Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
DYNA
Print version ISSN 0012-7353On-line version ISSN 2346-2183
Dyna rev.fac.nac.minas vol.74 no.153 Medellín Sep./Dec. 2007
TREATMENT OF FUE DIESEL WITH A PERMEABLE REACTIVE BARRIER TECHNOLOGY
TRATAMIENTO DE DIESEL CON LA TECNOLOGÍA DE BARRERA REACTIVA PERMEABLE
SANTIAGO ALONSO CARDONA GALLO
Escuela de Geociencias y Medio Ambiente, Universidad Nacional de Colombia, scardona@unal.edu.com
Recibido para revisar octubre 02 de 2006, aceptado mayo 18 de 2007, versión final junio 14 de 2007
RESUMEN: La investigación estudió el tratamiento de diesel combustibles de producción mexicana contenidos en agua con un sistema de barrera reactiva permeables a escala de laboratorio (siete columnas). Se uso un suelo agrícola como medio reactivo. Se aplico peroxido de hidrógeno al 50% industrial como fuente de oxigeno y nitrógeno en urea al 46% como nutriente. Se caracterizo el medio reactivo con los principales parámetros de interés (humedad, materia orgánica, pH, nitrógeno total, fósforo disponible, clasificación del suelo, conductividad eléctrica, sólidos suspendidos volátiles, densidad real y aparente, porosidad, textura, color, salinidad, conductividad hidráulica, capacidad de campo y densidad de bacterias. Se determinaron las cinéticas de degradación y la capacidad de adsorción del diesel en el medio reactivo. Las barreras reactivas permeables se diseñaron con los resultados cinéticos obtenidos en los reactores por lotes. Las columnas tenían dimensiones de 30 cm de longitud y 10 cm de diámetro. Las cinéticas de determinaron durante 18 días y las columnas se corrieron durante 70 días presentando remociones arriba del 80%. Se usaron concentraciones iniciales de diesel de 15,000 mg/L. Para la modelación de la adsorción se aplicaron las ecuaciones de Freundlich y Langmuir, donde esta ultima presentó un mejor ajuste a los datos a los datos experimentales y una mayor capacidad de adsorción. Para el suministro de los nutrientes y oxigeno se aplico el modelo propuesto por McCarty y la ecuación media para diesel propuesta por Jackson. Se determinó una velocidad de degradación de 0.0908 d-1, un coeficiente de distribución del diesel en el medio reactivo de 0.8 ml/g, una capacidad de adsorción de diesel en el medio reactivo de 13.50 mg/L y un factor de retardo de 3.69.
PALABRAS CLAVE: Diesel combustible, barreras reactivas permeables, biodegradación, nutrientes.
ABSTRACT: The objective of this investigation is to study the treatment of the diesel production of Mexican content in water with a system of permeable reactive barriers in laboratory (seven columns). An agricultural soil (ASo) was used as half reagent medium. It was applied peroxide of hydrogen to 50% industrial as source of oxygen and nitrogen in urea to 46% as nutrient. This characterized the reagent medium with the main parameters of interest (humidity, organic matter, pH, total nitrogen, available phosphorus, classification of the soil, volatile suspended solids, electric conductivity, real and apparent density, porosity, texture, color, hydraulic conductivity, field capacity and density of bacteria. The kinetic of degradation and the capacity of adsorption of the diesel was determined in the reagent medium by batches tests. The permeable reactive barriers were designed with the kinetic results obtained in the reactors by batch. The columns had 30 cm of length and 10 cm of diameter. The kinetics was determined during 18 days and the columns were run during 70 days where they presented removals up of 80 % in columns. An initial concentration of diesel of 15,000 mg/L was used. For the modeling of the adsorption the Langmuir equation was applied. For the supply of nutrients and oxygen the pattern proposed by McCarty and the half equation for diesel proposed by Jackson was applied. A velocity of degradation in the reagent medium of 0.0908 d- 1 a coefficient of distribution of the diesel in the reagent medium of 0.8 ml/L, a capacity of adsorption of diesel in the medium reagent of 13.50 mg/L and a factor of retard of 3.69 in the soil is presented.
