Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
DYNA
Print version ISSN 0012-7353On-line version ISSN 2346-2183
Dyna rev.fac.nac.minas vol.77 no.163 Medellín July/Sept. 2010
BIODEGRADATION PATHWAY PREDICTION OF POPS (PERSISTENT ORGANIC POLLUTANTS) AND BIOBARRIER TREATMENT
PREDICCIÓN DE LA RUTA DE BIODEGRADACIÓN DE COPS (CONTAMINANTES ORGÁNICOS PERSISTENTES) Y TRATAMIENTO POR BIOBARRERA
SANTIAGO CARDONA
Escuela de Geociencias y Medio Ambiente, Facultad de Minas, Universidad Nacional de Colombia Sede Medellín. Profesor Asociado. sacardona@unal.edu.co
EDGAR SUÁREZ
Escuela de Biociencias, Facultad de Ciencias, Universidad Nacional de Colombia Sede Medellín. esuarez@unal.edo.co
Received for review July 27th, 2009, accepted March 4th, 2009, final version May, 14th, 2009
ABSTRACT: In this paper, the biodegradation pathway of Dieldrin is simulated using an expert system. This insecticide is included in the Stockholm Convention, signed and ratified by Colombian government in 2008, and it is considered one of the most harmful human-made compounds. For this model contaminant a complete metabolic biodegradation sequence was built and a simulation-based strategy was formulated for its biodegradation in a practical case.
According to the simulated metabolic pathway, a sequential aerobic-anaerobic-aerobic reactor would be the best choice to achieve complete biodegradation. Using these results, the authors propose an innovative system for the biological treatment of POPs; this system was called Bio-Reactive Permeable Barrier (BioBarrier). In this work a description of the main and fundamentals aspects of BioBarrier system is also included, showing a new and potential possibility for the bio-treatment of hazardous pollutants.
KEYWORDS: Metabolic pathway, Heuristic, Persistent Organic Pollutant, BioBarrier.
RESUMEN: En este trabajo la ruta de biodegradación de Dieldrin es simulada usando un sistema experto. Este insecticida es incluido en el Convenio de Estocolmo, firmado y ratificado por el gobierno Colombiano en 2008, y es considerado uno de los compuestos sintéticos más tóxicos. Para este compuesto modelo, una secuencia metabólica completa de biodegradación es construida y una estrategia basada en simulación es formulada para su biodegradación en un eventual proceso aplicado.
De acuerdo con la simulación realizada, un proceso secuencial aerobio-anaerobio-aerobio sería la mejor opción para asegurar una completa biodegradación. A partir de estos resultados los autores proponen un sistema innovador para el tratamiento biológico de los Compuestos Orgánicos Persistentes COPs denominado Bio-Barrera Reactiva Permeable COPs (BioBarrera). En este trabajo, una descripción de los conceptos fundamentales del diseño y características de las BioBarreras es presentado adicionalmente, mostrando una nueva posibilidad para el tratamiento biológico de contaminantes peligrosos.
PALABRAS CLAVE: Ruta Metabólica, Heuristica, Compuesto Orgánico Persistente, BioBarrera.
1. INTRUDUCTION
Persistent Organic Pollutants (POPs) are organic (carbon based) compounds synthesized artificially (like pesticides and Poly-Chlorinate Biphenyls PCBs) or generated as by-products
mainly by human activities (like Dioxins and Furans) and are considered as the most dangerous pollutants if released into the environment. POPs have four characteristics in common [1]: are toxic, environmentally persistent, soluble in fatty tissues and easily transported through the environment.
The Stockholm Convention on Persistent Organic Pollutants, signed by Colombian government in 2001 and ratified in 2008, focuses on the reduction and safe elimination of 12 POPs (Dirty Dozen), including eight pesticides (aldrin, chlordane, DDT, dieldrin, endrin, heptachlor, mirex, and toxaphene), two industrial chemicals (polychlorinated biphenyls and hexachlorobenzene) and two combustion by-products (dioxins and furans) [1].
Being hydrophobic, POPs are adsorbed strongly in soil texture, making them very difficult to treat. In water bodies, these compounds resist natural breakdown and impact aquatic life. They are present at low concentration in water (making them refractory to conventional treatments), usually varying between nanogram and picogram per liter to almost micrograms per liter. New technologies for POPs removal include adsorption onto novel lipophilic materials [2] and the use of electro-kinetic and ultrasound for the improvement of contaminant mobility in low permeable soils [3].
