Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
DYNA
Print version ISSN 0012-7353On-line version ISSN 2346-2183
Dyna rev.fac.nac.minas vol.72 no.157 Medellín Jan./Apr. 2009
EVALUATION OF SOLVENTS EFFICIENCY IN CONDENSATE BANKING REMOVAL
EVALUACION DE LA EFICIENCIA DE SOLVENTES EN LA REMOCION DEL BANCO DE CONDENSADO
TOMAS CORREA
Msc. en Gas Natural y Gerencia de Energía, Instituto Tecnológico Metropolitano ITM, tomascorrea@itm.edu.co
DJEBBAR TIAB
Dr. en Ingeniería de Petróleos, Universidad de Oklahoma, USA, dtiab@ou.edu
DORA PATRICIA RESTREPO
Dr. en Ingeniería de Petróleos, Universidad Nacional de Colombia, dprestre@ou.edu
Recibido para revisar septiembre 14 de 2008, aceptado noviembre 4 de 2008, versión final noviembre 11 de 2008
ABSTRACT: This work describes experimental design and tests performed to simulate gas condensate reservoir conditions below dew point in the laboratory using three different compositions of synthetic gas condensate. Methanol, propanol and methylene chloride are the solvents used to remove the condensate banking and improve the gas effective permeability near to the wellbore. Solvents are injected in Berea sandstone rock with similar petrophysical properties in order to compare the efficiency at removing the condensate banking. It was observed that all of the solvents improved the gas effective permeability after removing banking condensate; however, methanol was the more efficient solvent to remove it while methylene chloride had the lowest values of gas effective permeability after removing the banking condensate.
KEY WORDS: Gas condensate reservoir, miscible displacement, experimental work, enhanced recovery, gas effective permeability, condensate banking removal.
RESUMEN: Este estudio describe el montaje experimental y las pruebas realizadas en el laboratorio para simular las condiciones de un yacimiento de gas condensado por debajo del punto de burbuja usando tres diferentes composiciones sintéticas de gas condensado. Metanol, Propanol y cloruro de metileno son los solventes usados para remover el banco de condensado y mejorar la permeabilidad efectiva al gas en la cara del núcleo. Ellos son inyectados en areniscas Berea con propiedades petrofísicas similares con el fin de comparar el grado de eficiencia en la remoción del banco de condensado. Los experimentos muestran que los tres solventes mejoraron la permeabilidad efectiva al gas después de remover el banco de condensado; sin embargo el metanol fue el solvente más eficiente para remover el banco de condensado, mientras el cloruro de metileno mostró los valores más bajos de permeabilidad efectiva al gas indicando menor eficiencia en la remoción el banco de condensado.
PALABRAS CLAVE: Yacimientos de gas condensado, desplazamiento miscible, trabajo experimental, recobro mejorado, permeabilidad efectiva al gas, remoción del banco de condensado.
1. INTRODUCCION
Many of the largest natural gas reservoirs (30-35%) have reservoir conditions which result in retrograde condensation due to pressure decreases during the production of gas. During depletion of these gas condensate reservoirs, as the pressure drop below the dew point pressure, liquid drops out of the gas phase and forms condensate banking near the wellbore, reducing the gas productivity significantly. The condensate continues accumulating in portions of the pore space that otherwise would be available for gas flow, thus blocking the gas flow. Once the condensate saturation exceeds the residual saturation, the condensate continuously forms and flows towards the wellbore. Liquid saturations near the wells can reach 50 to 60% under pseudo steady state flow of gas and condensate. Productivity reductions of 40-80% have been reported for some fields. Reductions in relative permeability greater than 95% in laboratory cores at low capillary number have been reported for both low and high permeability rocks.
The degree of condensate banking depends indirectly on a combination of several factors including fluids properties (interfacial tension, densities and wetting characteristics), formation characteristics, flow rate and pressure [1].