KEYWORDS: Fuel diesel, permeable reactive barriers, biodegradation, nutrients.
1. INTRODUCTION
The permeable reactive barriers are structures located under the surface in order to treat the contaminated underground water in places of dangerous waste. These barriers are put in situ with the gaps construction through the flow path of the underground water and where the natural gradients of contaminants transportation make them pass by the place of the reactive cell. The gap is filled with one or several materials carefully selected, in order to destroy or stabilize the specific kind of contaminants (Gavaskar et al., 2000). The main advantage in the walls treatment is that they are passive systems which treat the contaminants in situ (Fiorenza et al., 2000). Several methods have been developed for the installation of permeable walls treatment.
Walls treatment or permeable reactive barriers (PRB) were firstly reported by McMurry and Elton (1985), which involve construction of permanent, semi permanent or replaceable units across the flow path of a dissolved phase Contaminant plume (Guerin et al., 2001; Gavaskar et al., 2000; EPA, 1998). As the contaminated groundwater moves passively through the treatment wall, contaminants are removed by physical, chemical and/or biological processes, including precipitation, sorption, oxidation/reduction, fixation or degradation. This treatment system has presented excellent results with different organic and inorganic contaminants in several reactive medium where it has removed different kind of contaminants such as chlorinate solvents, metals, radio nucleotides, petroleum, hydrocarbon, volatile organic compounds, acid of mines, uranium VI and other contaminants (Gavaskar et al., 2000; Fiorenza et al., 2000; EPA, 1998). These barriers may contain agents that are placed either in the path of contaminant plumes to prevent further migration or immediately down gradient of the contaminant source to prevent plume formation (Gavaskar et al., 2000). The studies have been done in systems by batches, pilot programs, in situ and commercially (Cardona, 2004, Gavaskar et al., 2000; Fiorenza et al., 2000; Blowes et al., 1997).
Such systems consist of low hydraulic conductivity cut-off walls (e.g.1x10-6 cm/s) with one or more gaps that obtain permeable reaction zones (Guerin et al., 2001) There are studies where the utilization of organic reactive means have been reported where the organic contaminants removal (moss, sawdust, aquifer sediments, organic matter, municipal compost, leaves, peat, addition of bacterial inoculants, soils with microorganisms and activated sludges) with a bacteria content between 1x105-1x108 CFU/g and the addition of nutrients, agricultural fertilizer and a source of oxygen, air and oxygen (Atlas y Bartha 1972; Ho et al., 1995; Cardona, 2004, Ganzert, 1991; Powell et al., 1995; Robertson et al., 1995; Fiorenza y Ward, 1997; Waybrantt et al., 1998; Puls et al., 1999; Hebert et al., 2000; Kao et al., 2000; Fiorenza et al., 2000; Márquez-Rocha et al. (2001); Namkoomg et al., 2002).
The aim of this paper is the diesel removal present in water, which was done in laboratory using columns with a reactive medium of agricultural soil (ASo) where corn, sorghum, barley and oats are cultivated. The soil presented a bacterial density of 6.4x105 CFU/g, nitrogen as urea (46%) was added and industrial hydrogen of hydrogen to the 50% as oxygen source. Phosphorus was not added because the ASo exceeded recommendations for studies of bioremediation. The remotions presented were up the 80%.
2. MATERIALS AND METHODS
The study was carried out in two stages. The agricultural soil was characterized, where the kinetic degradation, the capacity of adsorption were obtained. With all of them the columns were designed and operated.