In this paper, the metabolic biodegradation pathway for Dieldrin was predicted using the UMBBD expert system. The main reactions, enzymes and microorganisms taking part in the biodegradation were identified and finally the concept of BioBarrier is introduced and suggested for future applications for the bio-treatment of POPs.
2. METODOLOGY
2.1 Simulation of metabolic pathwaysThe need for a tool capable of predicting metabolic routes has a core explanation since most of the 10 million of synthetic chemicals are not being subjected to biodegradation studies in order to determine their environmental fate and impact; in addition, industries continue synthesizing new materials faster than regulatory agencies and academic researchers are able to study their environmental facts. Companies should invest on prediction of biodegradation pathways in order to avoid releasing a new component that is later found to be dangerous and should be withdraw from commerce. Regulatory agencies could also do a better job if they would get access to more rapid and reliable information regarding to the biodegradation mechanism and health-environmental impacts of dozen of new chemicals every year [4]. The Simulation of the metabolic pathway for the biodegradation of Dieldrin was performed using the University of Minnesota's Biocatalysis/Biodegradation Database (UMBBD) and the rule-based Pathway Prediction System (UM-PPS) [5]. This expert system is built up from rules which experts have proved over time according with experimentation and scientific evidence (figure 1). This database contains 194 pathways, 1324 reactions, 1229 compounds, 50 organic functional groups, 855 enzymes and 506 microorganisms; the expert system is based on 267 biotransformation rules. Developments and improvements of the UM-PPS were published recently [6].
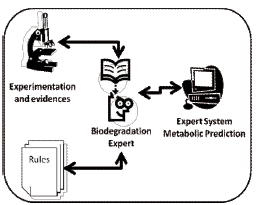
Figure 1. Development of an expert system based on experience and Heuristic
Each new single transformation is prioritized depending on their aerobic biotransformation likelihood. Priority score is as follow: very likely reaction (strong gray), likely reaction (gray), possible reaction (neutral clear), unlikely reaction (stronger gray), very unlikely reaction (black) and unknown. All biodegradation are considered to occur in environments like soil and water at neutral pH, 25°C and with no competing or other toxic compounds [4].
2.2 Model compound
Dieldrin is an insecticide used against insects and pest infesting mainly corn, cotton and potato. It has a low toxicity in plants, but high in insects, fish, birds and mammals. In humans it affects principally immune response, but has been linked with Parkinson's, breast cancer, and reproductive, and nervous system damage. The molecular structure of Dieldrin is presented in figure 2; chloride groups are responsible for its persistence and bioaccumulation capacity, besides, they constitute the major hindrance for microbial attack [1].
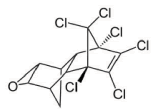
Figure 2 Chemical structure of Dieldrin
Microbial breakdown of Dieldrin has been conducted both under aerobic and anaerobic conditions; the efficiency of bacterial dechlorination in presence of oxygen has reached a maximum value of 40.4% with no registered presence of intermediaries and byproducts [7].
But not only bacterial strains can degrade Dieldrin. Evidence suggest than certain fungal species can hydrolyze this compound until CO2 plus some intermediaries, being the chlorinated segment the bottleneck for enzymatic attack [8]. Under anaerobic conditions, few works have been reported; the main transformation step involves just the reductive dechlorination of the Methylene Bridge and not complete mineralization [9]. As can be seen, the implementation of a sequential process for the effective and complete biodegradation of Dieldrin has not been probed.
3.1 The Predicted pathway
In figure 3 is shown the first predicted biodegradation step for Dieldrin both under aerobic and anaerobic conditions (conventions indicate probability of occurrence under aerobic conditions). Circles in each molecule indicate where the transformation is expected to occur. As several alternatives are available for each degradation step, just the ones taken place under aerobic conditions are selected, unless just anaerobic transformations are offered.