Many strategies have been proposed for stimulations in wells that show condensate banking effects: recycling gas, water injection, water alternating with gas (WAG), hydraulic fracture stimulation, viscosity reduction and chemical treatments, thereby delaying the onset of condensate formation around the wellbore [2],[3].
There are some mechanisms proposed to explain the enhancement in gas effective permeability and also the higher degree of cleaning and liquid removing obtained in laboratory and field studies [4]. Three of them are: miscible displacement, interfacial tension reduction and alteration of wettability. Miscible displacement uses solvents to remove water and hydrocarbons from the region near to the wellbore. Interfacial tension reduction uses surfactant injection with solvents. The surfactant reduces the interfacial tension between formation fluids and once the interfacial tension decreases, the solvent is injected. The third mechanism is the alteration of rock wettability. Li and Firoozabadi [5] used polymeric surfactants and Kumar [7] used fluorosurfactants to remove the condensate banking for altering the wettability of reservoir from liquid to intermediate gas wet. They concluded that this mechanism is more efficient to remove the banking condensate. Various chemicals were found that work well, and stimulations showed that this process could be economic. Firoozabadi and co-workers [5] first proposed to use chemicals to alter the wettability of the formation in the near wellbore region to mitigate the damage caused by condensate banking. Since most gas reservoirs are thought to be water wet, it is predicted that by changing the wettability to neutral wet (contact angle of ± 90), the relative permeability of the condensate banking and gas would both increase, resulting in substantial increase of productivity [6]. Kumar [7] conducted flow test at reservoir conditions to study the effect of various fluorosurfactants on wettability as well as the changes in critical parameters: gas relative permeability and capillary number (Krg = f(Krg/Kro, Nc)). In all instances, capillary forces trap some of this liquid in the pores resulting in a high liquid saturation and a reduction in the relative permeability of both the gas and condensate, which is the cause of the loss in production. Even for lean gas (1% liquid dropout) significant liquid condensate saturations can build up near the wells and can decrease production by a factor of two or three.
The objective of this study is to simulate three different gas condensate reservoir conditions in the laboratory (using three different compositions of synthetic gas condensate) below dew point and injecting three different solvents (methanol, propanol and methylene chloride) for each gas condensate reservoir conditions and evaluate which is the best solvent to remove the condensate banking. Gas effective permeability (KG) is measured in three different stages: a) Stage 1: at residual water saturation, b) Stage 2: when generating condensate banking, and c) Stage 3: after removing condensate banking. The more efficient solvent was selected based on comparison of gas effective permeabilities in stages 2 and 3.
2. EXPERIMENTNAL APPARATUS AND PROCEDURE
2.1 Core flood set up
Figure 1 shows a schematic diagram of the core flood apparatus. Positive displacement pumps were used to inject fluids at constant fluid rate. Multiple ports were used to measure pressure at the ends of the core holder. Two back pressures regulators were used to control the flowing pressure upstream and downstream. The core holder and flow lines are inside a temperature-controlled oven. Three different temperatures were used depending on the composition of synthetic gas.
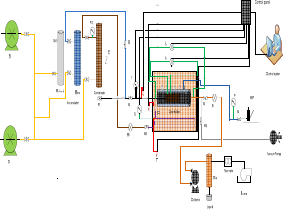
Figure 1. Scheme of core flood system
2.2 Gas mixtures properties
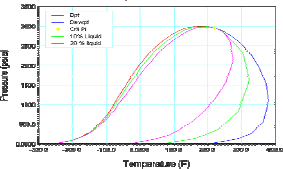
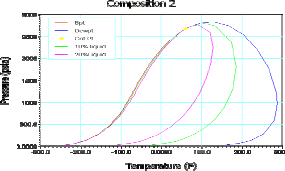
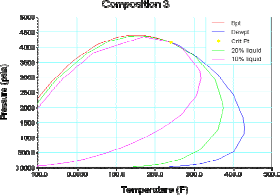
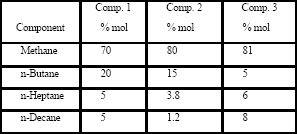
Table 1 shows the composition of three different synthetic gas condensate fluids that were used to perform experiments at 150°F, 200°F and 250°F. Peng Robinson equation was used to generate phase envelops in Hysys Program and figures 2, 3, 4 shows the phase envelopes. The selection of each composition was done base in previous experimental works [7].
