Ha S, Jeong S, Bok J, Cho K, Baik M, Yunjaie C. Cloning and characterization of bovine stearoyl CoAdesaturase 1 cDNAfrom adipose tissue. Biosci Biotechnol Biochem 2000; 64:1526-1530. [ Links ]
12. Dance JE, Matthews KR, Doran O. Effect of breed on fatty acid composition and stearoyl-CoA desaturase protein expression in the semimembranosus muscle and subcutaneous adipose tissue of cattle. Livest Sci 2009;125:291-297. [ Links ]
13. Daniel ZC, Wynn TR, Salter AM, Buttery PJ. Differing effects of forage and concentrate diets on the oleic acid and conjugated linoleic acid content of sheep Tissues: The role of stearoyl-CoA desaturase. J Anim Sci 2004; 82:747-758. [ Links ]
14. Herrera JA, Shahabuddin AK, Faisal M, Ersheng G, Wei J, Lixia D, GandahoT, López P. Efectos de la suplementación oral con calcio y ácido linoléico conjugado en primigrávidas de alto riesgo. Colomb Med 2004; 35:31-37. [ Links ]
15. Iwakiri Y, Sampson DA, Allen KG. Suppression of cyclooxygenase-2 and inducible nitric oxide synthase expression by conjugated linoleic acid in murine macrophages. Essent Fatty Acids prostaglandin Leukotr 2002; 67:435-443. [ Links ]
16. Jamberenghi AC, Paglialonga G, Gnoni A, Zanotti F, Giannico F, Vonghia G, Gnoni GV. Changes in lipid composition and lipogenic enzyme activities in liver of lambs fed ω-6 polyunsaturated fatty acids. Comp Biochem Physiol Part B. 2007; 147:498-503.
17. Kaestner KH, Ntambi JM, Kelly TJ, Lane MD. Differentiationinduced gene expression in 3T3-L1 preadipocytes. A second differentially Expressed gene encoding stearoyl-CoA desaturase. J Biol Chem 1989; 264:14755-14761. [ Links ]
18. Kay JK, Mackle RT, Auldist MJ, Thompson NA, Bauman DE. Endogenous synthesisof cis-9, trans-11conjugatedlinoleicacid in dairy cows fed fresh pasture. J Dairy Sci 2004; 87:369-378. [ Links ]
19. Kelsey JA, Corl BA, Collier RJ, Bauman DE. The effect of breed, parity, and stage of lactation on conjugated linoleic acid (CLA) in milk from dairy cows. J Dairy Sci 2003; 86: 2588-2597. [ Links ]
20. Kinsella JE. Stearoyl CoA as a precursor of oleic acid and glycolipids in mammary microsomes from lactating bovine: possible reculatory step in milk triglyceride synthesis. Lipids, 1972; 7:349-355. [ Links ]
21. Kritchevsky D. In: Yucawecs MP, Mossoba MM, Kramer JK, Pariza MW, Nelson GJ, editors. Advanced conjugated linoleic acids in reseach, Vol 1 Champaign (IL): AOCS Press; 1999. p. 397-403. [ Links ]
22. Lengi AJ, Corl BA. Identification and characterization of a novel bovine stearoyl-CoA desaturase isoform with homology to humanSCD5. Lipids 2007;42:499-508. [ Links ]
23. Lock AL, Garnsworthy PC. Seasonal Variation in milk conjugated linoleic acid and Δ9-desaturase activity in dairy cows.LivestProd Sci2003; 79:47-59.
24. Mele M, Conte G, Castiglioni B, Chessa S, Macciotta NP, Serra A, Buccioni A, Pagnacco G, Secchiari P. Stearoyl Coenzyme A desaturase gene polymorphism and milk fatty acid composition in Italian Holsteins.J DairySci2007; 90:4458-4465. [ Links ]
25. Miyazaki M, Ntambi JM. Role of stearoyl-coenzyme A desaturase in lipid metabolism. Prostaglandins Leukotrienes Essent FattyAcids2003; 68:113-121. [ Links ]
26. Miyazaki M, Jacobson MJ, Man WC, Cohen P, Asilmaz E, Friedman JM, Ntambi JM. Identification and characterization of murine SCD4, a novel heart-specific stearoyl-CoA desaturase isoform Regulated by leptin and dietary factors. J Biol Chem 2003; 278:33904-33911. [ Links ]
27. Miyazaki M, Bruggink SM, Ntambi JM. Identification of mouse palmitoyl-coenzyme A delta9-desaturase. J Lipid Res 2006; 47:700-704. [ Links ]
28. Moioli B, Contarini G, Avalli A, Catillo G, Orrù L, De Matteis G, Masoero G, Napolitano F. Effect of Stearoyl-coenzyme A desaturase polymorphism on fatty acid composition of milk. J Dairy Sci 2007;90:3553-3558. [ Links ]
29. Munday JS, Thompson KG, James KAC. Dietary conjugated linoleic acids Promote fatty streak formation in the C57BL / 6 mouseatherosclerosis model.Br JNutr 1999;81:251-255. [ Links ]
30. Nicolosi RJ, Rogers EJ, Kritchevsky D, Scimeca JA, Huth PJ. Dietary conjugated linoleic acids makes plasma lipoproteins and early aortic atherosclerosis in hypercholesterolemic hamsters. Artery1997; 22:266-277. [ Links ]