The selected soil of this study is an agricultural soil from the Rancho San Francisco, located in San Andres Mixquic, Mexico , D.F., of the Veterinary Faculty of the National Autonomous University of Mexico (UNAM). In the ASo, corn, sorghum, barley and oats are cultivated. The soil characteristic allows to obtaining physical and chemical properties in order to adapt them to the investigation. The soil analysis was carried out according to validated procedures (Soil Science Society of America, Inc., SSA, and American Society of Agronomy, Inc., ASA, 1984). The water content, organic carbon of fraction, pH, total nitrogen, available phosphorus, soil classification, electric conductivity, volatile suspended solids, real and apparent density of the soil, porosity, texture, color, hydraulic conductivity, field capacity and bacteria density were determined. The capacity of adsorption of diesel in the ASo was carried out with the Langmuir model. Diesel volatilization tests in closed and open roads with concentrations of 56,000 mg/L of diesel were determined respectively. The kinetic degradation of diesel during 18 days with three concentrations (28,500, 31,500 and 40,000 mg/L) adding and no adding nitrogen and peroxide of hydrogen by hands were determined.
In order to design the columns as barriers to the contaminated water with diesel the results of the tests of kinetic degradation and oxidation were taken as the basis, as well as the values obtained on adsorption and volatilization. With these results the time of half life, resident time, and thickness of the reactive medium, kinetic constant, transverse area and volume of the columns were obtained. To design the model of substratum consumption which follows a kinetic of first order to the biodegradation and the conceptual model of flow reactor, piston of engineering presented by Smith (1986), 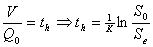 , The previous equation presents the residence time to determinate how long the contaminated water will be in the column before it can reach the desired concentration. The diesel, the nitrogen, and the peroxide of hydrogen were supplied according to the stoichiometric relation of aerobe degradation proposed by McCarty (1998) and the equation for diesel recommended by Jackson (1990): C15H30. 4C15H30 + 45O2 + 9NH3 → 9C5H7O2N + 42H2O + 15CO2. 1 kg of C15H30 1.71 kg de O2 = 3.64 kg of H2O2 and 0.15 kg of nitrogen. The needs of phosphorus were of 0.025 kg. The previous requirement is suitable to those found in the literature. 100:15: 1 for C:N:P (carbon, nitrogen and phosphorus) respectively (Zegarra, 2000).
, The previous equation presents the residence time to determinate how long the contaminated water will be in the column before it can reach the desired concentration. The diesel, the nitrogen, and the peroxide of hydrogen were supplied according to the stoichiometric relation of aerobe degradation proposed by McCarty (1998) and the equation for diesel recommended by Jackson (1990): C15H30. 4C15H30 + 45O2 + 9NH3 → 9C5H7O2N + 42H2O + 15CO2. 1 kg of C15H30 1.71 kg de O2 = 3.64 kg of H2O2 and 0.15 kg of nitrogen. The needs of phosphorus were of 0.025 kg. The previous requirement is suitable to those found in the literature. 100:15: 1 for C:N:P (carbon, nitrogen and phosphorus) respectively (Zegarra, 2000).
The columns were operated with downward flow and saturated during eight days. The mixture of diesel and distillated water was carried out in a container of 50 liters with continuous agitation of 100 rpm. The solution fed seven columns of 30 cm of length; the columns were built in transparent acrylic with 10 cm of diameter. The design flow of the reactors was 360 ml/d. The diesel concentration was of 15,000 mg/L. The degradation velocity obtained in the tests by batches was 0.0908d-1. The added nitrogen as urea was 16 ml/d and the peroxide of hydrogen was 5.9 ml/d. The samples were taken every seven days from the affluent and the effluent; subsequently they were analyzed in the gases chromatograph with a mass selector 5890 serie 11 Hewlett Packard. The packing material to the biodegradation was of 514 g of ASo for each column.