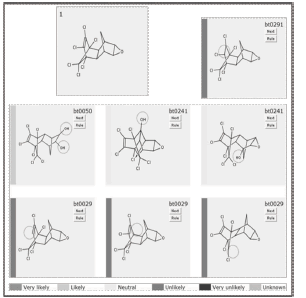
Figure 3. Predicted first biodegradation step for Dieldrin breakdown
From figure 3 it is observed that under aerobic conditions the first transformation implies the introduction of hydroxyl groups in different regions of the molecule away from the chlorine groups. On the other hand, when aerobic conditions are not present, dechlorination is expected to happen. This gives several insights regarding the whole degradation sequence. Firstly, as the dechlorination of the molecule is likely to occur under anaerobic conditions, the aerobic treatment must be complemented with one or several anaerobic steps. Secondly, as the chlorine groups are toxic, the anaerobic step should be carried out at earlier stages of the degradation process.
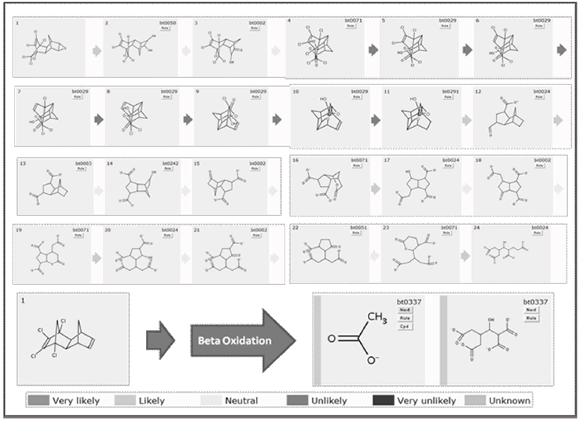
The complete simulation of the biodegradation of Dieldrin is presented in appendix 1. Several attempts were necessary before getting complete mineralization of the former molecule. Another routes were built in which the final transformations led to a complex molecules for which no biodegradation rule is known yet (results not presented). After releasing a contaminant in water bodies or soils, the first transformation is expected to occur under aerobic conditions, before the depletion of the available oxygen. In the first transformation note how microbial strains attack epoxy group and not chloride segment (toxic segment) and converts it into glycol likely groups (reaction 1), a transformation mediated by epoxide hydrolases and organisms like Mycobacterium bovis, Streptomyces sviceus, Sphingomonas wittichii and Rhodococcus erythropolis.

Reaction 1. Epoxide biotrasformation
Efficient attack of the ring system should continue in presence of oxygen or at least low pressure O2. Steps 3 and 4 (appendix 1) are probably major transformations for ring cleavage, for which it is necessary to introduce a ketone group into the structure before the Baeyer-Villiger oxidation (reactions 2 and 3). The former step is mediated by dehydrogenases, some depending of NADP+. Second transformation is also dependent on NADPH and is done mainly by monoxygenases.
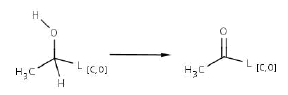
Reaction 2. Biotransformation of secondary alcohol to ketone (or ester group)
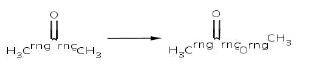
Reaction 3. Baeyer-Villiger oxidation of saturated rings (from cyclic ketone to cyclic ester)
Afterwards, anaerobic conditions are expected to occur. In this environment, two important tasks must be done with the scope of reducing toxicity: dechlorination and elimination of saturated bonds. Referring to steps 5 to 10, almost complete chloride removal could be reached in concordance with reductive dehalogenation reaction presented in reaction 4, performed by reductive dehalogenases and reductases present in organisms like Sphingomonas sp.
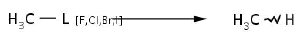
Reaction 4. Conversion of organohalide group into aliphatic one
Microbial strains commonly active in sediments (Shewanella piezotolerans, Clostridium botulinum), and families of enzymes like reductases and dehydrogenases, which require at least that the alkene group contains one free hydrogen, can perform the reaction 5 (step 11).

Reaction 5. Alkene to alkane transformation
Even though anaerobic conditions are present, it is necessary to provide enough oxygen to the system for the next transformations (step 12). Here, the process engineer should decide between pump technologies, natural aeration systems or oxygen releasing compounds (among other technologies). After the insertion of an oxygen molecule and in the presence of a ketone group inside the ring (ester group), it is possible to break it down, leading to an aliphatic chain (refer to reaction 6).