Table 1. Components of synthetic gas compositions
2.3 Rock properties
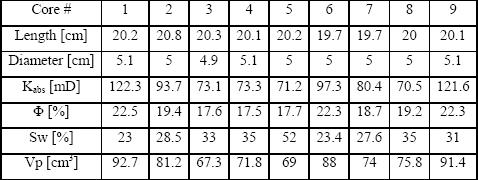
Berea sandstone was used in the core flood experiments. Table 2 list the properties of nine cores used in the tests. The cores were dried in an oven at 95oC for 48 hours and wrapped in aluminum foil to eliminate the diffusion of gases and possible interaction of fluids with the viton sleeve.
2.4 Compatibility Test
Compatibility test was the main tool to be sure that the used solvents didnt generate additional formation damage when mixed with formation fluids. The objective was to determine if the fluids generate any kind of precipitated solids, gums, or colloidal particles when they are mixed. Bottle test were performed and no precipitated solids were observed, therefore the fluids used in this experimental study guarantee no damage in the core caused by fluids incompatibility.
2.5 Core flood Procedure
The cores were placed into a core holder inside the oven at three different temperatures depending on the synthetic gas composition. An overburden pressure of 2,000 psi was applied and vacuum pump was turned on for three hours to assure no air is kept inside the core. After vacuum three pore volumes of Brine were injected a constant flow rate (1 cc/min) to guarantee 100% water saturation.
The experimental procedure designed for each run is as follow: 1. Flooding the core with brine, 2. Displacing brine using nitrogen to get residual water saturation, 3. Measuring gas effective permeability using methane( flow rate was between 1lt/sec to 5lt/sec), 4. Generating banking condensate, 5. Measuring gas effective permeability using methane, 6. Removing of banking condensate, 7. Measuring gas effective permeability using methane, and 8. Collect produced fluids.
2.6 Gas effective permeability
Gas effective permeability is the ability to preferentially flow or transmit a particular fluid when other immiscible fluids are present in the reservoir [8]. It was necessary to assure that the only fluid that was moving through the core was methane so residual liquid saturation was reached and gas effective permeability could be measured in the laboratory. Inlet and outlet pressures of the core and average flow rate (it was measured as a function of injected gas pore volume) were measured as well. Gas effective permeability was measured in three different stages: at specific water saturation (stage 1), after condensate banking generation (stage 2) and after removing banking condensate (stage 3).
2.7 Miscible displacement
Miscible displacement in hydrocarbon reservoir has been described as the displacement of heavier hydrocarbons from pore space in a rock using a solvent action that prevent formation of interfaces between formation fluids. Miscible displacement is considered to be very efficient because it eliminates capillary forces. In the absence of capillary pressure, no interface exists between miscible fluids of different composition [9]. They fall generally into two classes: process in which the injected fluid and in-place-fluid form a single-phase solution for all compositions and processes in which the injected fluid and in-place-fluid dont form a single equilibrium phase but which may generate a zone of contiguous single-phase by multi-contacts miscibility [10]. There are some studies [11] which are focused in the second class of miscible displacement to explain the improvement in mobility at the region near to the wellbore after injection solvents.
This project considered three different solvents to remove the gas condensate banking: methanol, propane and methylene chloride. Methanol has been widely used in experimental works and fields worldwide basically because it demonstrated to be effective when mixed with hydrocarbons and most water formations. There are not published results of experimental works using propane and methylene chloride as solvents even though they are used broadly as solvents in the chemical industry and both of them are miscible in water. In this way this work seeks other alternative mixtures of solvents to get more efficient solutions in order to remove banking condensate.