The ASo bacterial density was determined, extracting core from the columns. The cores were taken at the end of the study of the diesel biodegradation. A core was extracted from each column of the half of the reactive medium in a sterile environment. The density was determined using the technique of account plate as colonies former unities (CFU), CFU/g proposed by the Soil Science Society of America, Inc., and the American Society of Agronomy Inc. of the USA (1984). The scanning electron microscopy (SEM) of the surface of the ASo was carried out in order to observe the characteristics of the soil before and after of the columns processing. The SEM was taken in the Cell Physiology Institute, UNAM with an equipment Jeal model JSM-5410LV scanning microscope. The metal balance of importance in the permeable reactive barriers present in the affluent and effluent of the 7 continuous columns was carried out. The objective is to know the precipitation or dragging of the metals by the distillated water used to prepare the solution. The metals analyzed were magnesium, manganese, calcium, iron, potassium, sodium and silica. The analysis was carried out with a spectrophotometer of plasma emission (DUP-ICP) Thermo Jarrel Ash Corporation in the Engineering Institute, UNAM.
3. RESULTS
Firstly the results of the characterization of the applied ASo as a reactive medium are presented; secondly the kinetic biodegradation of the diesel and the adsorption capacity of the ASo, and finally the design and working of the columns.
Table 1. Characterization of the agricultural soil of interest (Cardona, 2006).
Tabla 1. Caracterización del suelo agrícola de interés (Cardona, 2006).
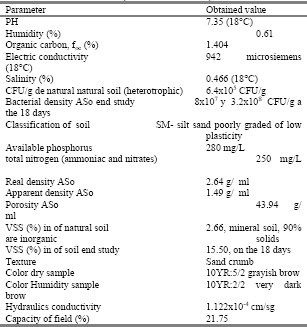
Table 2. Comparison of velocity, time of half time, removable mass percentage and number of half lives in three runs by batches (Cardona, 2006).
Tabla 2. Comparación de la velocidad, tiempo de vida media, porcentaje de masa removida y número de vidas medias en tres corridas intermitentes (Cardona, 2006).
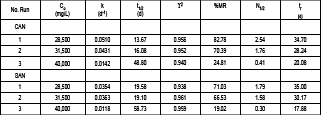
The constant of velocity of biodegradation to the columns design was 0.0908 d-1 and it was obtained from a linear adjustment of the velocities calculated experimentally. The sorption capacity (Cads) of the ASo was 13.5 mg/g. The coefficient of distribution (Kd) for diesel in the ASo was of 0.80 ml/g and the retard factor was 3.69.
SEM: The natural ASo without contact of diesel with a bacterial density of 6.4x105 CFU/g with the ASo at the end of the diesel biodegradation process, with a bacterial density between 8x107 to 3.2x108 CFU (Fig 2) was compared. The SEM presents a change in the layer and density of the biofilm of the ASo. Therefore the ASo presented a possible assimilation of the carbon source (diesel), nutrients (nitrogen as urea) and the oxygen source (H2O2) applied by hands that shows that is the appropriated to the process. (Pardieck et al., 1992, Fiorenza et al., 1997). In this study the characteristics of the stay of the soil and the biomass increasing of the ASo are observed. The soil surface has the same physical configuration and the same characteristics of the film of microorganisms in the agricultural soil. Given this it shows how the characteristics of the soil did not change adding H2O2 as oxygen source (Figs 2A and 2B).

Figure 1. Comparison of the unchangeable soil, photo A and soil the end of the biodegradation process of the day 18, photo B.
Figura 1. Comparación entre el suelo inalterado, foto A y el suelo al final del proceso de biodegradación al día 18, foto B.
Table 3. Summary of parameters of columns design.
Tabla 3. Parámetros de diseño de las columnas.
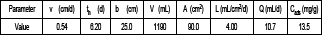
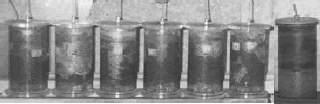
Figure 2. Columns working with agricultural soil as packing.
Figura 2. Desempeño de las columnas con suelo agrícola empacado.
The results of biodegradation obtained from the columns are presented in the following table.
Table 4. Residual concentrations (mg/L) of diesel biodegradation in continuous columns.