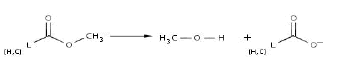
Reaction 6. Transformation of an ester group into alcohol plus carboxylate (alterntively lactone to hydroxycarboxylate)
The above reaction will produce cis products in rings with double bonds. A clearer example is presented in reaction 7. Enzymes involved in this reaction are hydrolases and carboxylic ester hydrolases, which are present in organisms such as Pseudomonas sp, Mesorhizobium loti and Acinetobacter calcoaceticus.
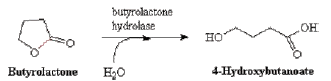
Reaction 7. Breakdown of the ring system under neutral conditions
Neutral conditions should be maintained to allow the system to introduce hydroxyl groups (alcohol) and ketones (ester group) for further ring cleavage. In step 13, carboxylation (reaction 8) is performed by dehydrogenases dependent on NADH present in species like Aromatoleum aromaticum, Nitrobacter sp., Pseudomonas mendocina and Aspergillus niger.

Reaction 8. Transformation from aldehyde to carboxylate group
In step 14, introduction of OH seems to be probable due to the high charge density in the opposite segment of the molecule. This transformation is described in reaction 9. Hydroxylation of secondary aliphatic carbon atoms in a ring system, adjacent to a sp2 carbon or bound to N or O, may be mediated by monooxyganases, dioxygenases and dehydrogenases present in several microorganisms like Brachymonas petroleovorans, Desulfococcus oleovorans, Aromatoleum aromaticum, Persephonella marina and Pseudomonas putida.

Reaction 9. Transformation from secondary aliphatic to secondary alcohol.
From the last intermediary compound, it is necessary to preserve aerobic conditions. Some patterns are identified as responsible for ring cleavage, as was mentioned above: first of all an hydroxyl group is inserted into the ring, next it is oxidized to ketone and lastly an oxygen atom is putted into the ring structure to conform an ester group (follow steps 14 to 16). Here it is most probable for the ring to be broken to aliphatic chains (step 17). This efficient sequence is completed again by the same microbial strains in steps 18 to 21. However, metabolite in step 21 exhibits so dense electrical charge that is too difficult to access inside the ring; others strains, moreover, are able to slash carboxylates and release space for further enzymatic attack (like Piruvate decarboxylase or decarboxylases). That kind of conversion (reaction 10) is commonly performed by fungal organisms such as Aspergillus niger and Aspergillus fumigates, and should correspond to evidences of partial mineralization reported by [8].
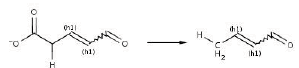
Reaction 10. Conversion from 2-or 3-substituted carboxylates to RH plus CO2.
The main scope of any bioremediation project is to take the former contaminant and direct it to a low size, non toxic and easily assimilable end products. In steps 23 and 24, rings structures are finally converted into aliphatic ones. Last compound (similar to fatty acids) are susceptible for further b-oxidation (reaction 11).
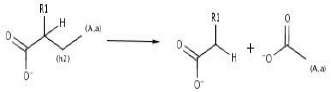
Reaction 11. b-oxidation (central metabolism).
Acetate and Malonate are the final compounds obtained after b-oxidation transformations. Acetate easily reaches Glycolysis/Gluconeogenesis pathway and Malonate gets into core metabolism through b-Alanine metabolism (figures not shown).
3.1 Treatment by BioBarriers
BioBarrier concept is founded on Permeable Reactive Barriers (PRB) technology, a passive packed bed-like system implemented for groundwater remediation [10]. Among other reactive media, PRB have used Zero Valent Metals (ZVM) as a catalyst for chemical modification (e.g. declhorination) and then detoxification of spills in soils [11]. A typical PRB is outlined in figure 4. Several contaminants, including chlorinated compounds, have been treated using ground Barriers and many design variations appeared since first application [12].
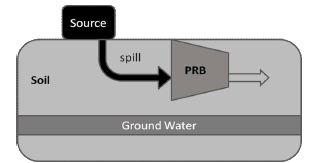
Figure 4. Basic Permeable Reactive Barriers
BioBarriers, a difference from traditional PBR, uses native microorganisms growing over solid particles as a catalyst instead of ZVM, making them a real cheap technology. For accurate designing of BioBarrier, we propose to start from metabolic prediction in order to have an idea of the process strategy which will make mineralization possible.