31. Ntambi JM, Buhrow SA, Kaestner KH, Christy RJ, Sibley E, Kelly TJ Jr, Lane MD. Differentiation-induced gene expression in 3T3-L1 preadipocytes. Characterization of a differentially Expressed gene encoding stearoyl-CoAdesaturase. J Biol Chem 1988; 263:17291-17300. [ Links ]
32. Ntambi JM. The regulation of stearoyl-CoA desaturase (SCD). Prog LipidRes 1995;34:139-150. [ Links ]
33. O'Shea M, Lawless F, Stanton C, Devery R. Conjugated linoleic acid inbovine milk fat:a food-basedapproach tocancer chemoprevention. Trends in Food Sci & Technol 1998; 9:192-196. [ Links ]
34. Pavan E, Duckett SK. Corn oil supplementation to steers grazing endophyte-free tall fescue. II. Effects on longissimus muscle and subcutaneous adipose fatty acid composition and stearoyl-CoA desaturase activity and expression. J Anim Sci 2007; 85:1731-1740. [ Links ]
35. Peterson DG, Kelsey JA, Bauman DE. Analysis of variation in cis-9, trans-11 conjugated linoleic acid (CLA) in milk fat of dairycows. JDairy Sci 2002;85:2164-2172. [ Links ]
36. Royal MD, Garnsworthy PC. Estimation of genetic variation in Δ9-desaturase enzyme activity in dairy cows. Proc Br SocAnim Sci 2005:52.
37. Sehat N, Kramer JK, Mossoba MM, Yurawecz MP, Roach JA, Eulitz K, Morehause KM, Ku Y. Identification of conjugated linoleic acid isomers in cheese by gas chromatography, silver ion high performance liquid chromatography and mass spectral reconstructed ion profiles. Comparison of chromatographic elution sequences. Lipids 1998;33:963-971. [ Links ]
38. Soyeurt H, Dehareng F, Mayeres P, Bertozzi C, Gergler N. Variation of Δ9-desaturase activity in dairy cows. J Dairy Sci 2008; 91:3211-3224.
39. Taniguchi M, Utsugi T, Oyama K, Mannen H, Kobayashi M, Tanabe Y, Ogino A, Tsuji S. Genotype of stearoyl-CoA desaturase isAssociated with fatty acid composition in Japanese Black cattle. Mamm Genome2004; 14:142-148. [ Links ]
40. Taugbøl O, Karlengen IJ, Bolstad T,Aastveit AH, Harstad OM. Cobalt supplied per os reduces the mammary Δ9-desaturase index ofbovine milk. JAnimSci 2008;86:3062-3068.
41. Thida MA, Ozols J, Strittmatter PJ. Construction and sequence of cDNA for rat liver stearoyl coenzyme A desaturase. J Biol Chem1986; 261:13230-13235. [ Links ]
42. Thompson NA, Chand A, Kay JK. Δ9-desaturase activity and the association with conjugated linoleic acid (CLA) concentration in bovine milk. N Z Proc Soc Anim Prod 2003: 25-30.
43. Vasta V, Priolo A, Scerra M, Hallett KG, Wood JD, Doran O. Δ9-desaturase protein expression and fatty acid composition of longissimus dorsi muscle in lamb fed green herbage or concentrate with or without added tannins. Meat Sci 2009; 82:357-364.
44. Wang J, Yu L, Schmidt RE, Su C, Huang X, Gould K, Cao G. Characterization of HSCD5, a novel human stearoyl-CoA desaturase unique to primates. Biochem Biophys Res Commun 2005; 332:735-742. [ Links ]
45. Ward RJ, Travers MT, Richards SE, Vernon RG, Salter AM, Buttery PJ, Barber MC. Stearoyl-CoA desaturase mRNA is transcribed from a single gene in the Ovine genome. Biochim Biophys Acta 1998;1391:145-156. [ Links ]
46. Yahyaoui MH, Sanchez A, Folch JM. Rapid communication: Partial nucleotide sequence of the goat stearoyl coenzyme A desaturase cDNA and gene structure. JAnim Sci 2002; 80:866-867. [ Links ]
47. Yang M, Cook ME. Dietary conjugated linoleic acid Decreased cachexia, macrophage tumor necrosis factor-alpha production, and modifies splenocyte cytokines production. Exp Biol Med (Maywood) 2003; 228:51-58. [ Links ]
48. Yu Y, Correll PH, Heuvel JP. Conjugated linoleic Decreases production of pro-inflammatory products in macrophages: evidence for a PPAR?-dependent mechanism. Biochim Biophys Acta2002; 158:88-99. [ Links ]
49. Zhang L, Ge L, Parimoo S, Stenn K, Prouty SM. Human stearoyl-CoA desaturase: alternative transcripts generated from a single gene by usage of tandem polyadenylation sites. BiochemJ1999; 340:255-264 [ Links ]
50. Zheng Y, Prouty SM, Harmon A, Sundberg JP, Stenn KS, Parimoo S. Scd3-a novel gene of the stearoyl-CoA desaturase family with restricted expression in skin. Genomics 2001; 71:182-191. [ Links ]