Tabla 4. Concentraciones residuales (mg/L) de diesel en las columnas de biodegradación.
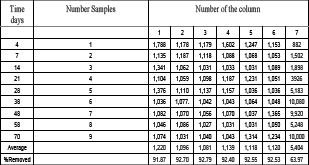
The columns 1 to 6 were working with diesel, nitrogen and peroxide of hydrogen. The column 7 worked in a sterile agricultural soil and only diesel was added to it.
Bacterial density in the columns: The table 7 shows the results obtained from the core culture of the columns and is compared with the agricultural soil before the study. In the data obtained an increase of microorganisms is appreciated.
Table 5. Bacterial density in continuous column.
Tabla 5. Densidad bacterial en las columnas continuas.
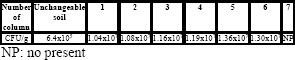
4. DISCUSSION
The characterization of the ASo showed the desired qualities to be used in a process of treatment in contaminated underground water by diesel. It shows the great influence of the concentration in diesel transformation in the batches which make smaller or bigger the biotransformation rate by the agricultural soil. In the system of biodegradation is showed that the removable contaminants percentages are smaller with the increasing of the concentration due to the inhibition caused by the hydrocarbons on the bacterial soil. In this study the diesel biodegradation was presented by the consortium of microorganisms of an ASo. Similar velocities of biotransformation were observed on the literature ( Jackson, 1994). The CFU/g showed the growing of the microbial biomass being the appropriate tools to observe the change in the microorganisms as agent of biodegradation. The H2O2 had an appropriate behavior as source of O2 in the aerobe process of the diesel removal, which is consequent with the CFU/g. Both methods with nutrients or without them show a smaller difference for low concentrations due to the high presence of phosphorus. However to higher a concentration more accentuated difference is presented. The kinetic biodegradation was properly presented by the equation of grade one. The diesel adsorption has a proper behavior according to the Langmuir equation. Any important volatilization in the diesel tests was not appreciated. The reactors out of the site allow observing the evolution of the mineralization of the diesel.
The results also allow to observe that the columns with the agricultural soil as a reactive medium presented high discharge with the addition of N and H2O2 which is similar with other reports of studies where organic reactive medium has been used (moss, sawdust, aquifer sediments, organic matter, municipal compost, leaves, peat, addition of bacterial inoculants, soils with microorganisms and activated sludges), with a bacterial content between 1x105 -1x108 and the addition of nutrients, agricultural fertilizer and an oxygen source, air or oxygen (Atlas y Bartha (1972); Ho et al., (1995); Ganzert, (1991); Powell et al., (1995); Robertson et al., (1995); (Fiorenza y Ward (1997); Waybrantt et al., (1998); Puls et al., (1999); Hebert et al., (2000); Kao et al., (2000); Fiorenza et al., (2000); Márquez-Rocha et al. (2001); Namkoomg et al., (2002)). The diesel adsorption by the ASo was an important process at the beginning of the process because the high adsorption of the hydrocarbon, however it became constant in the time.
The columns presented a similar behavior with the addition of N and H2O2. The process of efficiency of discharge of the seven columns is 89.4 % which is very satisfactory considering the high concentration of diesel (15,000 mg/L). The design parameters to the permeable reactive barrier as a chemical reactor, the reaction of velocity time, time of half life, thickness of the reactive medium, adsorption capacity and bacterial density of the ASo were determined. The evaluation of the conditions of the aerobe biodegradation allowed deducing that diesel can be biodegradable by the bacteria of an agricultural soil, which was used as a reactive medium in the columns by batches. The study of sweep electronic microscope presented the same structure of the agricultural soil before and after the process.