Since metabolic information indicates each of the transformations suffered by an initial compound, every single transformation step gives insights than can improve both design and operation of the treatment process. Some reports, in which BioBarrier concept is applied, the packed column (barrier) is operated as a single unit, providing same conditions along the barrier length [13]; this approach does not consider the fact that target compounds are not the same through it. Spending money having a longer column, or providing nutrients in regions where microorganisms don't need them, or having a system which doesn't guaranty complete toxicity, removal and/or mineralization could be avoided with appropriate designs.
We believe this can be overcome by having prior metabolic and microbial knowledge about the target compound.
The design of BioBarriers must be thought as a building, made of several bricks, one after another. Each brick corresponds to a single metabolic transformation and therefore should be treated as a particular reactor, which means, to provide particular inducers, nutrients, conditions and microbial strains. The length of each brick therefore must agree with the kinetic of biotransformation, transport properties and BioBarrier characteristics (e.g. porosity and permeability). In figures 5 and 6 the concept of BioBarrier design is presented.
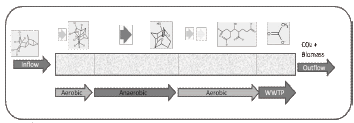
Figure 5. BioBarrier metabolic simulation-based approach design
According to the simulated pathway, the biodegradation of Dieldrin would occur efficiently under an Aerobic-Anaerobic-Aerobic sequential bioreactor. The length of the BioBarrier (and each process step) depends on how many biochemical transformations must occur and the kinetics of each reaction. Last metabolites (Acetate and Malonate) can be treated either in a final compartment inside the column or within a domestic Waste Water Treatment Plant (WWTP) (figure 6).
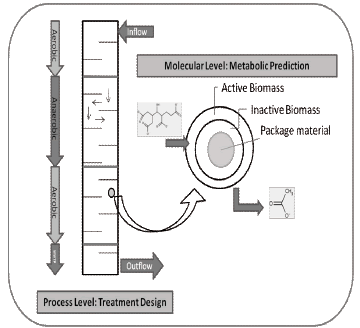
Figure 7. Molecular and process level approximation for BioBarrier Design
An optimal design for a BioBarrier (process level) can be achieved by a complete examination of the site, including reaction rates of the contaminants and properties of package material. Reaction rates or half-lives of contaminants in contact with the bio-reactive medium are necessary to determine the reactive length path that will provide adequate residence time for the degradation of each compound (according to the simulation).
Both batch and continuous column systems have been used in the past to determine reaction rates. Because continuous tests better simulate the dynamic flow of contaminants, testing in columns is by far the most common method. The use of databases and expert systems can, however, save money and time, as less experimentation and column experiments are required.
Traditional design equations have considered mostly a pseudo first-order rate kinetic approach without taking into account other degradation kinetics or transport process [10]. We propose the implementation of a basic phenomenological design equation (equation 1). This relationship can accurately predict transport through porous media (diffusion and also dispersion) and relate biodegradation, giving exact concentration of each species over a space coordinate. The application of this phenomenological equation will vary according with every metabolic step predicted before, so we can obtain all the information of species over time and space through BioBarrier.

As an example of Ri term, we will consider typical Monod kinetics; making a mass balance through biofilm layer (attached into package material), we obtain:
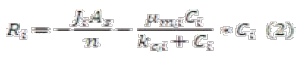
Where:
Ji: flux of substrate into a biofilm
As: Superficial area of biofilm
µmi, kci: Constant for Monod kinetics
The value of Ji can be calculated applying Fick's law inside the layer:
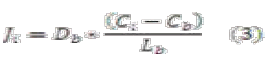
Where:
Db: Diffusivity in the biofilm
Lb: Thickness of biofilm
The solution of above equations could be done using finite differences method and considerations like unidirectional transport, constant dispersion and porosity or zero concentration of each compound inside deep biofilm.
The Combination of metabolic prediction (molecular level) and design equations (process level) should constitute and accurate approximation for an efficient design of hazardous wastes treatment process using BioBarriers (figure 6).