3. RESULTS AND DISCUSION
3.1 Effect of Solvents Treatment
Table 3 shows the basic chemical and physical properties of the solvents used to remove the banking condensate. Figures 5 to 13 are plots of gas effective permeability vs. pore volume of gas methane injected to measure gas effective permeability during the three different stages for every solvent. Tables 4, 5 and 6 summarize results of gas effective permeability for each run.
Table 3. Chemical and Physical Properties of Solvents
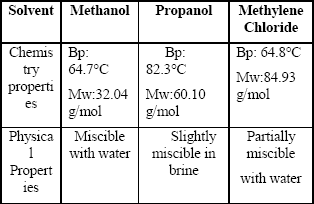
Table 4. Gas effective permeability using methanol
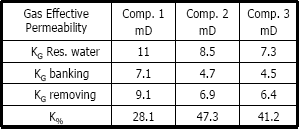
Table 5. Gas effective permeability using propanol
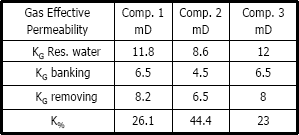
Table 6. Gas effective permeability using methylene chloride
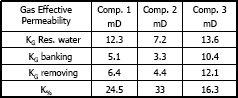
3.2 Methanol Injection
Figures 5, 6 and 7 show a plot of gas effective permeability for each stage vs. pore volumes injected for the three different compositions of gas condensate. Table 4 shows gas effective permeability for each stage for the three different composition of gas condensate fluid. When removing banking condensate using methanol the gas effective permeability improved and the percentages with respect to the banking condensate were 28.1 %, 47.3 % and 41.2 %, respectively. This means gas effective permeability after injection of methanol to remove banking condensate improved 28.1 %, 47.3 % and 41.2 % with respect to the gas effective permeability of the banking condensate.
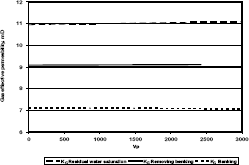
Figure 5. Gas effective permeability vs. pore volumes for composition 1, solvent methanol
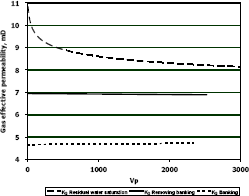
Figure 6. Gas effective permeability vs. pore volumes for composition 2, solvent methanol
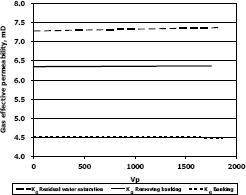
Figure 7. Gas effective permeability vs. pore volumes for composition 3, solvent methanol
The highest increase in gas effective permeability was obtained for composition 2 which is 47.3% as showed in table 4.
3.3 Propanol Injection
Figures 8, 9 and 10 present gas effective permeability for each stage vs. pore volumes injected for three different composition of gas condensate. Table 5 shows gas effective permeability for each stage for the three different composition of gas condensate fluid. When removing banking condensate using propanol the gas effective permeability improved and the percentages with respect to the banking condensate were 26.1%, 44.4% and 23%, respectively.
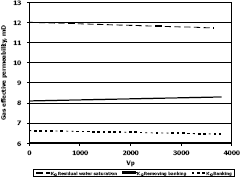
Figure 8. Gas effective permeability vs. pore volumes for composition 1, solvent propanol
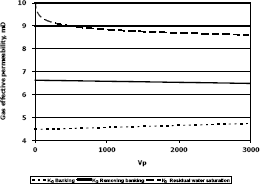
Figure 9. Gas effective permeability vs. pore volumes for composition 2, solvent propanol
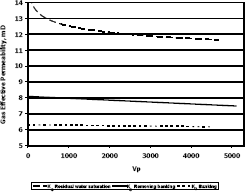
Figure 10. Gas effective permeability vs. pore volumes for composition 3, solvent propanol
This means gas effective permeability after injection of propanol to remove banking condensate improved 26.1%, 44.4% and 23% with respect to the gas effective permeability of the banking condensate.