5. CONCLUSIONS
The study presented the treatment of contaminated water by diesel through the PRB with an agricultural soil, which has not been studied and where a good behavior of discharge of diesel was presented. The study achieves its objectives since one can confirm that a permeable reactive barrier is efficient on the treatment of contaminated underground water by diesel. The agricultural soil with the characteristics presented is suitable to be used in a treatment of contaminated underground water by diesel. The initial concentration of diesel has a big influence in the biotransformation rate. In the conditions of the carried out study the biodegradation of diesel by the consortium of microorganisms of the agricultural soil is presented. The absorption of the Langmuir model presented a good adjustment to the experimental data. Volatilization in the test with diesel was not appreciated. The biodegradation was properly presented with a kinetic of first grade. The sweep electronic microscopy presented the same structures of the agricultural soil before and after of the process. The diesel discharge efficiency in the PRB is greater than 89.4% in the case of the addition of nutrients and peroxide of hydrogen.
6. ACKNOWLEDGMENTS
This work was supported by the Researcher Rosario Iturbe Argüelles of Engineering Institute the National University of Mexico .
REFERENCES
[1] ATLAS, R. Y BARTHA, R. Degradation and mineralization of petroleum in sea water: Limitation by nitrogen and phosphorous. Biotechnology and Bioengineering. Vol. XIV, 309-318. 1972. [ Links ]
[2] CARDONA, S. Tratamiento de hidrocarburos con un sistema de barrera reactiva permeable. Tesis de Doctorado. Instituto de Ingeniería, Universidad Nacional Autónoma de México. México, D.F. 2004. [ Links ]
[3] CARDONA, S. Biodegradation of diesel fuel in water by microbial consortium. Submitted to Journal of Environmental Engineering. In Press. 2006. [ Links ]
[4] BLOWES, D., PTACEK, C. AND JAMBOR, J. In-situ remediation of Cr (VI)-contaminated groundwater using permeable reactive walls: laboratory studies. Environ. Sci. Techno.Vol. 31, No. 12, 3348-3357. 1997. [ Links ]
[5] EPA. Permeable Reactive Barrier Technologies for Contaminant Remediation. Environmental Protection Agency, EPA. EPA/600/R-98/125. Cincinnati, Ohio. 1998. [ Links ]
[6] FIORENZA, S. AND WARD, CH. Microbial adaptation to hydrogen peroxide and biodegradation of aromatic hydrocarbons. J. of Industrial Microbiology & Biotechnology. 18: 140-151. 1997. [ Links ]
[7] FIORENZA, S., OUBRE, C. AND WARD, C. Sequenced reactive barriers for groundwater remediation. Lewis Publishers, CRC Press LLC. Boca Raton, Florida. 2000. [ Links ]
[8] GANTZER, Ch. Reductive dechlorination catalyzed by bacterial transition-metal coenzymes. Environ. Sci. Techno. 25, 715-722. 1991. [ Links ]
[9] GAVASKAR, A., GUPTA, N., SASS, B., JANOSY, R. AND SULLIVAN, D. Design Guidance for Application of Permeable Reactive Barriers for Groundwater Remediation, F08637-95-D-6004 DO 5503. BATTELLE, Strategic Environmental Research and Development Program, SERDP of U.S. Air Force Research Laboratory, AFRL. Washington, D.C. 20503. 2000. [ Links ]
[10] GUERIN, T., MCGOVERN, T. AND HORNER, S. A funnel and gate system for remediation of dissolved phase petroleum hydrocarbons in groundwater. Land Contamination & Reclamation, vol. 9 (2), 1-13. 2001. [ Links ]
[11] HERBERT JR, R., BENNER, S. and BLOWES, D. Solid phase iron-sulfur geochemistry of a reactive barrier for treatment of mine drainage. Applied Geochemistry, 15, 1331-1343. 2000. [ Links ]