4. CONCLUSIONA complete biodegradation pathway for Dieldrin as a representative compound of Persistent Organic Pollutants was done using the UMBBD expert system. The simulated catabolism shows that it is possible to obtain a complete mineralization of former contaminant and that Aerobic-Anaerobic-Aerobic process strategy seems to be most successful for designing of a treatment plant. Basic relation between metabolic simulation and BioBarrier concept were also presented and insights regarding to advantages of this new approach and main phenomenological design equations, were finally outlined as a promissory approach for the design of hazardous waste treatment process.
5. RECOMMENDATIONS
The ideas presented in this report belong to a project under development in School of Environment and Geosciences, Faculty of Mines at Universidad Nacional de Colombia Sede Medellín. In order to complete foundations of BioBarriers design, it is important to collect more information regarding to kinetic parameters (reaction rates or half-lives) of each enzymatic transformation and consider others environmental and process parameters that can influence barrier performance. Numerical simulation should be performed and optimized further on, so unnecessary parameters will be removed or other important variables will be added. Further work is necessary in order to collect experimental data that allows verification of simulated results.
Appendix 1. Complete simulated pathway for the biodegradation of Dieldrin
[1] Resource futures international. Persistent Organic Pollutants and the Stokholm Convention: A resource Guide. World bank and CIDA, 2001. [ Links ]
[2] Liu, H., Ru, J., Qu, J., Dai, R., W, Z. and Hu, C. Removal of persistent organic pollutants from micro-polluted drinking water by triolein embedded absorbent. Bioresource Technology. Vol. 100, 2995-3002, 2009. [ Links ]
[3] Pham, T.D., Shrestha, R.A., Virkutyte, J. and Sillanpää, M. Combined ultrasonication and electrokinetic remediation for persistent organic removal from contaminated kaolin. Electrochimica Acta. Vol. 54, 1403-1407, 2009. [ Links ]
[4] Wacked, L.P. and Hershberger, C.D. Biocatalysis and Biodegradation. ASM press. 2001. [ Links ]
[5] University of Minnesota Biocatalysis and Biodegradation Database (UM-BBD) at www.umbbd.ahc.umn.edu. Last accessed may 5 2010. [ Links ]
[6] Gao, J., Ellis, L.B.M. and Wackett, L.P. The University of Minnesota Biocatalysis/ Biodegradation Database: improving public access. D488-D491 Nucleic Acids Research, Vol. 38, Database issue, 2010. [ Links ]
[7] Gao, B., Jia, L. and Xie, J. Aerobic degradation of dieldrin by Enterobacter sp. LY402. Journal of Biotechnology. Vol. 136S, S678-S707, 2008. [ Links ]
[8] Bixby, M.W., Boush, G.M. and Matsumura, F. Degradation of Dieldrin to Carbon Dioxide by a Soil Fungus Trichoderma Koningi. Bulletin of Environmental Contamination & Toxicology. Vol. 6, N° 6, 491-494, 1971. [ Links ]
[9] Chiu, T.C., Yen, J.H., Hsieh, Y.N. and Wang, Y.S. Reductive transformation of dieldrin under anaerobic sediment culture. Chemosphere. Vol. 60, 1182-1189, 2005. [ Links ]
[10] Gavaskar, A.R. Design and construction techniques for permeable reactive barriers. Journal of Hazardous Materials. Vol. 68, 41-71, 1999. [ Links ]
[11] Liu, S.J., Jiang, B., Huang, G.Q. and LI, X.G. Laboratory column study for remediation of MTBE-contaminated groundwater using a biological two-layer permeable barrier. Water Research. Vol. 40, 3401 - 3408, 2006. [ Links ]
[12] Blowes, D.W., Ptacek, C.J., Benner, S.G., Mcrae, C.W.T., Bennett, T.A. and Puls, R.W. Treatment of inorganic contaminants using permeable reactive barriers. Journal of Contaminant Hydrology. Vol. 45, 123-137, 2000. [ Links ]
[13] Hunter, W.J., Shaner, D.L. Nitrogen limited biobarriers remove atrazine from contaminated water: Laboratory studies. Journal of Contaminant Hydrology. Vol. 103, 29-37, 2009 [ Links ]