The highest increase in gas effective permeability was obtained for composition 2 which is 44.4% as showed in table 5.
3.4 Methylene chloride injection
Figures 11, 12 and 13 present gas effective permeability for each stage vs. pore volumes injected for the three different composition of synthetic gas condensate. Table 6 shows an average of gas effective permeability for each stage for the three different composition of gas condensate fluid. When removing banking condensate using propanol the gas effective permeability improved and the percentages with respect to the banking condensate were 24.5%, 33% and 16.3%, respectively. This means gas effective permeability after injection of propanol to remove banking condensate improved 24.5%, 33% and 16.3% with respect to the gas effective permeability of the banking condensate. The highest increase in gas effective permeability was obtained for composition 2 which is 33.0% as showed in table 6.
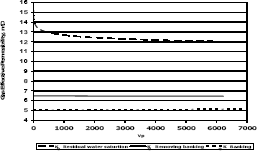
Figure 11. Gas effective permeability vs. pore volumes for composition 1, solvent methylene chloride
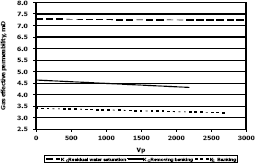
Figure 12. Gas effective permeability vs. pore volumes for composition 2, solvent methylene chloride
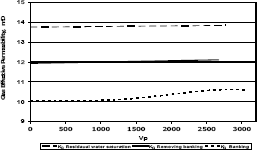
Figure 13. Gas effective permeability vs. pore volumes for composition 3, solvent methylene chloride
The three solvents demonstrated to remove the banking condensate in all of the gas synthetic gas condensate compositions as showed in figures 5 to 13 because values of gas effective permeability were higher after removing banking condensate compared with respect to gas effective permeability of banking condensate. These results are close to previous experimental studies that showed that alcohols can be used as solvents to remove banking condensate and enhance gas relative permeability [12], [13].
Figure 14 shows that values of gas effective permeability when using methanol were the highest compared with other solvents. It could be explained because methanol has dual miscibility with water and hydrocarbons while propanol and methylene chloride are partially miscible in some water formation. Since all of solvent used are miscible in hydrocarbons, multi contact miscible displacement could be the mechanism that displace the liquid hydrocarbon in the banking condensate. The efficiency of solvent depends on many variables such as properties of formation fluids, pore volume of the solvent injected and formation characteristics.
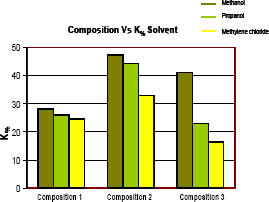
Figure 14. Gas permeability percentage increase for 3 compositions and 3 solvents
Permeability of the rock will affect drastically the efficiency of solvent. Cores with low permeability have small pores so interfacial forces and capillary pressures will be stronger than in those cores with high permeability. Pore size distribution, therefore permeability, will affect miscibility when solvents are injected because solvents could be concentrated in pores of intermediate and high diameter removing hydrocarbons in those pores efficiently.
5. CONCLUSIONS
- Laboratory design allowed the evaluation of effectiveness in removing damage caused by banking condensate using three different solvents: methanol, methylene chloride and propanol.
- All of the solvents used for removing banking condensate presented high values of gas effective permeability when compared with the values of the gas curve of effective permeability before treatment using solvents, therefore they removed condensate blocking.
- The best solvent to remove condensate banking for all gas condensate compositions was methanol because it gives the highest increases in the gas effective permeability.
- Ø Methylene chloride, although removed condensate blocking presented the lowest increases in gas effective permeability.
6. RECOMMENDATIONS
- Mixing methanol with other solutions should be done in order to improve the miscibility of methanol in hydrocarbons and water formation and also will reduce the cost associated with pure solvents.
- Additional work needs to be done to study the phase behavior of hydrocarbons hydrocarbons-water- methanol mixtures that may be used under different reservoir conditions, in particular at higher temperatures and different compositions.