[12] HO, T., JOHN, D. and FORSTER, C. Batch niquel removal from aqueous solution by sphagnum moss peat. Ground Water, Vol. 29, No. 5, 1327-1332. 1995. [ Links ]
[13] HORNER, S. A funnel and gate system for remediation of dissolved phase petroleum hydrocarbons in groundwater. Land Contamination & Reclamation, vol. 9 (2), 1-13. 2001. [ Links ]
[14] JACKSON, J. Bioremediation of diesel contaminated soils. Processing of 3th annual Symposium of the Hydrology Society, Casa Grande, Arizona. 309-316. September 20-21. 1990. [ Links ]
[15] JACKSON, J. Using microbial kinetics in the bioremediation of contaminated soils. In: Donald L. Wise and Bebra J. Trantolo (Eds). Remediation of Hazardous Waste Contaminated Soils. Marcel Dekker, Inc. New York, pp. 681-989. 1994. [ Links ]
[16] KAO, M. and LEI, S. Using a peat biobarrier to remediate PCE/TEC contaminated aquifers. Water Research. Vol. 34, No. 3, pp. 835-845. 2000. [ Links ]
[17] MÁRQUEZ-ROCHA, F., HERNÁNDEZ-ROGRÍGUEZ, V. and LAMELA, M. Biodegradation of diesel oil in soil by a microbial consortium. Water, Air and soil Pollution, 128: 313-320. 2001. [ Links ]
[18] MCCARTY, P. Bioengineering issues related to in situ remediation of contaminated soils and groundwater. In Environmental Biotechnology, Reducing risks from environmental chemicals trough biotechnology. Edited by Gilbert S. Omenn. Plenum Press. New York. 1988. 1988. [ Links ]
[19] MCMURTY, D. and ELTON, R. (1985) New approach to in situ treatment of contaminated groundwater. Environmental Progress, 4 (3), 3413- 3423. In Guerin, T., McGovern, T. and [] Horner, S. A funnel and gate system for remediation of dissolved phase petroleum hydrocarbons in groundwater. Land Contamination & Reclamation, vol. 9 (2), 1-13. 2001. [ Links ]
[20] NAMKOONG, W., HWANG, E., PARK, J. AND CHOI, J. Bioremediation of diesel-contaminate soil with composting. Environmental Pollution, 119, 23-31. 2002. [ Links ]
[21] PARDIECKN, D., BOUWER, E. and STONE, A. hydrogen peroxide use to increase oxidant capacity for in situ bioremediation of contaminated soils and aquifers: A review. J. of Contaminant Hydrology, 9, 221-242. 1992. [ Links ]
[22] POWELL, R., PULS, R., HIGHTOWER, S. and SABATINI, D. Coupled iron corrosion and chromate reduction: mechanisms for subsurface remediation. Environ. Sci. Techno. 29, 1913-1922. 1995. [ Links ]
[23] PULS, R., PAUL, C. and POWELL, R. The application of in situ permeable reactive (zero-valent iron) barrier technology for the remediation of chrolate contaminated groundwater: a field test. Applied Geochemistry. 14, 989-1000. 1999. [ Links ]
[24] ROBERTSON, W. and CHERRY, J. In situ denitrification of septic-system nitrate using reactive porous media barriers: field trials. Ground Water, Vol. 33, 995.
[25] SMITH, J. Chemical engineering kinetics, 3ª edition. McGraw Hill. New York. 1986. Soil Science Society of America, Inc. y American Society of Agronomy, Inc. Methods of soil analysis. Part 1, 2 y 3. Soil Science Society of America, Inc. y American Society of Agronomy, Inc. Madison, Wisconsin, USA . 1984. [ Links ]
[26] WAYBRANT, K., BLOWES, D. and PTACEK, C. Selection of reactive mixtures for use in permeable reactive walls for treatment of mine drainage. Environ. Sci. Techno. Vol. 32, No. 13, 1972-1979. 1998. [ Links ]
[27] ZEGARRA, H. Optimización de la biodegradación de diesel industrial en un suelo contaminado mediante la metodología de superficie de respuesta. Tesis de Maestría Ingeniería Ambiental. División de Estudios de Posgrado de la Facultad de Ingeniería de la UNAM. México, D.F. 2000. [ Links ]