NOMENCLATURE
Bp: Boiling Point
KG: water residual: gas effective permeability at residual water mD
KG: banking: gas effective permeability before treatment, mD
KG: removing: gas effective permeability after treatment, mD
Kabs: Absolute permeability to liquid, mD
K%: Increase in percent of gas effective permeability when compared gas effective permeability of removing banking with gas effective permeability of banking condensate.
Krg: gas relative permeability
Kro: oil relative permeability
Mw: molecular weight
Nc: capillary number
Sw: Residual water saturation
Vp: pore volume
Greek Symbols
Φ: Porosity, %
REFERENCES
[1] KUMAR V., POPE G.A., SHARMA M. M., Improving the gas and Condensate Relative Permeability Using Chemical Treatments , SPE 100529 presented by at the 2006 SPE Gas Technology Symposium Held in Calgary, Alberta, Canada, 15-17 may 2006. [ Links ]
[2] DU L., WALKER J., POPE G., SHARMA M., WANG P., Use of Solvents to Improve the Productivity of Gas Condensate Wells, SPE 62935 presented at the 2000 SPE Annual technical conference and exhibition held in Dallas, Texas, 1-4 October 2000. [ Links ]
[3] FAM L., HARRIS B., JANALUDDIN A., KAMMATH J., MOTT R., POPE G., SHANDRYGIN A., WHITSON C., Understanding Gas Condensate Reservoirs, Oilfield Review. Winter 2005-2006. Chevron [ Links ]
[4] AL-ANAZI H., WALKER J., POPE G., SHARMA M., HACKNEY D., A successful Methanol Treatment in Gas-Condensate Reservoir: Field Application, SPE 80901 presented at SPE production and Operations symposium held in Oklahoma City , USA, 22-25 March 2003. [ Links ]
[5] FIROOZABADI F., Wettability Alteration to intermediate Gas Wetting in gas/Condensate Reservoirs at high temperatures, SPE 96184, presented at the SPE Annual Technical Conference and Exhibition, Dallas, October 9-12, 2005. [ Links ]
[6] KAMATH J., Deliverability of gas Condensate Reservoir-field Experiences and predictions techniques, JPT Volume 59 Number 4, April 2007. [ Links ]
[7] KUMAR V., Chemical Stimulation of gas Condensate Reservoirs: An Experimental and Stimulation Study, PhD. Dissertation, The University of Texas at Austin. 2006. [ Links ]
[8] TIAB D., DONALDSON E., Petrophysics Second Edition. Elsevier Gulf Professional Publishing, Oxford 2004. [ Links ]
[9] SALAMA D., KANTZAS A., Experimental Observations of Miscible Displacement of Heavy Oils with Hydrocarbon Solvents , SPE 97854 presented at the 2005 SPE International Thermal Operations and heavy Oil Symposium Held in Calgary, Alberta Canada, 1-3 November 2005. [ Links ]
[10] RATHMELL, J., STALKUP F., HASSINGER, R., A laboratory investigation of miscible Displacement by carbon dioxide, SPE paper 3483, presented by the 46th annual Fall Meeting of the AIME, held in New Orleans, La., Oct. 3-6, 1971. [ Links ]
[11] WALKER J., Laboratory evaluation of alcohols and surfactants to increase production from gas condensate reservoirs, M.S. Thesis, the University of Texas Austin, December 2000. [ Links ]
[12] ZHAO L., Steam Alternating Solvent Process, SPE 86957 presented at the SPE International Thermal Operations and Heavy oil Symposium and western regional meeting held in Bakersfield, California, U.S.A. , 16-18 March 2004. [ Links ]
[13] KOOL H., AZARI M., SOLIMAN M., PROETTT M., IRANI C., DYBDAHL B., Testing of Gas Condensate Reservoirs-Sampling, Test Design and Analysis SPE 68668 presented at the SPE Asia Pacific Oil and Gas Conference and Exhibition held in Jakarta, Indonesia, 17-19 April 2001. [ Links ]